

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine kinase (Rattus norvegicus (rat)) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain cytosolic Adk using [2-3H]adenosine incubated for 15 mins by scintillation spectrometry | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Rattus norvegicus (rat)) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat brain ADK expressed in Escherichia coli | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59098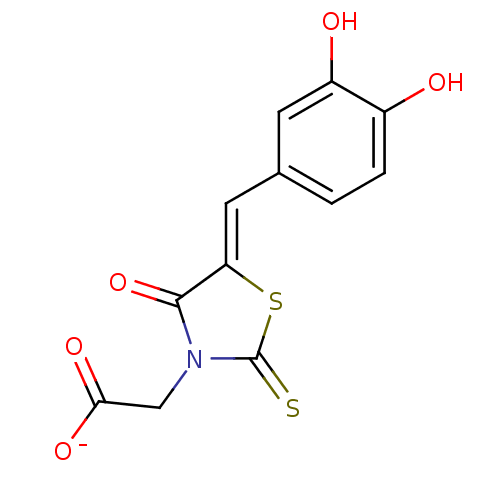 (Bi-ligand, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of recombinant human Adk using [U-14C]adenosine by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055641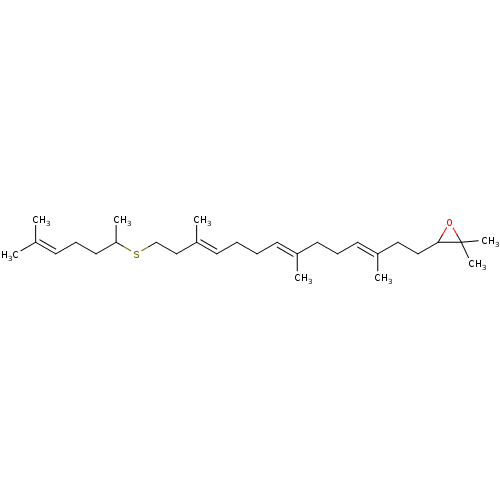 (2,2-dimethyl-3-((3E,7E,11E)-3,7,12-trimethyl-14-(6...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59099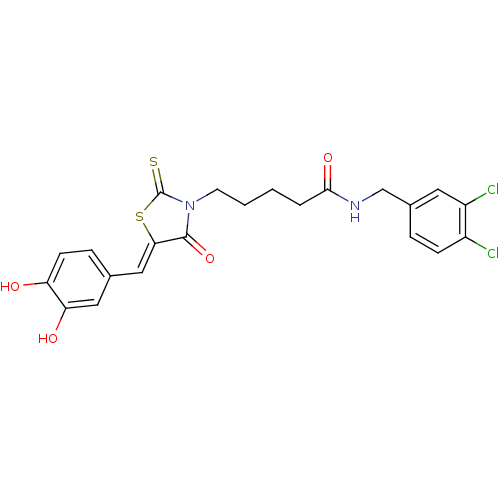 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59101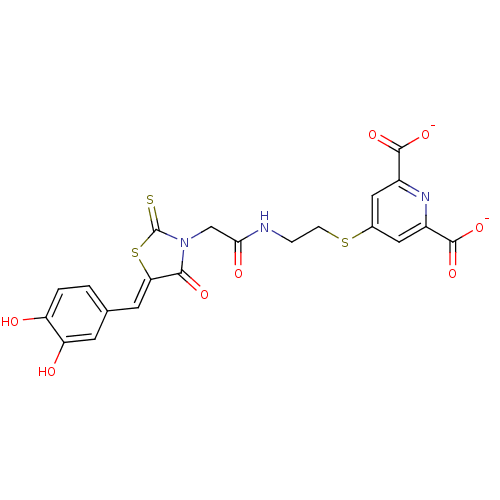 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50533824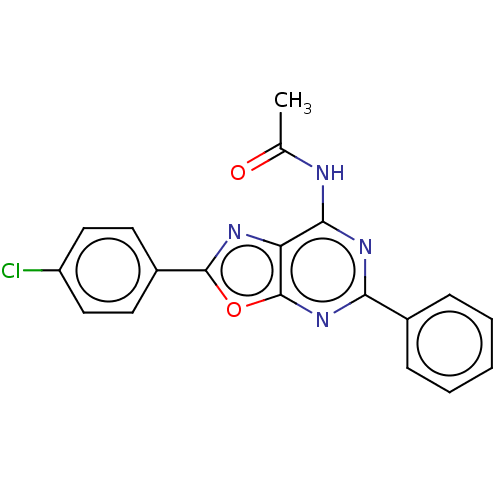 (CHEMBL4574016) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055642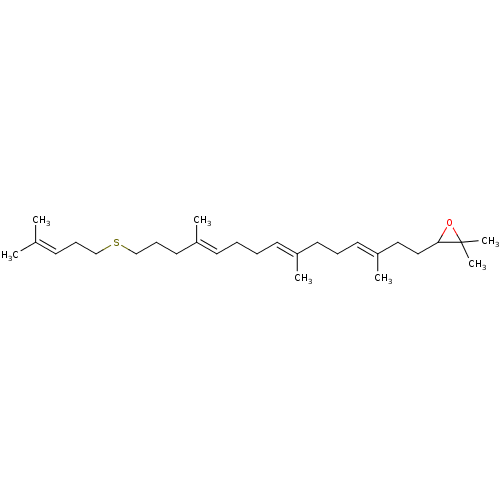 (2,2-Dimethyl-3-[(3E,7E,11E)-3,7,12-trimethyl-15-(4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533828 (CHEMBL4570031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59100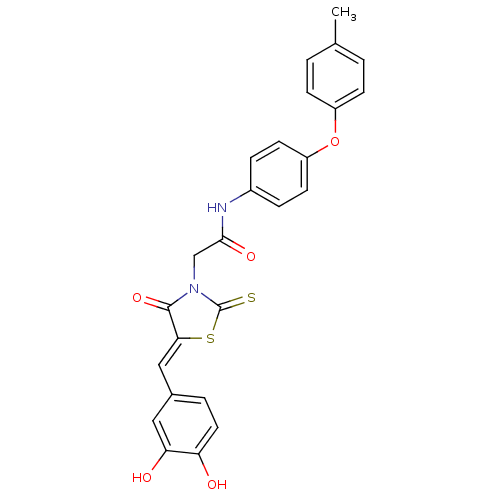 