

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM50239965 (Bromhexine | CHEBI:77032) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM420291 (0591-5329 | 4-(Methoxycarbonyl)phenyl thiophene-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM50022172 ((7-Hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid meth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59162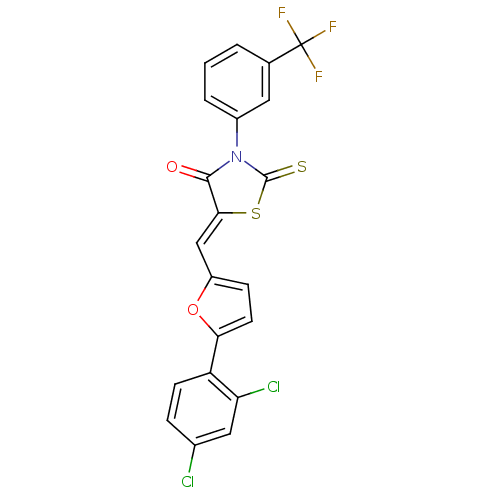 (8047577) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
University of Washington | Assay Description Inhibition of factor X activation assay. A commercially available chromogenic assay that measures the rate of factor X activation to measure inhibit... | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59159 (6190013) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Washington | Assay Description Inhibition of factor VIII ELISA. The interaction between factor VIII and PS was measured by using an enzyme-linked immunoadsorbent assay (ELISA). | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59162 (8047577) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59162 (8047577) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Washington | Assay Description Inhibition of factor VIII ELISA. The interaction between factor VIII and PS was measured by using an enzyme-linked immunoadsorbent assay (ELISA). | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM420294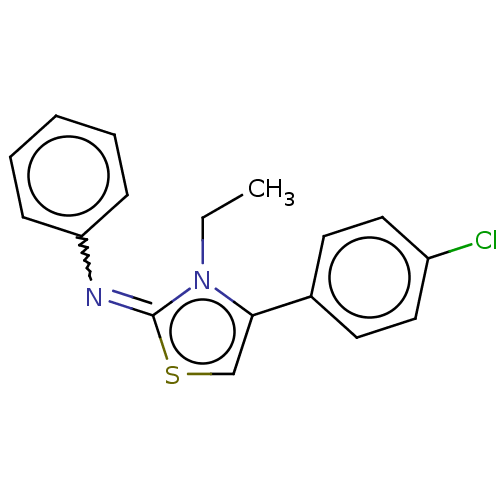 (8008-1235 | N-[4-(4-chlorophenyl)-3-ethyl-1,3-thia...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase UniChem | Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 2 (Homo sapiens (Human)) | BDBM420292 (4401-0077 | Antidepressant agent 1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE UniChem | Article PubMed | n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center | Assay Description To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... | Cancer Discov 4: 1310-25 (2014) Article DOI: 10.1158/2159-8290.CD-13-1010 BindingDB Entry DOI: 10.7270/Q2HD7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262769 (CHEMBL4062161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Merrill Bloedel Hearing Research Center, University of Washington , Seattle, Washington 98195, United States. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as decrease in peak tail current amplitude by path clamp method | J Med Chem 61: 84-97 (2018) Article DOI: 10.1021/acs.jmedchem.7b00932 BindingDB Entry DOI: 10.7270/Q2697615 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262740 (CHEMBL4074914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Merrill Bloedel Hearing Research Center, University of Washington , Seattle, Washington 98195, United States. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as decrease in peak tail current amplitude by path clamp method | J Med Chem 61: 84-97 (2018) Article DOI: 10.1021/acs.jmedchem.7b00932 BindingDB Entry DOI: 10.7270/Q2697615 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262771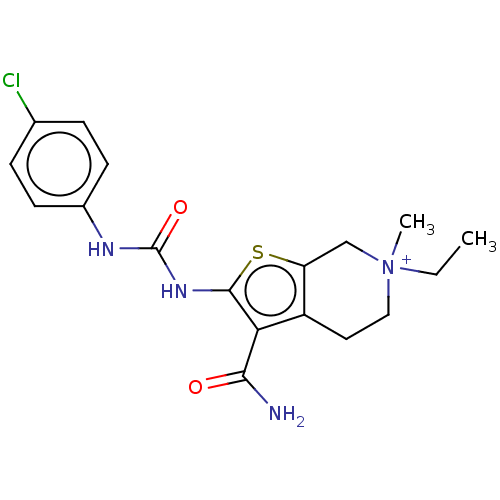 (CHEMBL4074997) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Merrill Bloedel Hearing Research Center, University of Washington , Seattle, Washington 98195, United States. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as decrease in peak tail current amplitude by path clamp method | J Med Chem 61: 84-97 (2018) Article DOI: 10.1021/acs.jmedchem.7b00932 BindingDB Entry DOI: 10.7270/Q2697615 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262772 (CHEMBL4085169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Merrill Bloedel Hearing Research Center, University of Washington , Seattle, Washington 98195, United States. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as decrease in peak tail current amplitude by path clamp method | J Med Chem 61: 84-97 (2018) Article DOI: 10.1021/acs.jmedchem.7b00932 BindingDB Entry DOI: 10.7270/Q2697615 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59164 (8048422) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Washington | Assay Description Inhibition of factor VIII ELISA. The interaction between factor VIII and PS was measured by using an enzyme-linked immunoadsorbent assay (ELISA). | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59164 (8048422) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59154 (5739991) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59163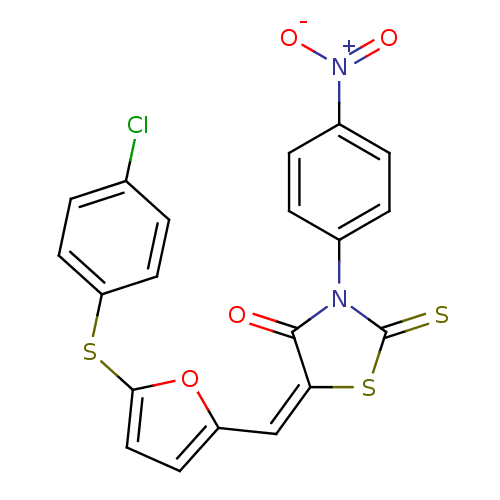 (6789536) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Washington | Assay Description Inhibition of factor VIII ELISA. The interaction between factor VIII and PS was measured by using an enzyme-linked immunoadsorbent assay (ELISA). | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59161 (5738875) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
University of Washington | Assay Description Inhibition of factor X activation assay. A commercially available chromogenic assay that measures the rate of factor X activation to measure inhibit... | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262768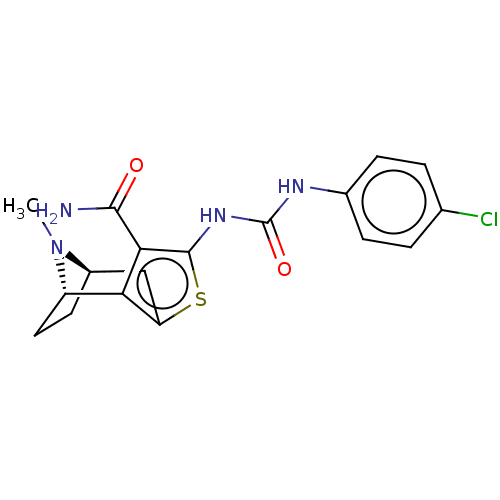 (CHEMBL4096279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Merrill Bloedel Hearing Research Center, University of Washington , Seattle, Washington 98195, United States. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as decrease in peak tail current amplitude by path clamp method | J Med Chem 61: 84-97 (2018) Article DOI: 10.1021/acs.jmedchem.7b00932 BindingDB Entry DOI: 10.7270/Q2697615 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59163 (6789536) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
University of Washington | Assay Description Inhibition of factor X activation assay. A commercially available chromogenic assay that measures the rate of factor X activation to measure inhibit... | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59161 (5738875) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59163 (6789536) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-3, mitochondrial (Homo sapiens (Human)) | BDBM50004764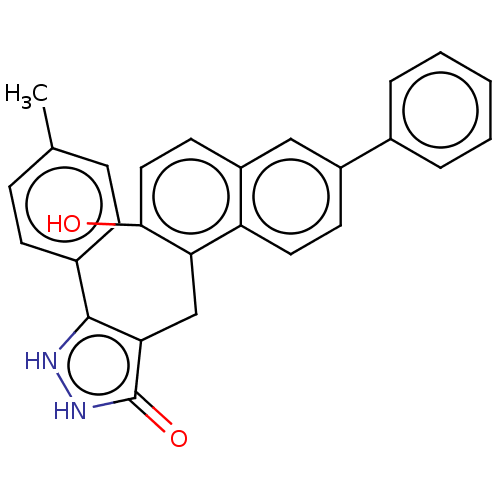 (CHEMBL3233733) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description Inhibition of SIRT3 (unknown origin) by luminescence assay | J Med Chem 57: 3283-94 (2014) Article DOI: 10.1021/jm4018064 BindingDB Entry DOI: 10.7270/Q28G8N7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59165 (8056354) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59165 (8056354) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.51E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Washington | Assay Description Inhibition of factor VIII ELISA. The interaction between factor VIII and PS was measured by using an enzyme-linked immunoadsorbent assay (ELISA). | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59153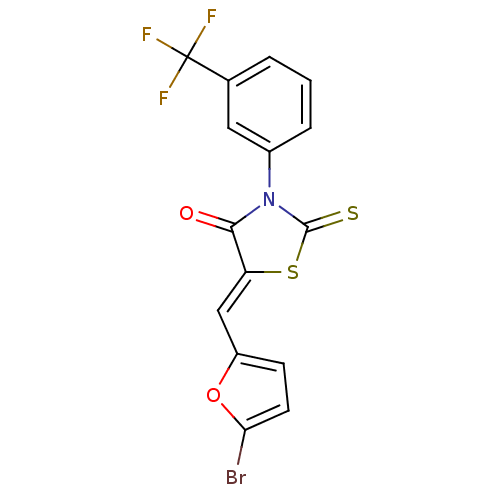 (5737176) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.66E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59164 (8048422) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
University of Washington | Assay Description Inhibition of factor X activation assay. A commercially available chromogenic assay that measures the rate of factor X activation to measure inhibit... | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59165 (8056354) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
University of Washington | Assay Description Inhibition of factor X activation assay. A commercially available chromogenic assay that measures the rate of factor X activation to measure inhibit... | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59161 (5738875) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.83E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Washington | Assay Description Inhibition of factor VIII ELISA. The interaction between factor VIII and PS was measured by using an enzyme-linked immunoadsorbent assay (ELISA). | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59159 (6190013) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
University of Washington | Assay Description Inhibition of factor X activation assay. A commercially available chromogenic assay that measures the rate of factor X activation to measure inhibit... | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59159 (6190013) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.48E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59153 (5737176) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
University of Washington | Assay Description Inhibition of factor X activation assay. A commercially available chromogenic assay that measures the rate of factor X activation to measure inhibit... | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent histone deacetylase SIR2 (Saccharomyces cerevisiae) | BDBM50146058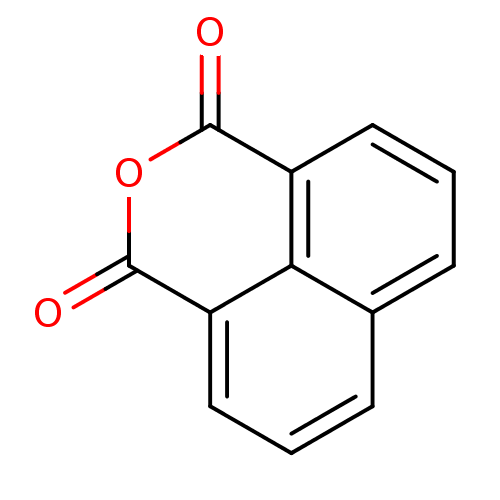 (Benzo[de]isochromene-1,3-dione | CHEMBL316059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description In vitro inhibition of sirtuin 2 was evaluated using yeast whole cell lysates | J Med Chem 47: 2635-44 (2004) Article DOI: 10.1021/jm030473r BindingDB Entry DOI: 10.7270/Q228071B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59153 (5737176) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.25E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Washington | Assay Description Inhibition of factor VIII ELISA. The interaction between factor VIII and PS was measured by using an enzyme-linked immunoadsorbent assay (ELISA). | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59154 (5739991) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
University of Washington | Assay Description Inhibition of factor VIII ELISA. The interaction between factor VIII and PS was measured by using an enzyme-linked immunoadsorbent assay (ELISA). | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59154 (5739991) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | 7.9 | n/a |
University of Washington | Assay Description Inhibition of factor X activation assay. A commercially available chromogenic assay that measures the rate of factor X activation to measure inhibit... | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59157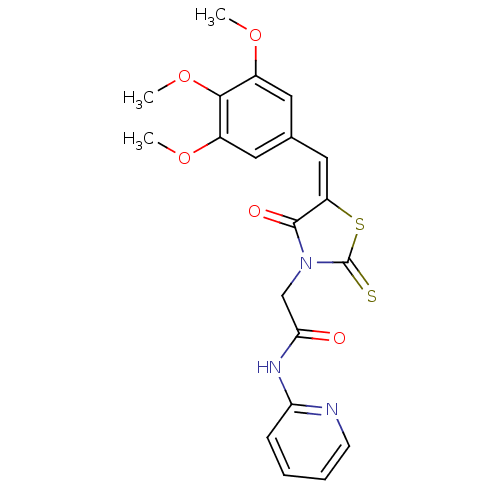 (6126765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent histone deacetylase SIR2 (Saccharomyces cerevisiae) | BDBM50146048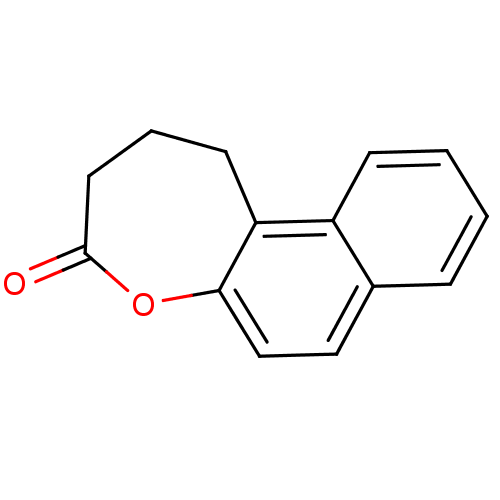 (2,3-Dihydro-1H-naphtho[2,1-b]oxepin-4-one | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description In vitro inhibition of sirtuin 2 was evaluated using yeast whole cell lysates | J Med Chem 47: 2635-44 (2004) Article DOI: 10.1021/jm030473r BindingDB Entry DOI: 10.7270/Q228071B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59160 (6209549) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262767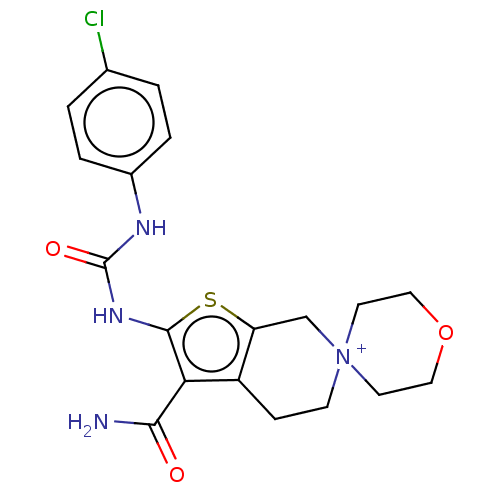 (CHEMBL4101606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Merrill Bloedel Hearing Research Center, University of Washington , Seattle, Washington 98195, United States. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as decrease in peak tail current amplitude by path clamp method | J Med Chem 61: 84-97 (2018) Article DOI: 10.1021/acs.jmedchem.7b00932 BindingDB Entry DOI: 10.7270/Q2697615 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50004887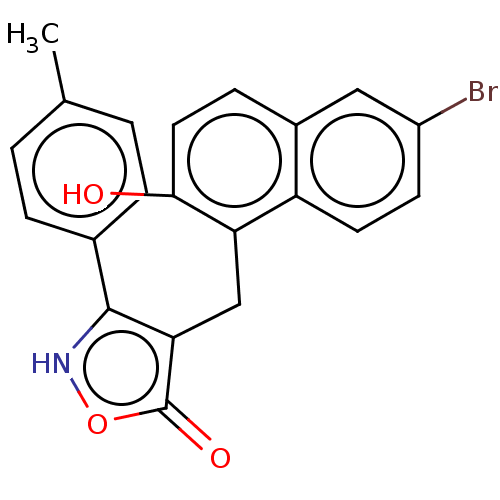 (CHEMBL3233749) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description Inhibition of SIRT2 (unknown origin) by luminescence assay | J Med Chem 57: 3283-94 (2014) Article DOI: 10.1021/jm4018064 BindingDB Entry DOI: 10.7270/Q28G8N7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262770 (CHEMBL4101684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Merrill Bloedel Hearing Research Center, University of Washington , Seattle, Washington 98195, United States. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells assessed as decrease in peak tail current amplitude by path clamp method | J Med Chem 61: 84-97 (2018) Article DOI: 10.1021/acs.jmedchem.7b00932 BindingDB Entry DOI: 10.7270/Q2697615 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM50004795 (CHEMBL3233748) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human SIRT1 expressed in Escherichia coli BL21(DE3) by luminescence assay | J Med Chem 57: 3283-94 (2014) Article DOI: 10.1021/jm4018064 BindingDB Entry DOI: 10.7270/Q28G8N7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59155 (5924029) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent histone deacetylase SIR2 (Saccharomyces cerevisiae) | BDBM50146067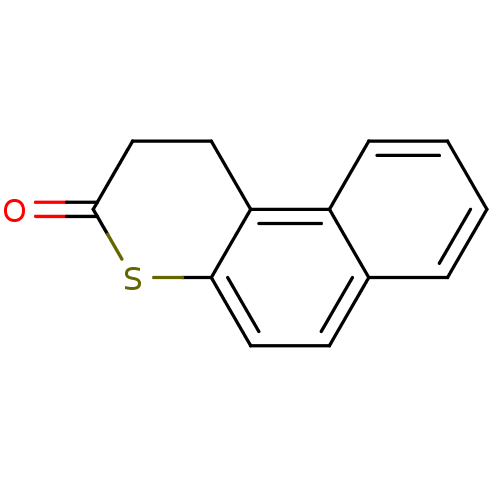 (1,2-Dihydro-benzo[f]thiochromen-3-one | CHEMBL3143...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description In vitro inhibition of sirtuin 2 was evaluated using yeast whole cell lysates | J Med Chem 47: 2635-44 (2004) Article DOI: 10.1021/jm030473r BindingDB Entry DOI: 10.7270/Q228071B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM50004777 (CHEMBL3233742) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human SIRT1 expressed in Escherichia coli BL21(DE3) by luminescence assay | J Med Chem 57: 3283-94 (2014) Article DOI: 10.1021/jm4018064 BindingDB Entry DOI: 10.7270/Q28G8N7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VIII (Homo sapiens (Human)) | BDBM59156 (6072484 | cid_726942) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Washington | Assay Description Inhibition of factor VIII C2 domain. | Chem Biol 11: 1413-22 (2004) Article DOI: 10.1016/j.chembiol.2004.08.006 BindingDB Entry DOI: 10.7270/Q29W0CX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent histone deacetylase SIR2 (Saccharomyces cerevisiae) | BDBM50146063 (2-Isobutyl-1,2-dihydro-benzo[f]chromen-3-one | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description In vitro inhibition of sirtuin 2 was evaluated using yeast whole cell lysates | J Med Chem 47: 2635-44 (2004) Article DOI: 10.1021/jm030473r BindingDB Entry DOI: 10.7270/Q228071B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent histone deacetylase SIR2 (Saccharomyces cerevisiae) | BDBM50146044 (2-Allyl-1,2-dihydro-benzo[f]chromen-3-one | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description In vitro inhibition of sirtuin 2 was evaluated using yeast whole cell lysates | J Med Chem 47: 2635-44 (2004) Article DOI: 10.1021/jm030473r BindingDB Entry DOI: 10.7270/Q228071B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent histone deacetylase SIR2 (Saccharomyces cerevisiae) | BDBM50146052 (5-Benzyloxy-1,2-dihydro-benzo[f]chromen-3-one | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center Curated by ChEMBL | Assay Description In vitro inhibition of sirtuin 2 was evaluated using yeast whole cell lysates | J Med Chem 47: 2635-44 (2004) Article DOI: 10.1021/jm030473r BindingDB Entry DOI: 10.7270/Q228071B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |