

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50059955 (4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Curated by ChEMBL | Assay Description Inhibitory activity of compound against rat liver Dihydrofolate reductase | J Med Chem 40: 3040-8 (1997) Article DOI: 10.1021/jm970055k BindingDB Entry DOI: 10.7270/Q2C53JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50059948 (4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Curated by ChEMBL | Assay Description Inhibitory activity of compound against rat liver Dihydrofolate reductase | J Med Chem 40: 3040-8 (1997) Article DOI: 10.1021/jm970055k BindingDB Entry DOI: 10.7270/Q2C53JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043393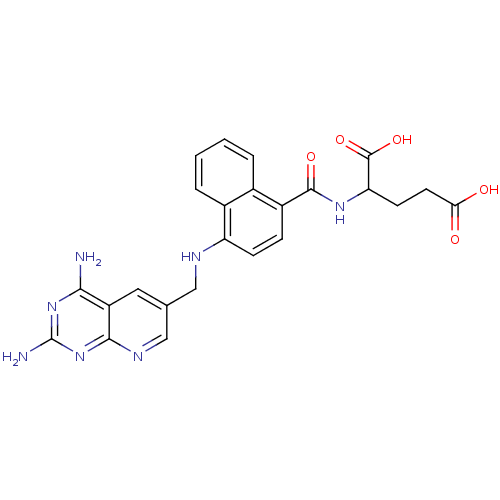 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043396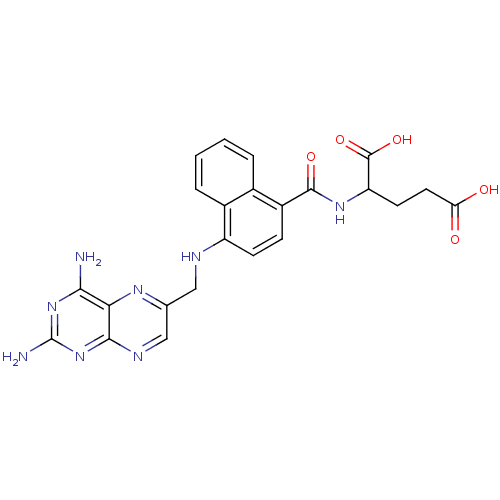 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043399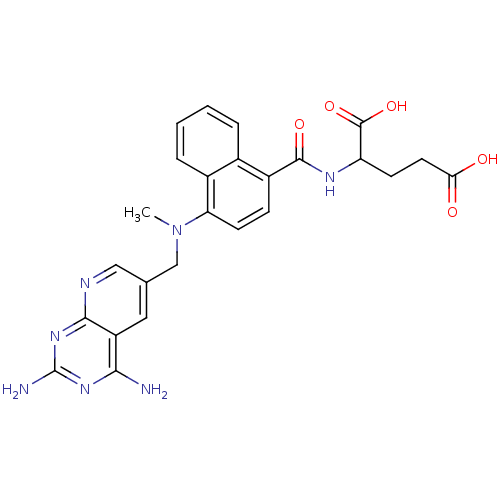 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of dihydrofolate reductase (DHFR) in L1210 cells | J Med Chem 35: 3002-6 (1992) BindingDB Entry DOI: 10.7270/Q2MP527Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50098576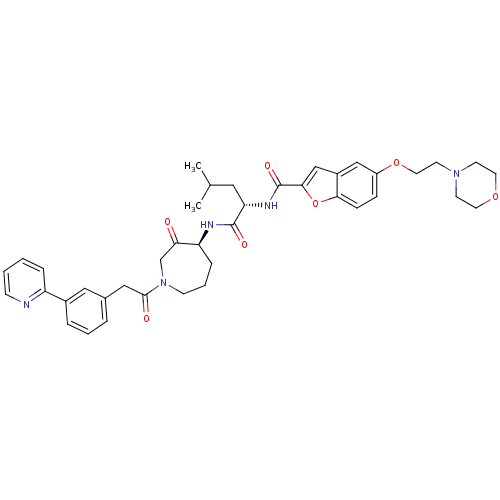 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043395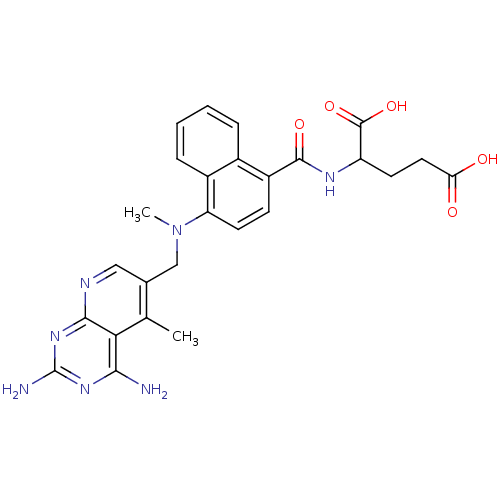 (2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043400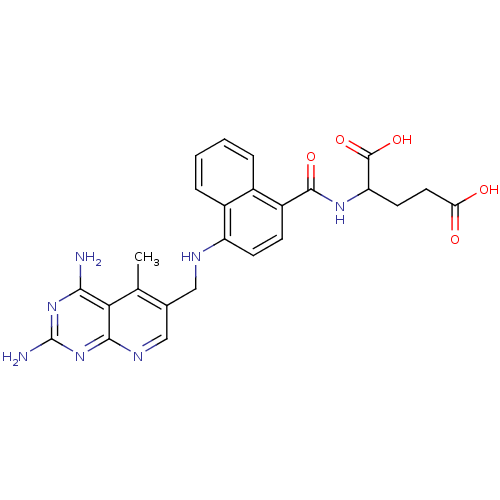 (2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043398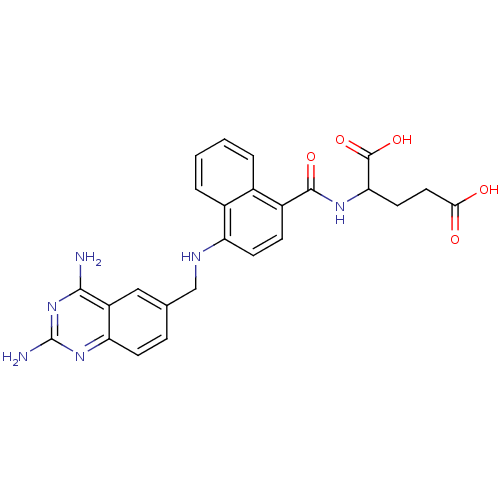 (2-({4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043394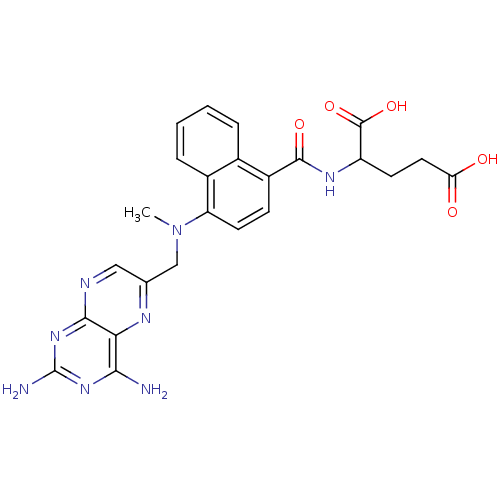 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50058420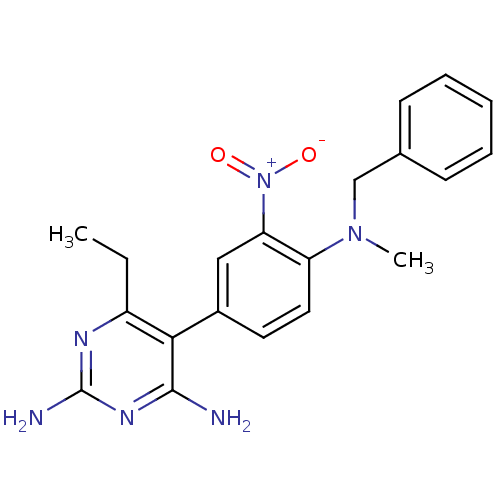 ((methylbenzoprim, MBP) 5-[4-(Benzyl-methyl-amino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Curated by ChEMBL | Assay Description Inhibitory activity of compound against rat liver Dihydrofolate reductase | J Med Chem 40: 3040-8 (1997) Article DOI: 10.1021/jm970055k BindingDB Entry DOI: 10.7270/Q2C53JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19770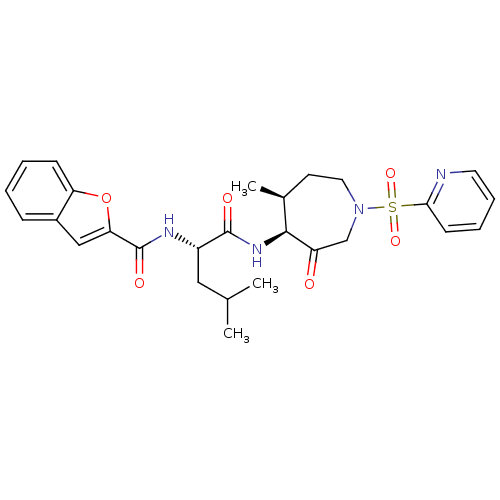 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50184069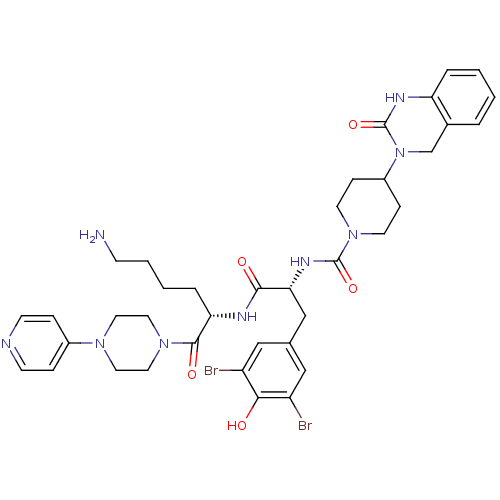 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50365814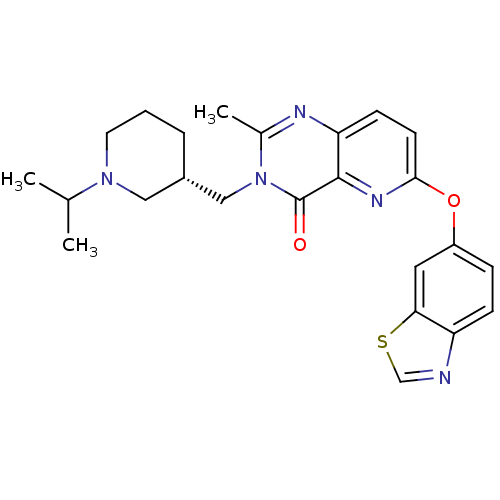 (CHEMBL1956993) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Prosidion Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-Ghrelin from human GHSR membranes overexpressing GSH-R1a by scintillation counting | Bioorg Med Chem Lett 22: 2271-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.078 BindingDB Entry DOI: 10.7270/Q20G3KMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Galanin receptor type 1 (Homo sapiens (Human)) | BDBM50273370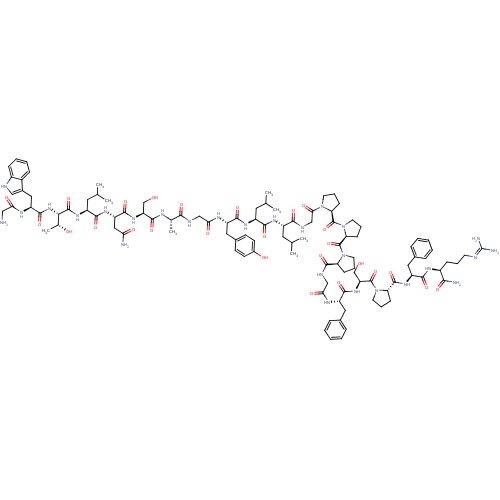 (CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Binding affinity to human GalR1 | J Med Chem 53: 1871-5 (2010) Article DOI: 10.1021/jm9018349 BindingDB Entry DOI: 10.7270/Q26D5TXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50365815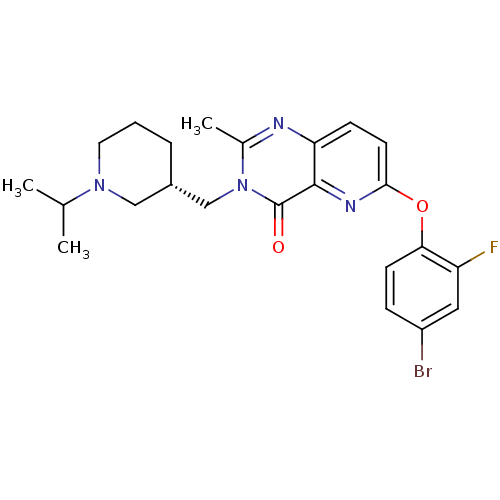 (CHEMBL1956994) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Prosidion Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-Ghrelin from human GHSR membranes overexpressing GSH-R1a by scintillation counting | Bioorg Med Chem Lett 22: 2271-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.078 BindingDB Entry DOI: 10.7270/Q20G3KMP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086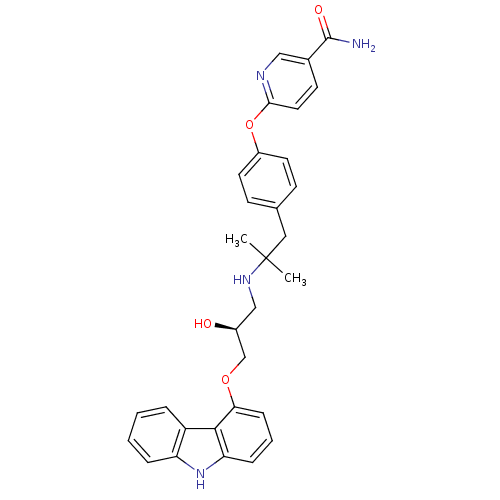 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M1 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M1 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333144 ((S)-(3-(dimethylamino)azetidin-1-yl)(5-ethyl-2-(6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10947229 (2021) BindingDB Entry DOI: 10.7270/Q2MC934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333145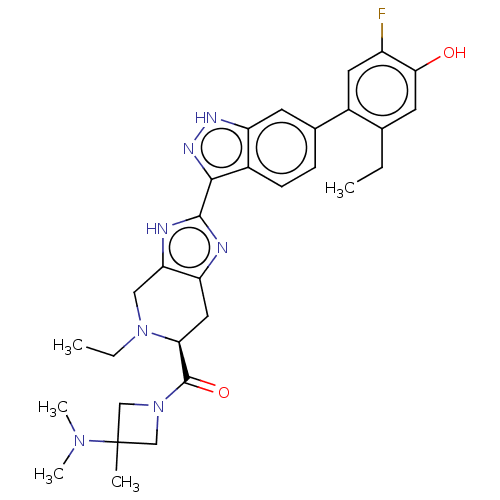 ((S)-(3-(dimethylamino)-3-methylazetidin-1-yl)(5-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS60BP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333144 ((S)-(3-(dimethylamino)azetidin-1-yl)(5-ethyl-2-(6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS60BP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM431909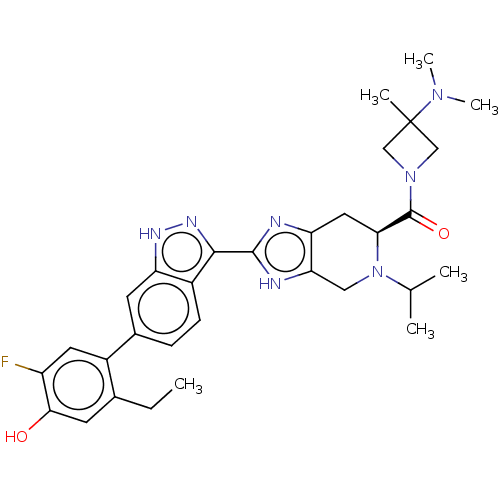 (US10550118, Example 8-16 | US10947229, Example C-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS60BP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM431909 (US10550118, Example 8-16 | US10947229, Example C-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10947229 (2021) BindingDB Entry DOI: 10.7270/Q2MC934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333145 ((S)-(3-(dimethylamino)-3-methylazetidin-1-yl)(5-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10947229 (2021) BindingDB Entry DOI: 10.7270/Q2MC934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043397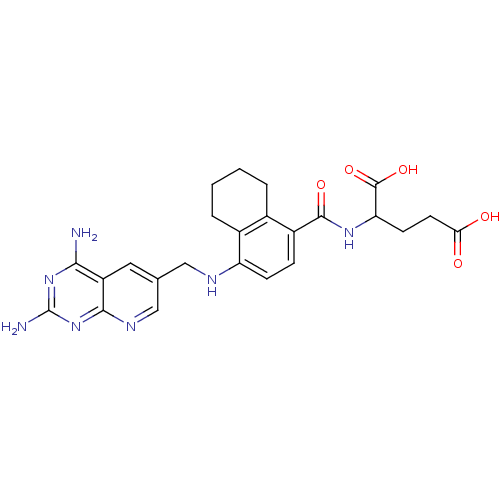 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50059957 (4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Curated by ChEMBL | Assay Description Inhibitory activity of compound against rat liver Dihydrofolate reductase | J Med Chem 40: 3040-8 (1997) Article DOI: 10.1021/jm970055k BindingDB Entry DOI: 10.7270/Q2C53JZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333121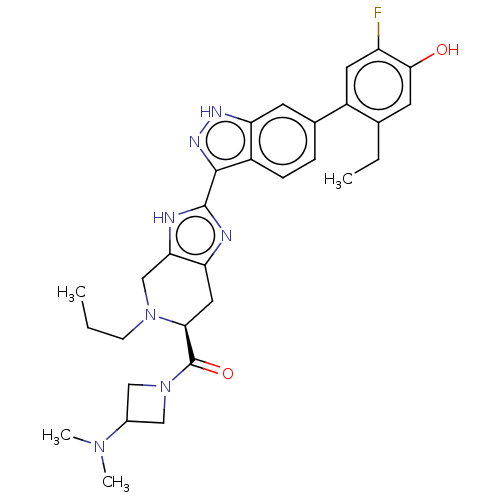 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10947229 (2021) BindingDB Entry DOI: 10.7270/Q2MC934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM473568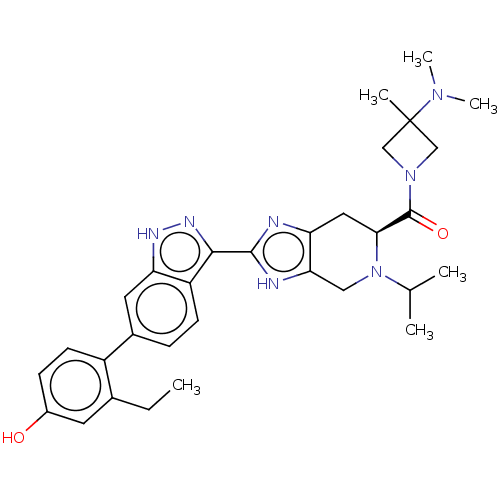 (US10844057, Example 11 | US10947229, Example 4 | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS60BP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350089 (((S)-2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24F1VV8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350089 (((S)-2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10836763 (2020) BindingDB Entry DOI: 10.7270/Q26D5X2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333120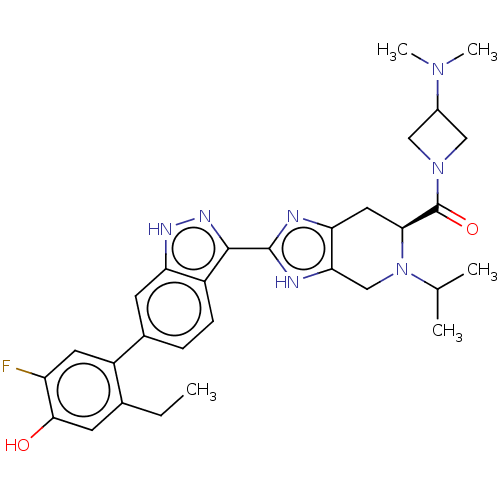 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10947229 (2021) BindingDB Entry DOI: 10.7270/Q2MC934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333121 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS60BP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM473568 (US10844057, Example 11 | US10947229, Example 4 | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10947229 (2021) BindingDB Entry DOI: 10.7270/Q2MC934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333120 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS60BP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350090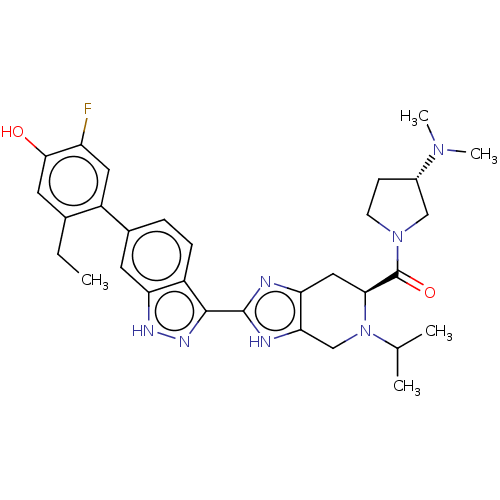 (((S)-3-(dimethylamino)pyrrolidin-1-yl)((S)-5-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10836763 (2020) BindingDB Entry DOI: 10.7270/Q26D5X2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350092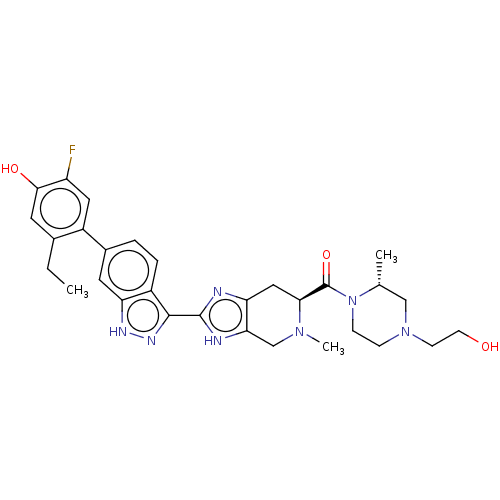 (((S)-2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24F1VV8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM473566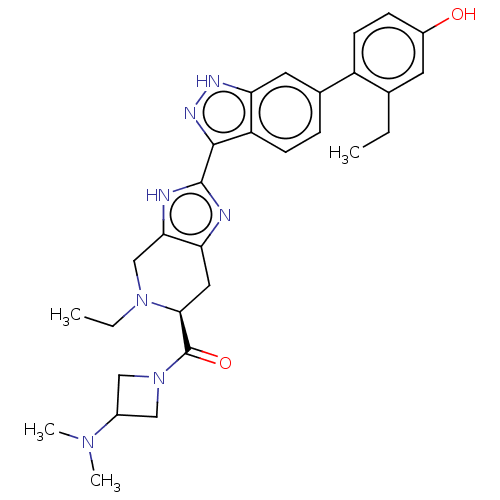 (US10844057, Example 9 | US10947229, Example 2 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS60BP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333122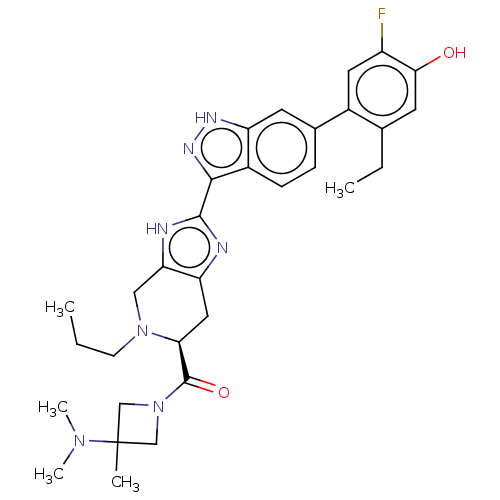 ((S)-(3-(dimethylamino)-3-methylazetidin-1-yl)(2-(6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2XS60BP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM473566 (US10844057, Example 9 | US10947229, Example 2 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10947229 (2021) BindingDB Entry DOI: 10.7270/Q2MC934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350092 (((S)-2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10836763 (2020) BindingDB Entry DOI: 10.7270/Q26D5X2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350090 (((S)-3-(dimethylamino)pyrrolidin-1-yl)((S)-5-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24F1VV8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59226 total ) | Next | Last >> |