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055643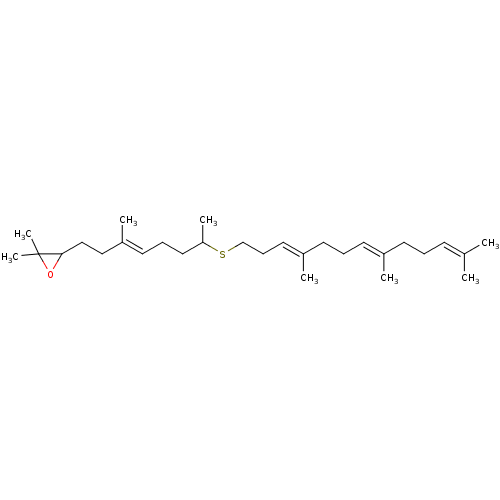 (2,2-Dimethyl-3-[(E)-3-methyl-7-((3E,7E)-4,8,12-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59101 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50533830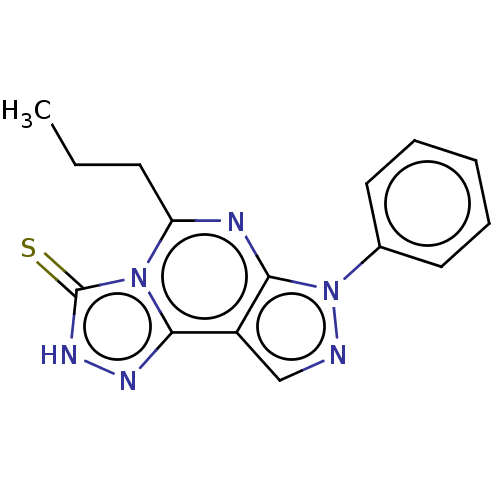 (CHEMBL4460655) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50533833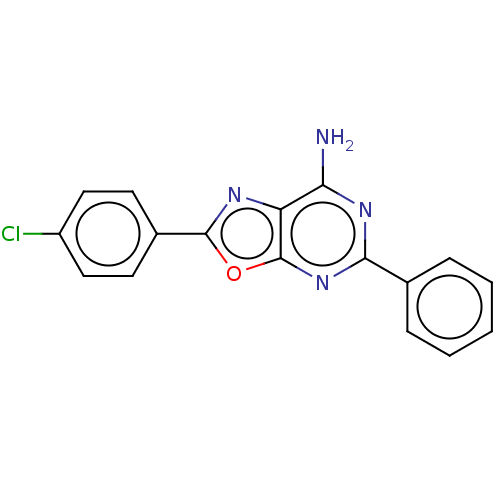 (CHEMBL4545415) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055642 (2,2-Dimethyl-3-[(3E,7E,11E)-3,7,12-trimethyl-15-(4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055641 (2,2-dimethyl-3-((3E,7E,11E)-3,7,12-trimethyl-14-(6...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055644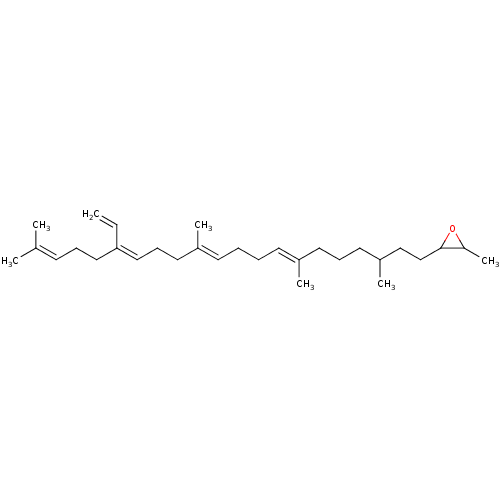 (2-Methyl-3-((7E,11E,15Z)-3,7,12,20-tetramethyl-16-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from rat liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533830 (CHEMBL4460655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055644 (2-Methyl-3-((7E,11E,15Z)-3,7,12,20-tetramethyl-16-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50055640 (3-[(3E,7E)-11-((E)-4,8-Dimethyl-nona-3,7-dienylsul...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Compound was tested for kinetic inhibition constant against Oxidosqualene-lanosterol cyclase from pig liver | J Med Chem 40: 201-9 (1997) Article DOI: 10.1021/jm960483a BindingDB Entry DOI: 10.7270/Q2P26X73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533823 (CHEMBL494210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533825 (CHEMBL4460819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533832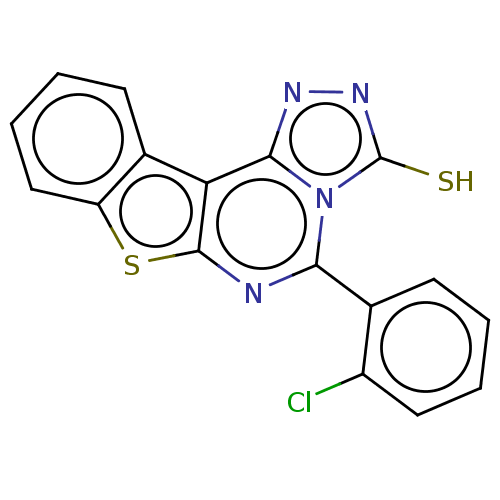 (CHEMBL4524434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59101 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533830 (CHEMBL4460655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533829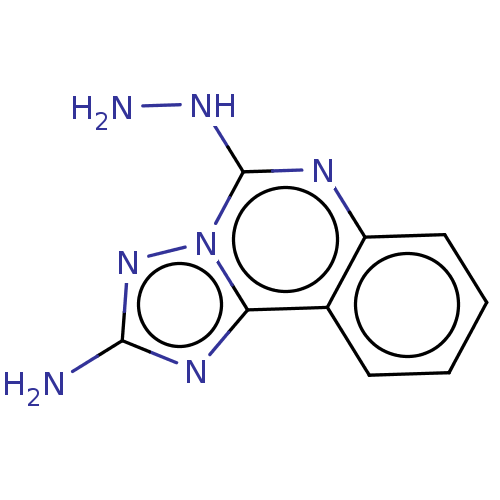 (CHEMBL4592393) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533827 (CHEMBL4457103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59099 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533823 (CHEMBL494210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533824 (CHEMBL4574016) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50533825 (CHEMBL4460819) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50533829 (CHEMBL4592393) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50533823 (CHEMBL494210) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50533828 (CHEMBL4570031) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533825 (CHEMBL4460819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533831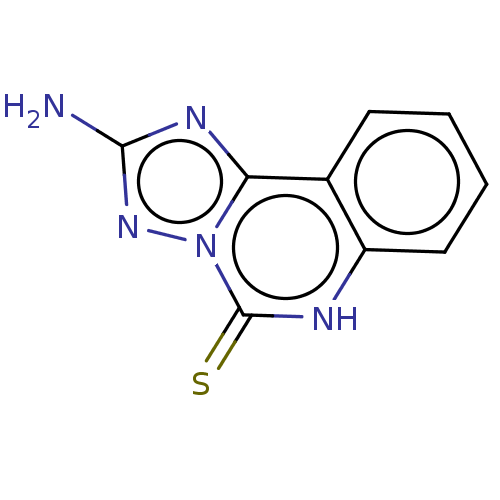 (CHEMBL4445820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533833 (CHEMBL4545415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533828 (CHEMBL4570031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533829 (CHEMBL4592393) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50533831 (CHEMBL4445820) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat cortex adenosine A1 receptor using [3H]CCPA measured after 90 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50533832 (CHEMBL4524434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat striatal adenosine A2A receptor using [3H]MXS-2 measured after 30 mins by liquid scintillation counting method | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533833 (CHEMBL4545415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59100 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533831 (CHEMBL4445820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533834 (CHEMBL4448716) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59100 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533824 (CHEMBL4574016) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50533826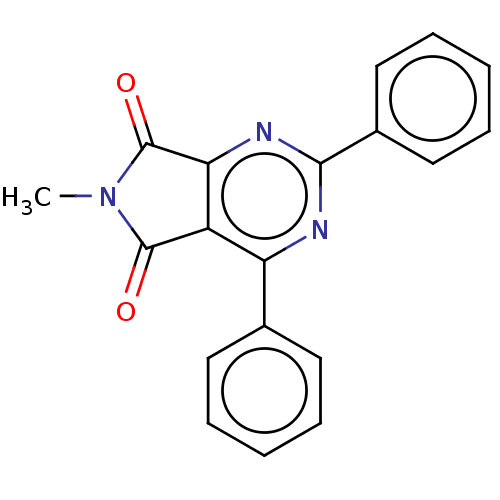 (CHEMBL4520524) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human Adk-short expressed in Escherichia coli BL21[DE3] assessed as [3H]AMP formation preincubated for 15 mins f... | Bioorg Med Chem 24: 5127-5133 (2016) Article DOI: 10.1016/j.bmc.2016.08.026 BindingDB Entry DOI: 10.7270/Q2TB1BDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |