

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Homo sapiens (Human)) | BDBM50068901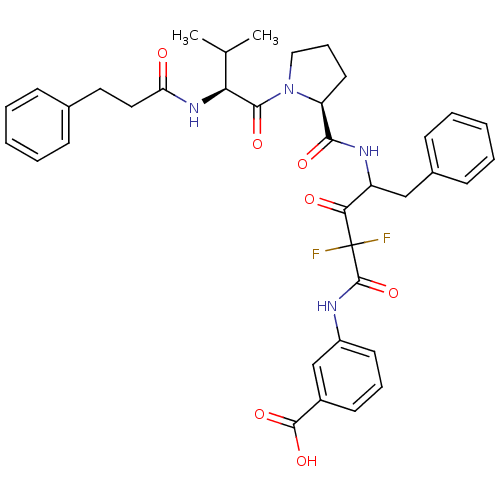 (3-[2,2-Difluoro-4-({(S)-1-[(S)-3-methyl-2-(3-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068918 ((S)-4-((2S,3S)-2-Benzyloxycarbonylamino-3-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068899 (3-(4-{[(S)-1-((S)-2-Benzoylamino-3-methyl-butyryl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068919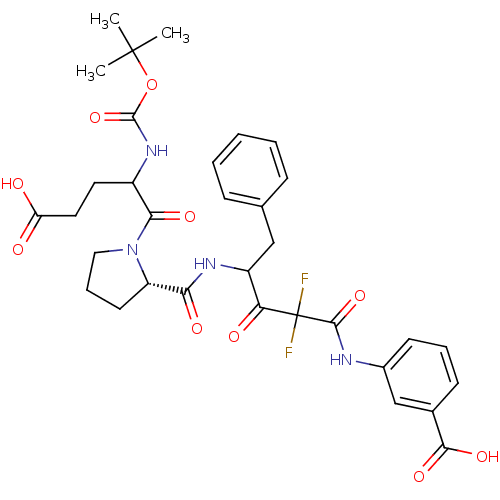 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068894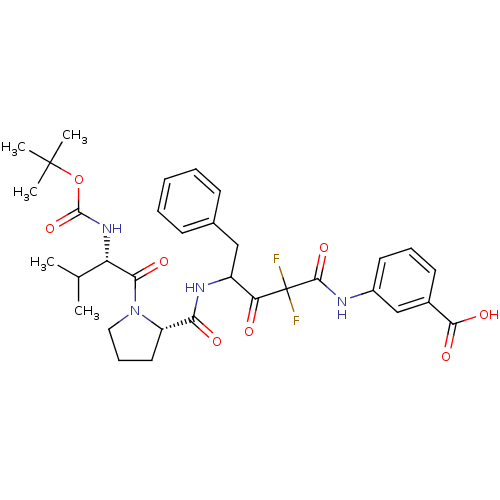 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068911 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068917 (5-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068889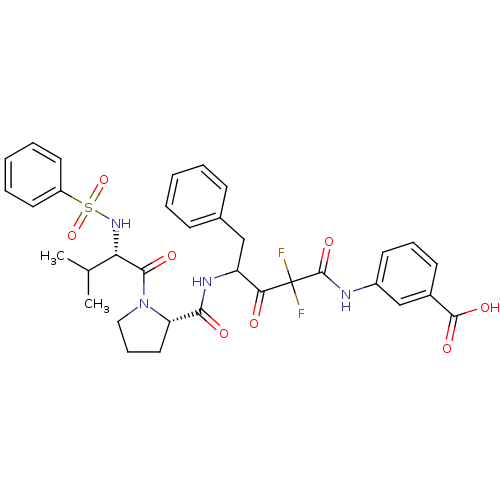 (3-(4-{[(S)-1-((S)-2-Benzenesulfonylamino-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068896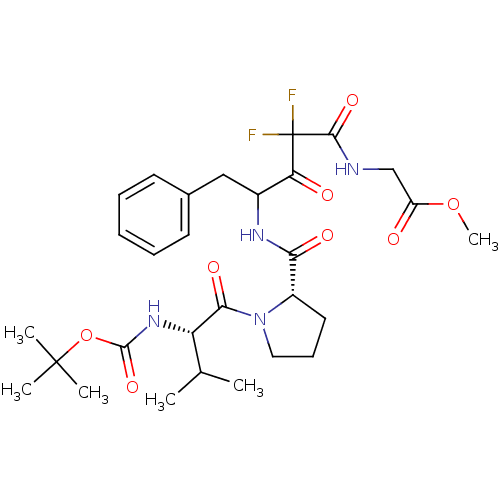 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068915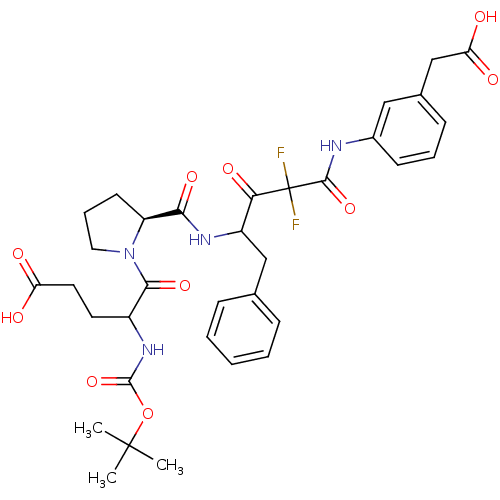 (5-{(S)-2-[1-Benzyl-3-(3-carboxymethyl-phenylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068887 (4-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068892 (3-(4-{[(S)-1-((S)-2-Acetylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068892 (3-(4-{[(S)-1-((S)-2-Acetylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068903 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068905 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068912 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-4-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068908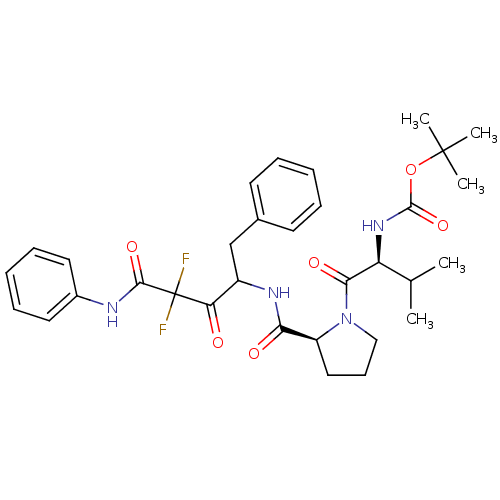 (CHEMBL352917 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068909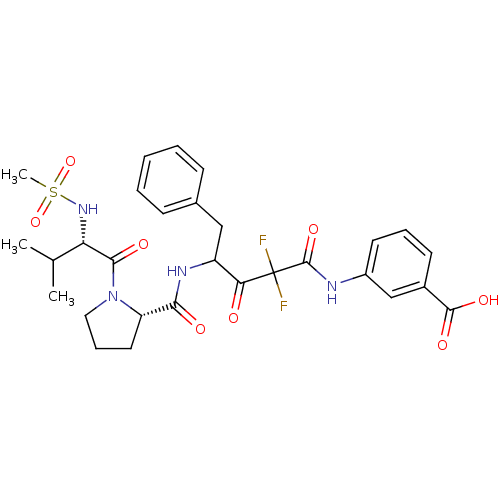 (3-(2,2-Difluoro-4-{[(S)-1-((S)-2-methanesulfonylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068897 (CHEMBL171119 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068901 (3-[2,2-Difluoro-4-({(S)-1-[(S)-3-methyl-2-(3-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068913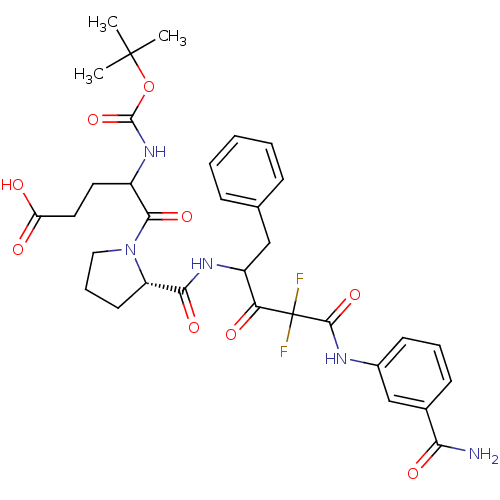 (5-{(S)-2-[1-Benzyl-3-(3-carbamoyl-phenylcarbamoyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068914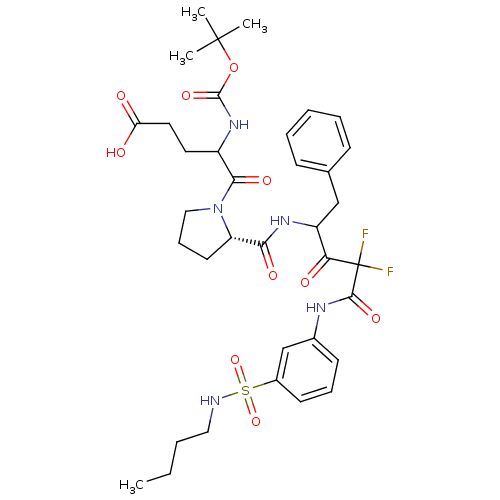 (5-{(S)-2-[1-Benzyl-3-(3-butylsulfamoyl-phenylcarba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068885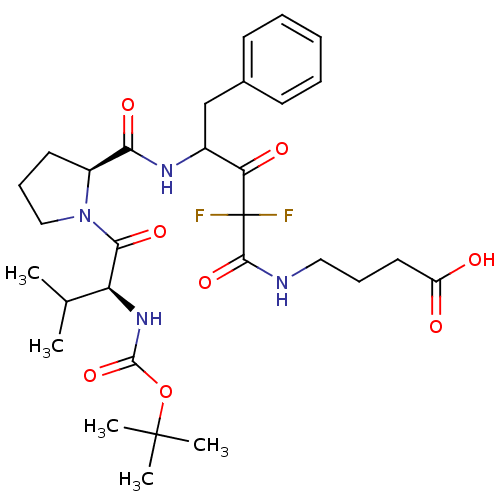 (4-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068920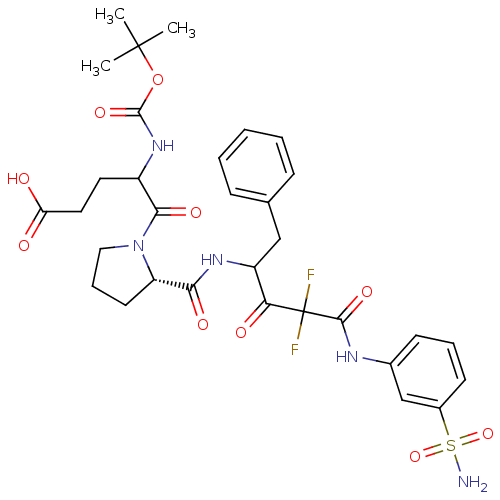 (5-{(S)-2-[1-Benzyl-3,3-difluoro-2-oxo-3-(3-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068891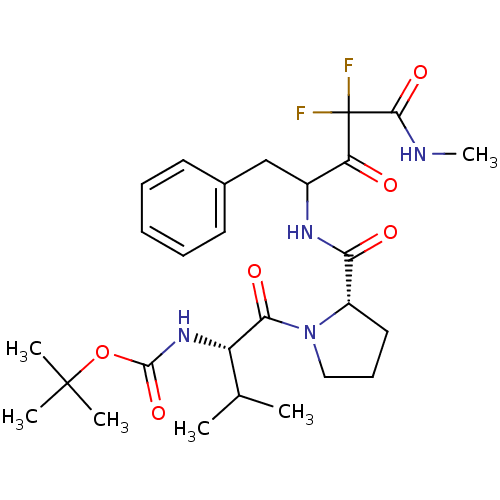 (CHEMBL170683 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068890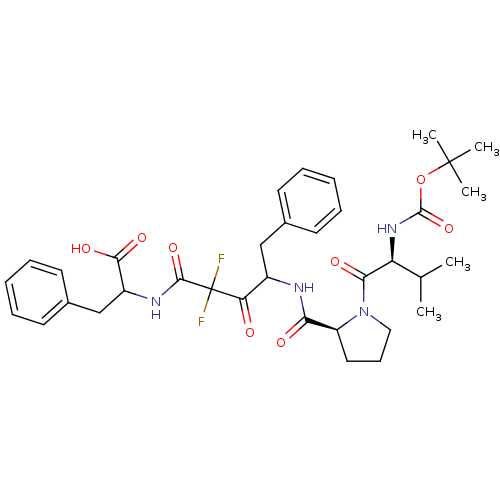 (2-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50068918 ((S)-4-((2S,3S)-2-Benzyloxycarbonylamino-3-methyl-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Bovine Chymotrypsinogen | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068904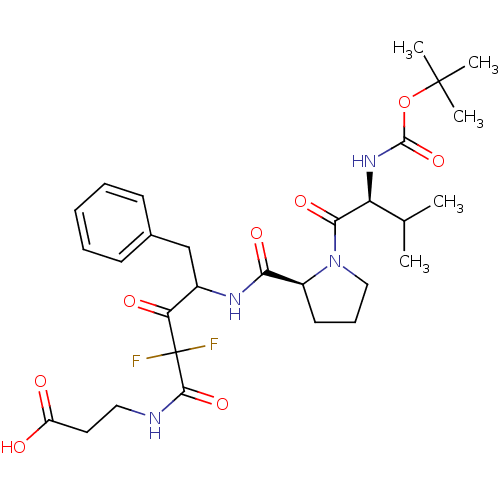 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068916 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-3-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068898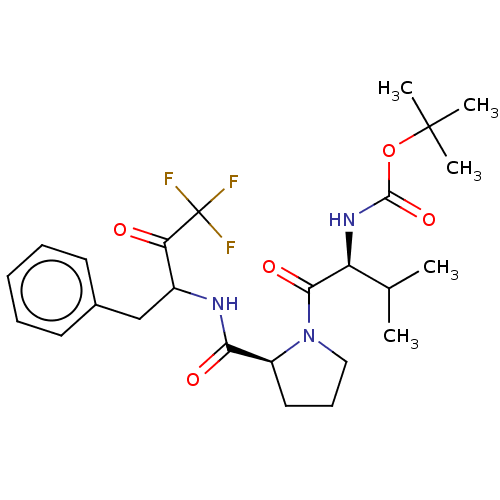 (BDBM50281588 | CHEMBL147013 | {1-[2-(1-Benzyl-3,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068906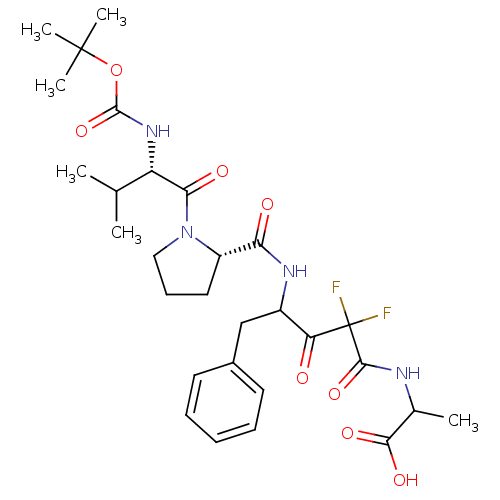 (2-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068908 (CHEMBL352917 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068910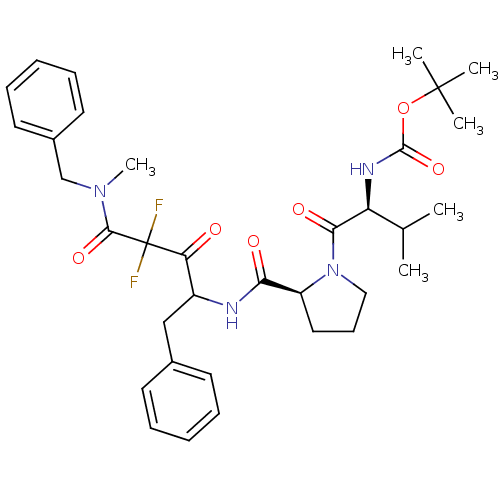 (((S)-1-{(S)-2-[1-Benzyl-3-(benzyl-methyl-carbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068888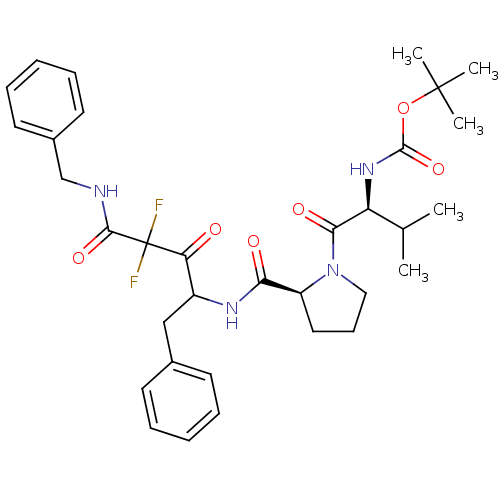 (CHEMBL170074 | {(S)-1-[(S)-2-(1-Benzyl-3-benzylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068888 (CHEMBL170074 | {(S)-1-[(S)-2-(1-Benzyl-3-benzylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 92.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068898 (BDBM50281588 | CHEMBL147013 | {1-[2-(1-Benzyl-3,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068887 (4-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068909 (3-(2,2-Difluoro-4-{[(S)-1-((S)-2-methanesulfonylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068899 (3-(4-{[(S)-1-((S)-2-Benzoylamino-3-methyl-butyryl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068905 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50068911 (3-(4-{[(S)-1-(2-tert-Butoxycarbonylamino-3-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 364 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Bovine Chymotrypsinogen | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068894 (3-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 364 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50068914 (5-{(S)-2-[1-Benzyl-3-(3-butylsulfamoyl-phenylcarba...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Bovine Chymotrypsinogen | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068895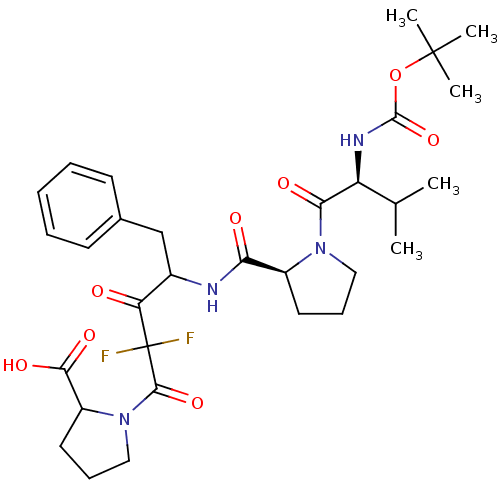 (1-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068886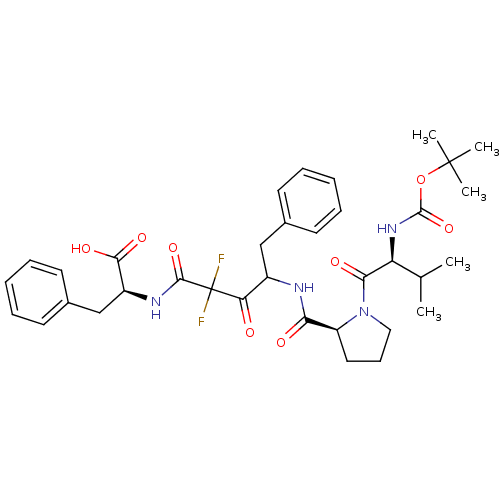 ((S)-2-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068889 (3-(4-{[(S)-1-((S)-2-Benzenesulfonylamino-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 554 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068900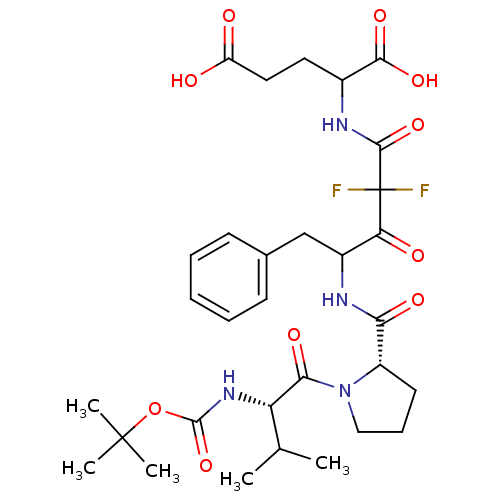 (2-(4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50068920 (5-{(S)-2-[1-Benzyl-3,3-difluoro-2-oxo-3-(3-sulfamo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Bovine Chymotrypsinogen | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068893 ((4-{[(S)-1-((S)-2-tert-Butoxycarbonylamino-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068891 (CHEMBL170683 | {(S)-1-[(S)-2-(1-Benzyl-3,3-difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |