

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144762 (US8952128, 21) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144761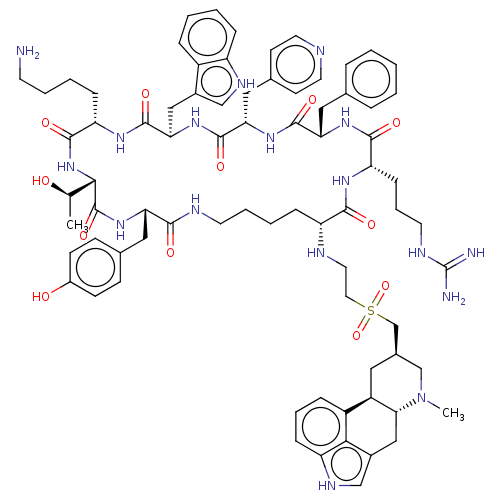 (US8952128, 20) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144757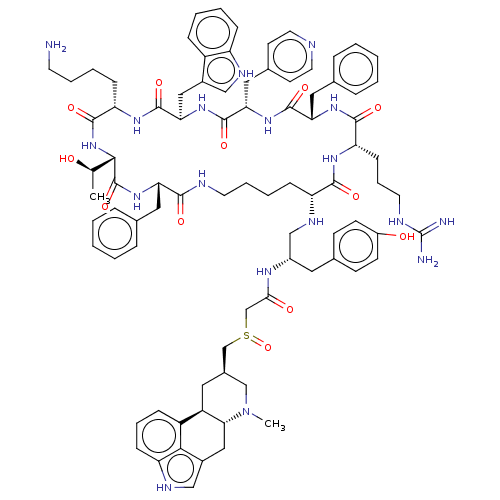 (US8952128, 15) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM104102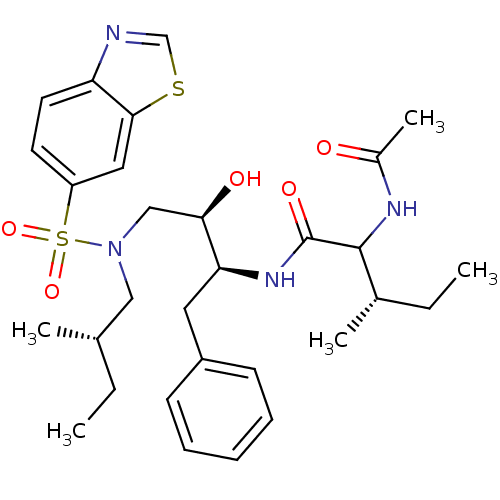 ((2S,3S)-2-(Acetylamino)-N-[(1S,2R)-3-[(6-benzothia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,I494L,I497V,V499I,D514N,E519D,R541K,D544E,Q553K,N572D,L573M] (Human immunodeficiency virus) | BDBM104102 ((2S,3S)-2-(Acetylamino)-N-[(1S,2R)-3-[(6-benzothia...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144770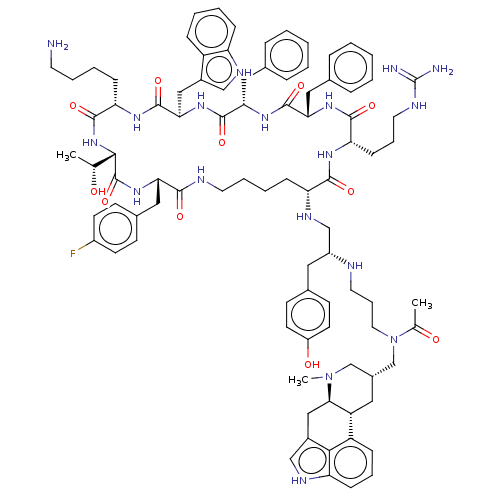 (US8952128, 28A) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144758 (US8952128, 16) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144737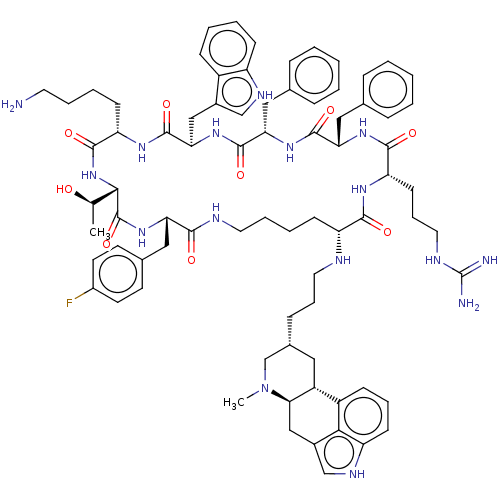 (US8952128, 1) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144755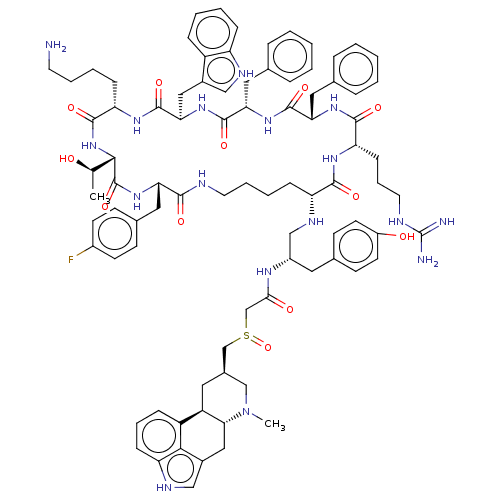 (US8952128, 13) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM104101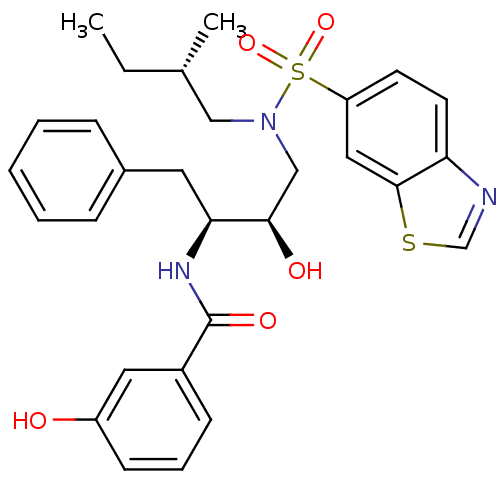 (MIT-2-AD-93 (AD-93)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,I497V,V499I,E519D,R541K,D544E,Q553K,A555V,G557S,I568V,L574M,L573M] (Human immunodeficiency virus) | BDBM104102 ((2S,3S)-2-(Acetylamino)-N-[(1S,2R)-3-[(6-benzothia...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,I494L,I497V,V499I,D514N,E519D,R541K,D544E,Q553K,N572D,L573M] (Human immunodeficiency virus) | BDBM104107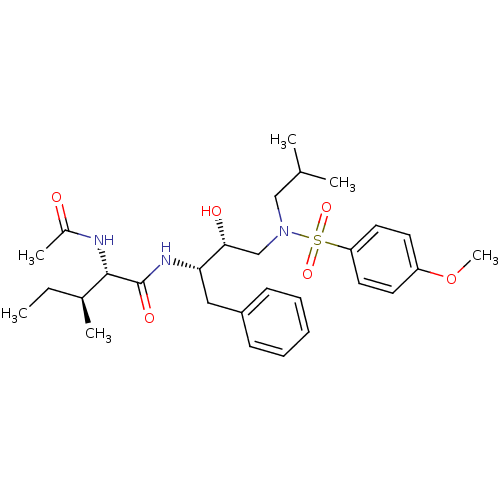 ((2S,3S)-2-(Acetylamino)-N-[(1S,2R)-2-hydroxy-3-[[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144752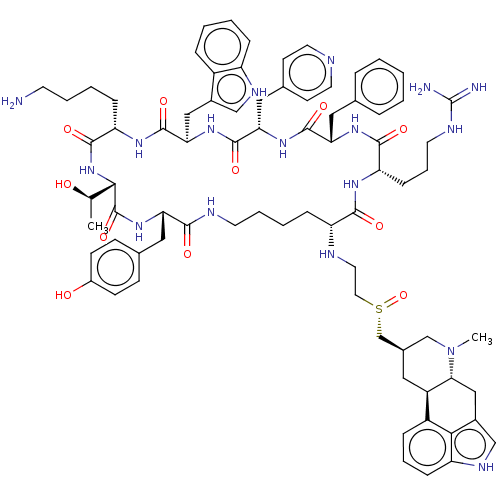 (US8952128, 11) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144756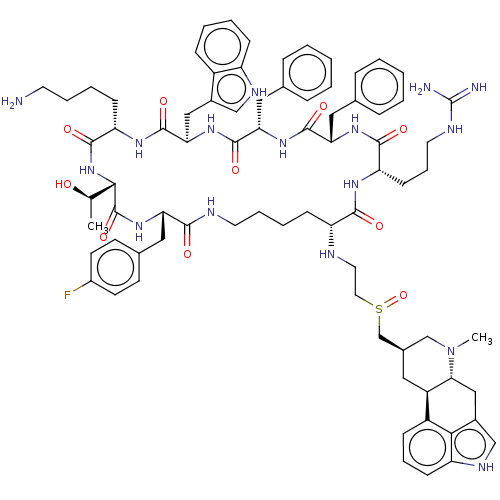 (US8952128, 14) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144766 (US8952128, 25) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 1403-10 (1994) BindingDB Entry DOI: 10.7270/Q2154FJQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144753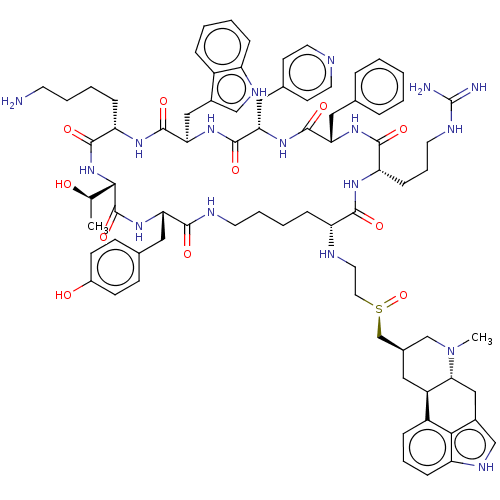 (US8952128, 12 | US8952128, 46) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | -58.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50007692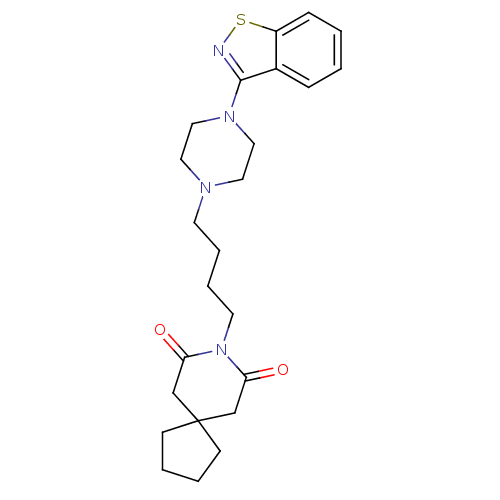 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 1403-10 (1994) BindingDB Entry DOI: 10.7270/Q2154FJQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM104104 (N-[(1S,2R)-3-[(6-Benzothiazolylsulfonyl)(3-phenylp...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144748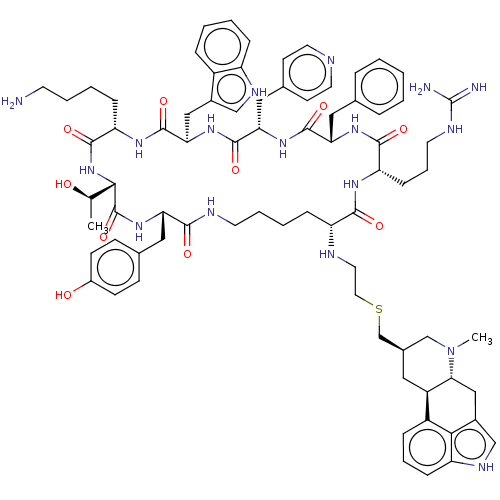 (US8952128, 7) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM144757 (US8952128, 15) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144759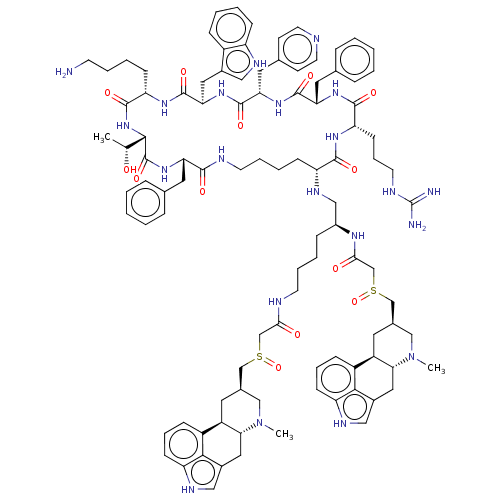 (US8952128, 17) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144765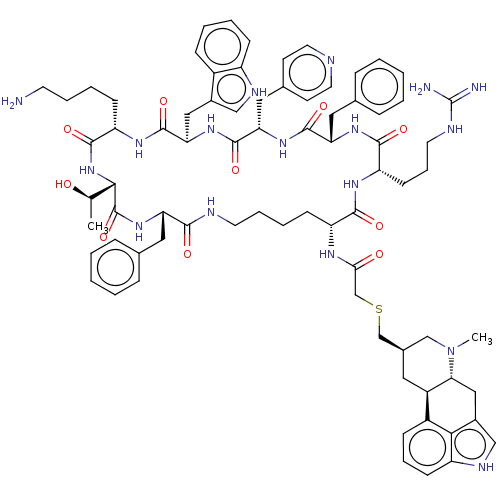 (US8952128, 24) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144764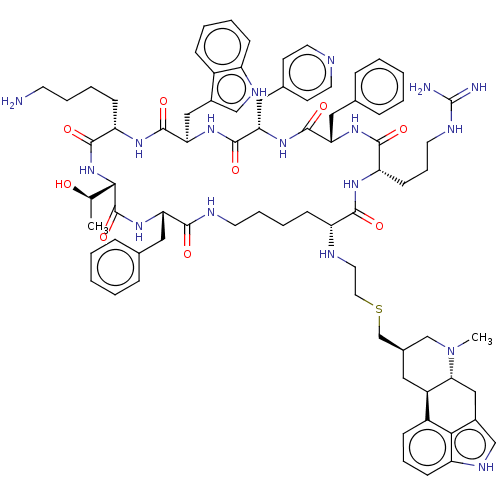 (US8952128, 23) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | -57.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM144752 (US8952128, 11) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | -57.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM104102 ((2S,3S)-2-(Acetylamino)-N-[(1S,2R)-3-[(6-benzothia...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144740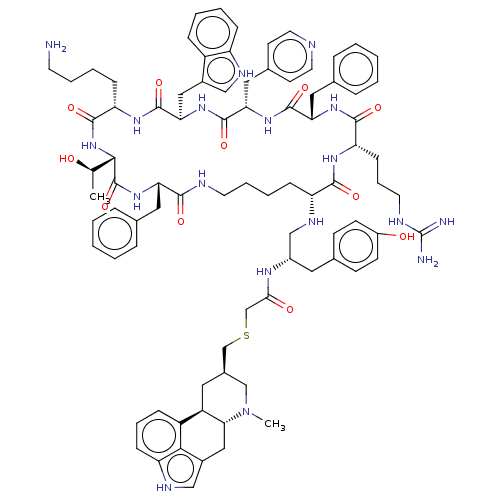 (US8952128, 4) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM104103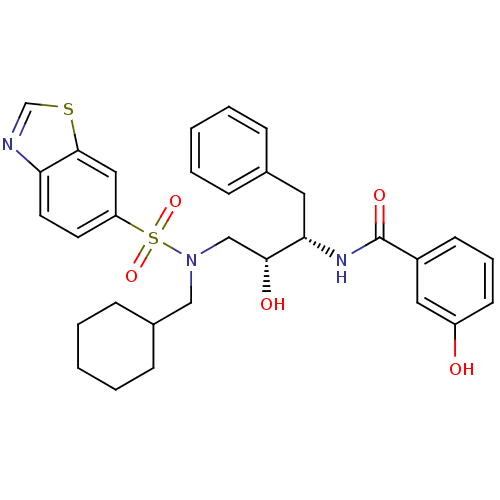 (N-[(1S,2R)-3-[(6-Benzothiazolylsulfonyl)(cyclohexy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144750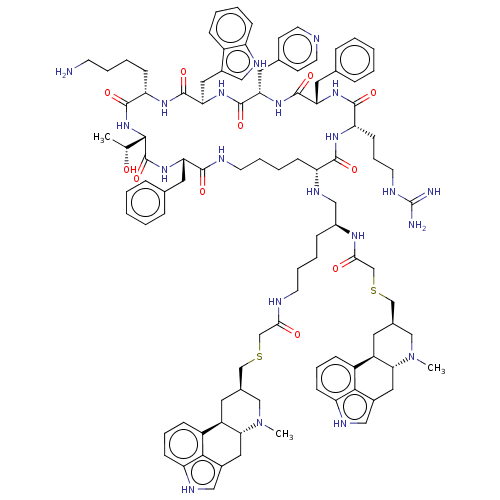 (US8952128, 9) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144772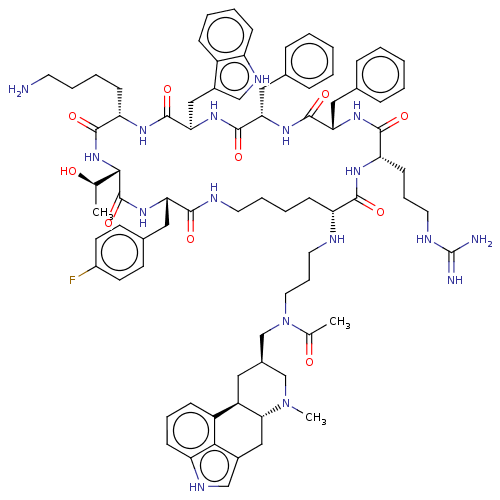 (US8952128, 29A) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144771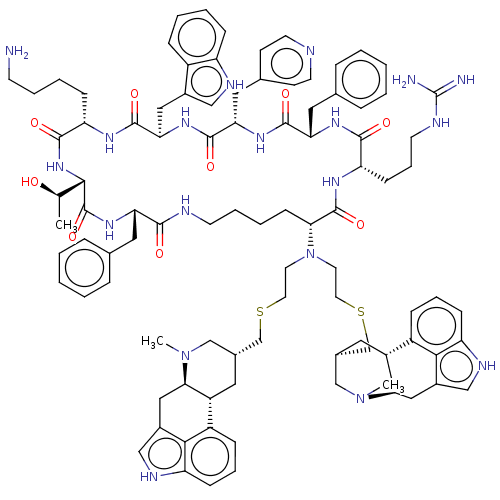 (US8952128, 29) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | -56.2 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM104107 ((2S,3S)-2-(Acetylamino)-N-[(1S,2R)-2-hydroxy-3-[[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM104107 ((2S,3S)-2-(Acetylamino)-N-[(1S,2R)-2-hydroxy-3-[[(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144745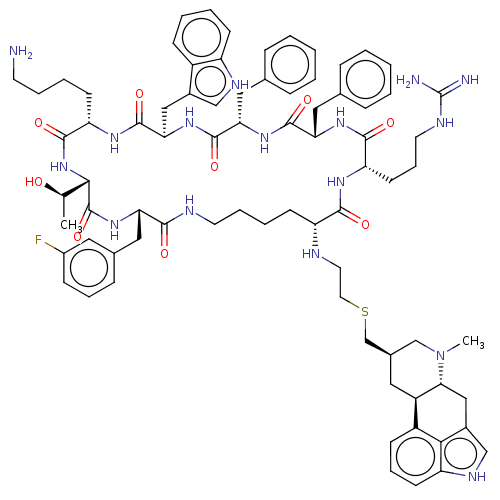 (US8952128, 6C) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM144751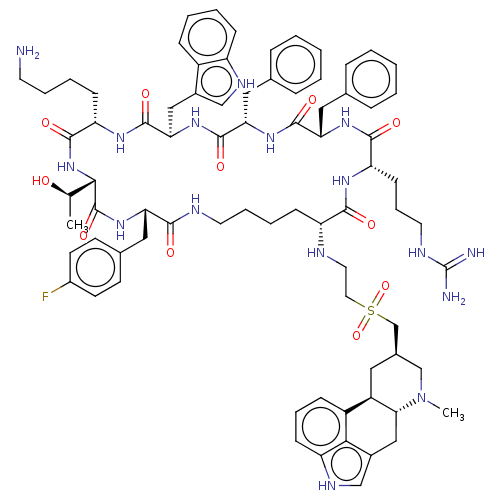 (US8952128, 10) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144760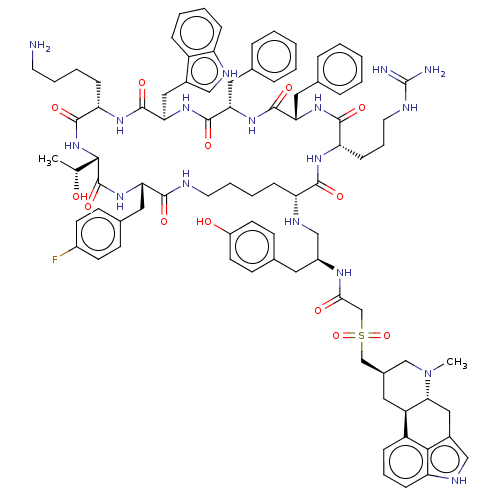 (US8952128, 19) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM21392 (3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by PDSP Ki Database | J Biol Chem 268: 18200-4 (1993) BindingDB Entry DOI: 10.7270/Q2V1239R | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144778 (US8952128, 35) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144751 (US8952128, 10) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM79181 (10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 1403-10 (1994) BindingDB Entry DOI: 10.7270/Q2154FJQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM104106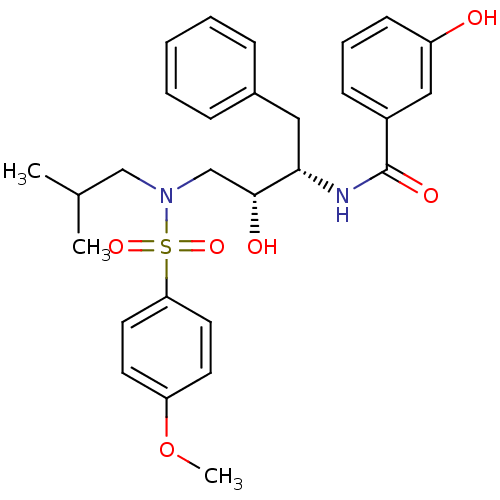 (MIT-2-KB-83 (KB-83)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology | Assay Description HIV protease inhibitor activities were determined by fluorescence resonance energy transfer (FRET) method. Protease substrate, Arg-Glu-(EDANS)-Ser-G... | ACS Chem Biol 8: 2433-41 (2013) Article DOI: 10.1021/cb400468c BindingDB Entry DOI: 10.7270/Q2R2101Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human androgen receptor expressed in monkey COS7 cells by whole cell binding assay | Bioorg Med Chem Lett 18: 3431-5 (2008) Article DOI: 10.1016/j.bmcl.2008.03.085 BindingDB Entry DOI: 10.7270/Q25M66J9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 50: 5049-52 (2007) Article DOI: 10.1021/jm070231h BindingDB Entry DOI: 10.7270/Q29Z935Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144749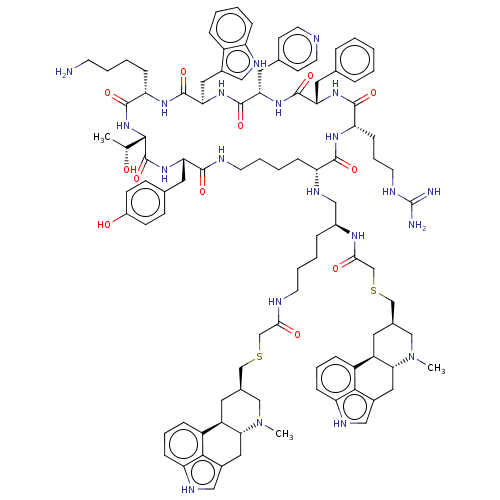 (US8952128, 8) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM144755 (US8952128, 13) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144776 (US8952128, 33) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.240 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144789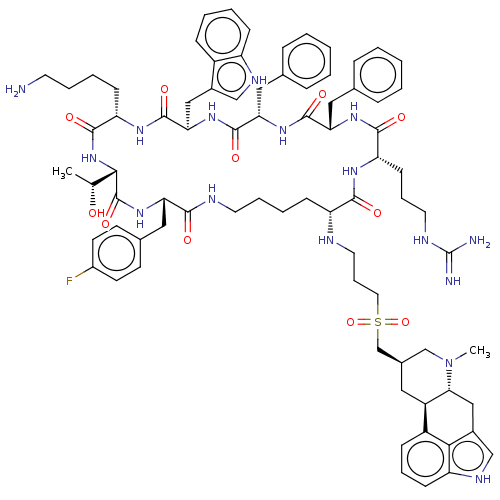 (US8952128, 48) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 1403-10 (1994) BindingDB Entry DOI: 10.7270/Q2154FJQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144744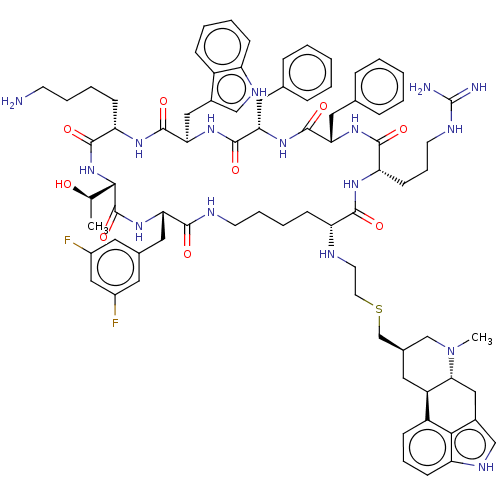 (US8952128, 6B) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM144773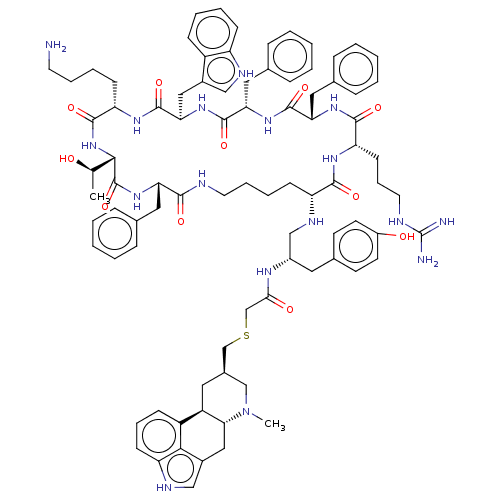 (US8952128, 30 | US8952128, 32) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.6 | 25 |
Ipsen Pharma S.A.S. US Patent | Assay Description Membranes for in vitro receptor binding assays were obtained by the following procedures. CHO-K1 cells expressing one of the somatostatin receptors w... | US Patent US8952128 (2015) BindingDB Entry DOI: 10.7270/Q2057DNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3816 total ) | Next | Last >> |