

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50223787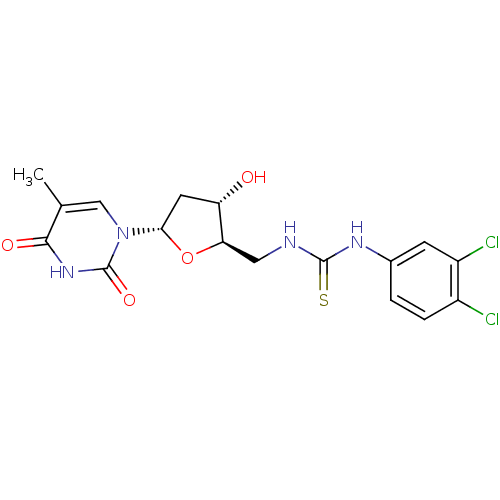 (CHEMBL235088 | N-(5'-deoxy-alpha-D-thymidin-5'-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis thymidylate kinase | J Med Chem 55: 852-70 (2012) Article DOI: 10.1021/jm201349f BindingDB Entry DOI: 10.7270/Q2BZ66HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205450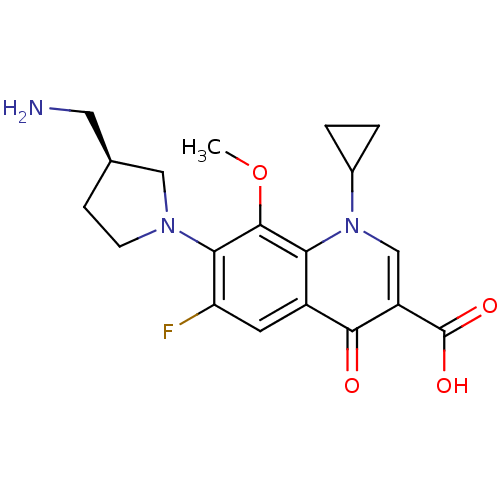 ((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205452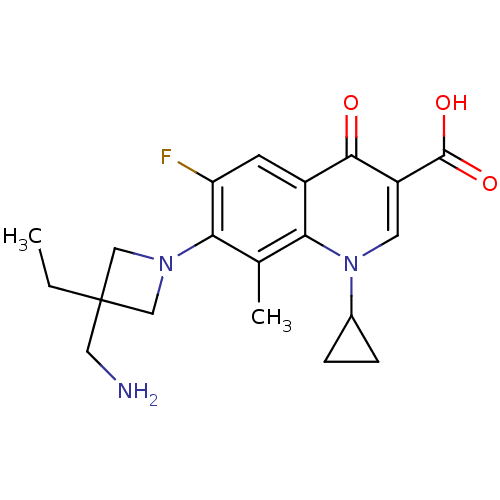 (7-(3-(aminomethyl)-3-ethylazetidin-1-yl)-1-cyclopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205465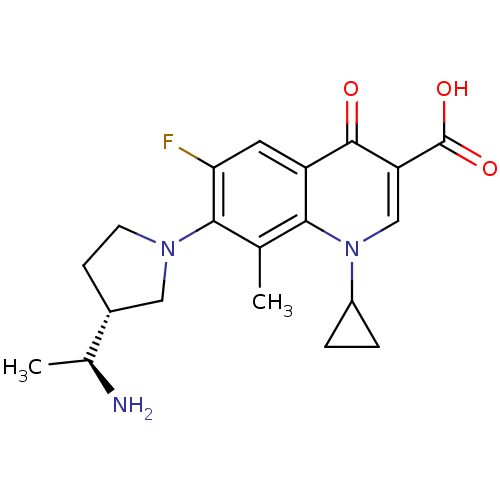 (7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205451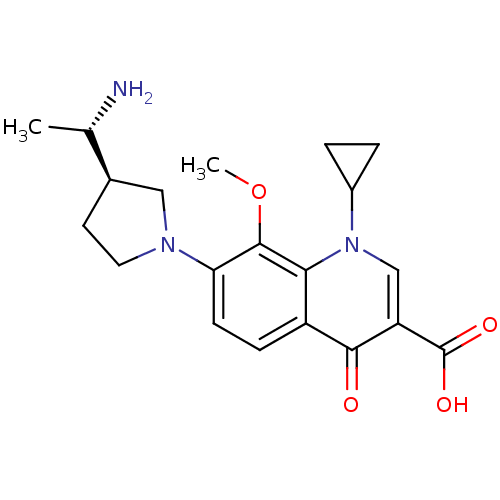 (7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205466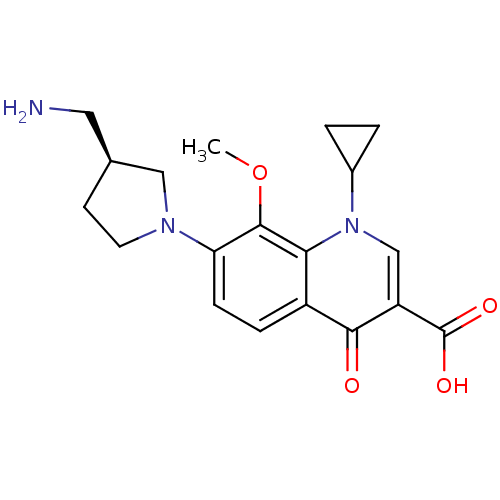 ((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205457 (7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205453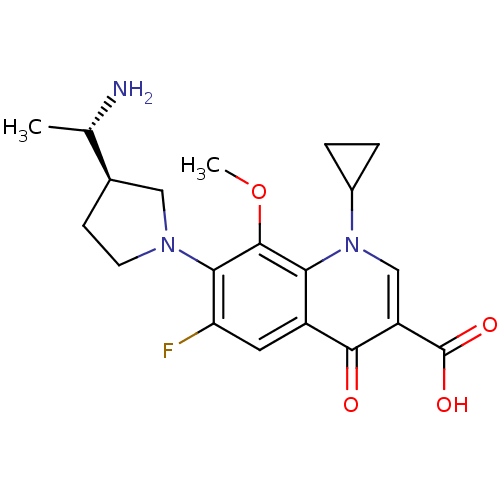 (7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205446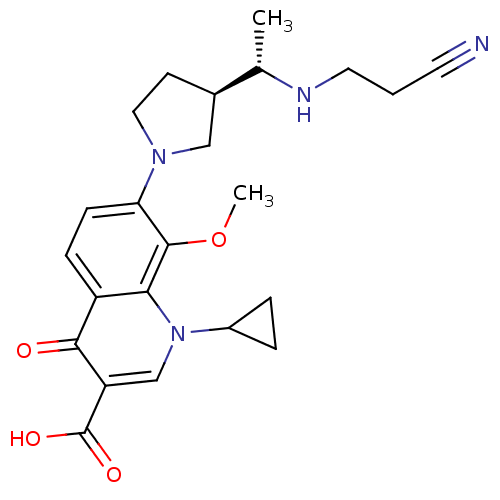 (7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205447 (10-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-9-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205461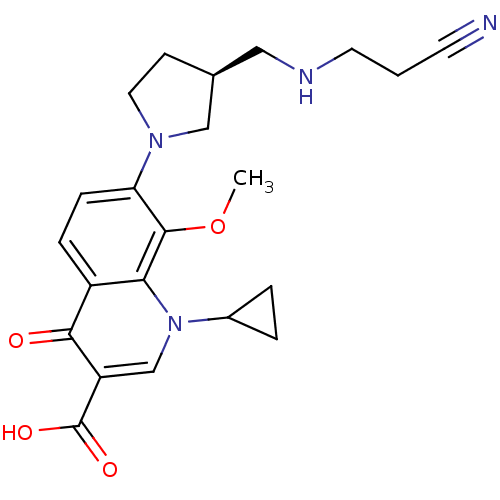 ((S)-7-(3-((2-cyanoethylamino)methyl)pyrrolidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205459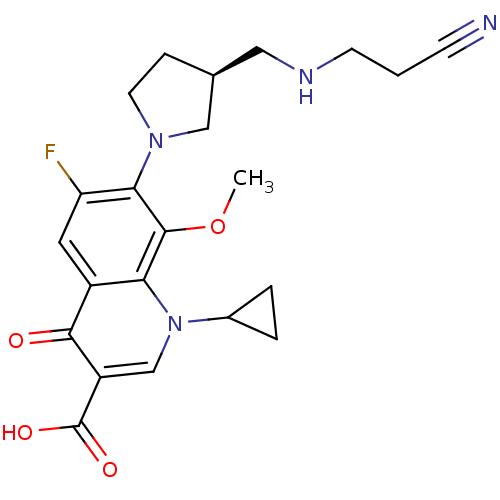 ((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205455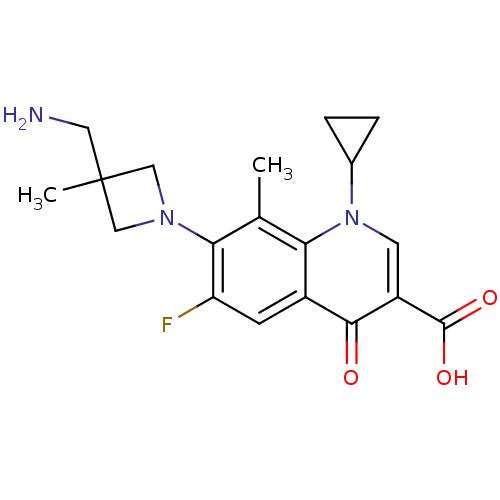 (7-(3-(aminomethyl)-3-methylazetidin-1-yl)-1-cyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205449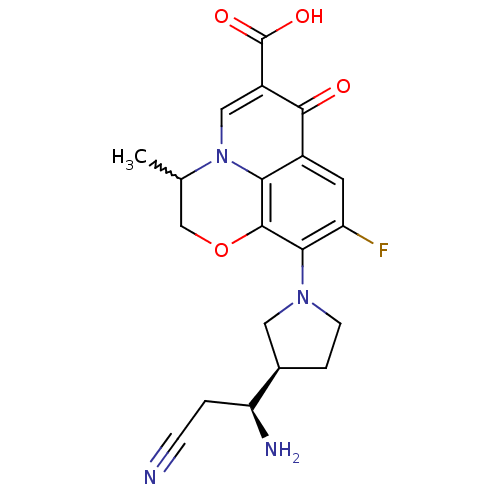 (10-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205458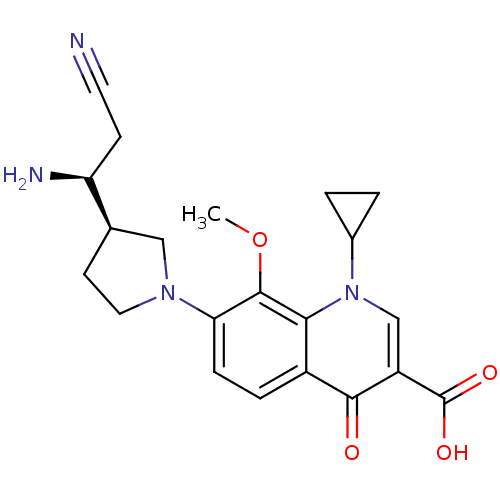 (7-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205460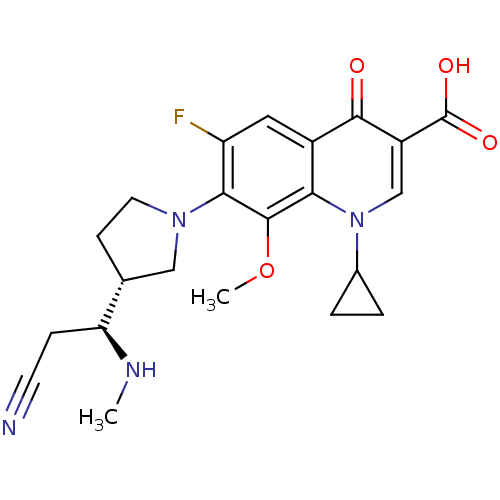 (7-((R)-3-((S)-2-cyano-1-(methylamino)ethyl)pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205454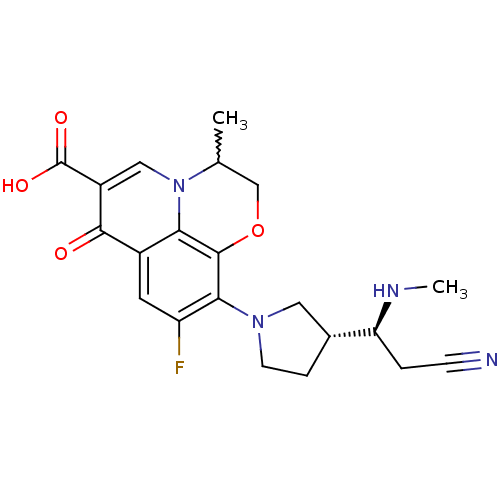 (10-((R)-3-((S)-2-cyano-1-(methylamino)ethyl)pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205448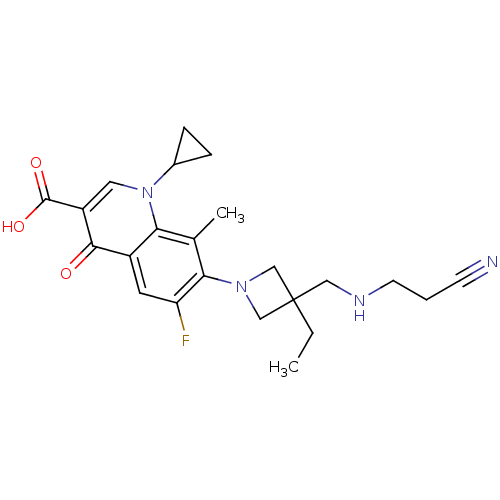 (7-(3-((2-cyanoethylamino)methyl)-3-ethylazetidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205467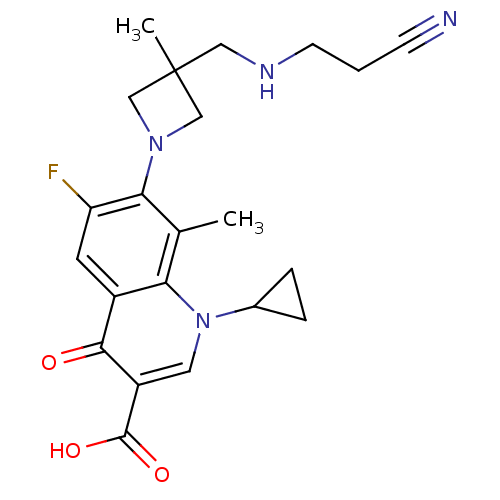 (7-(3-((2-cyanoethylamino)methyl)-3-methylazetidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50131445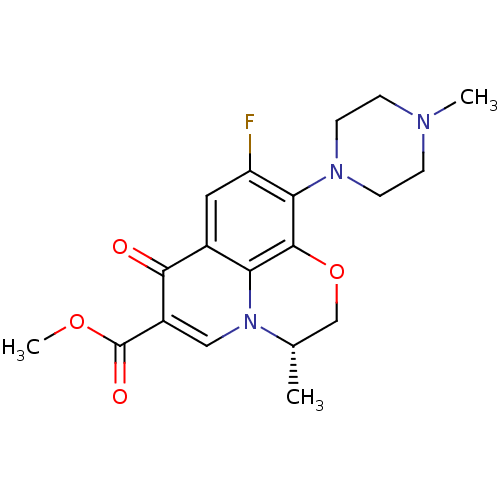 ((3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205456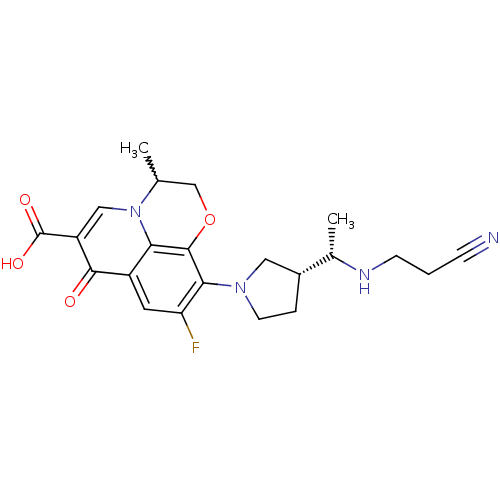 (10-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205463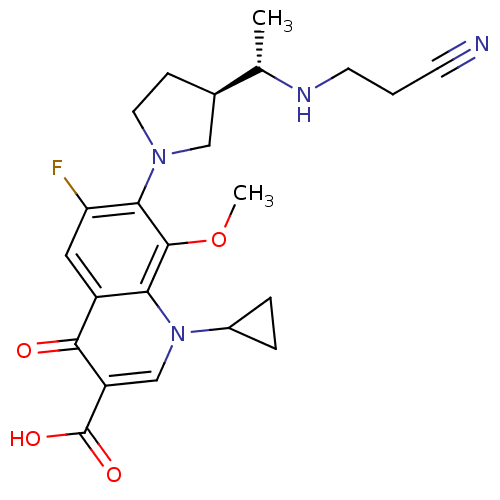 (7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205462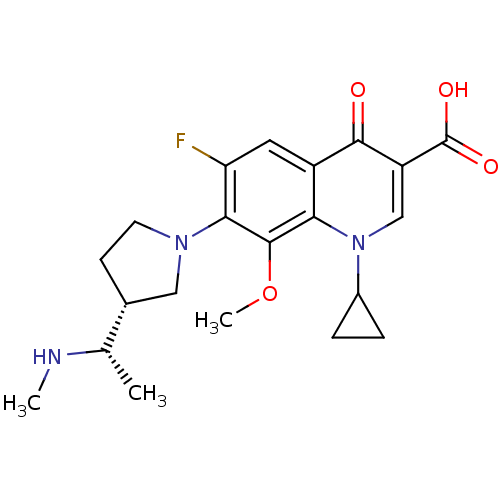 (1-cyclopropyl-6-fluoro-8-methoxy-7-((R)-3-((S)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50205464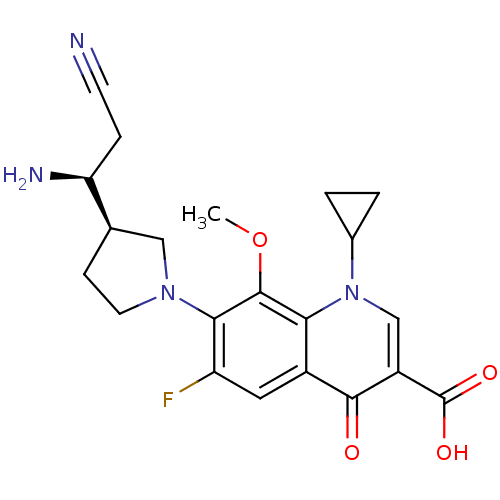 (7-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG by fliter binding assay | Bioorg Med Chem Lett 17: 2150-5 (2007) Article DOI: 10.1016/j.bmcl.2007.01.090 BindingDB Entry DOI: 10.7270/Q2GM86Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331355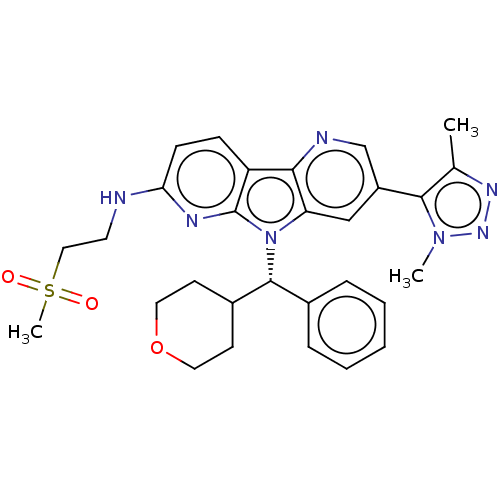 (N-(2-Methanesulfonylethyl)-5-[4-(2H3)methyl-1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331356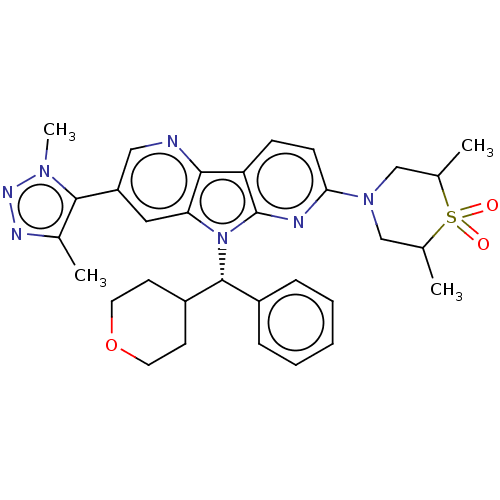 (4-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331380 (5-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331379 (4-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331385 ((2-{[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331389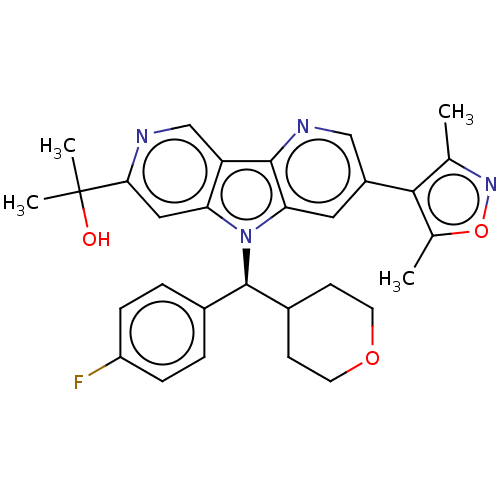 (2-[5-(Dimethyl-1,2-oxazol-4-yl)-8-[(S)-(4-fluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331412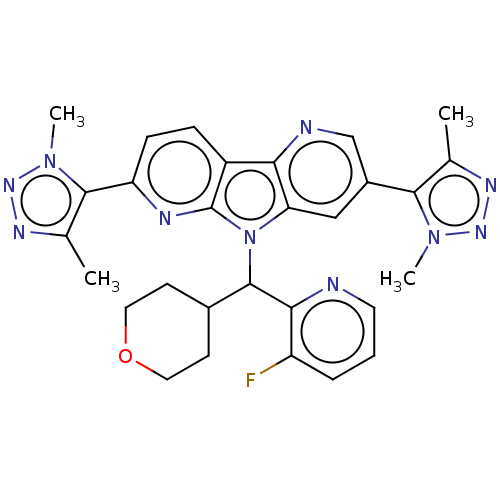 (8-[(3-Fluoropyridin-2-yl)(oxan-4-yl)methyl]-5,11-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331417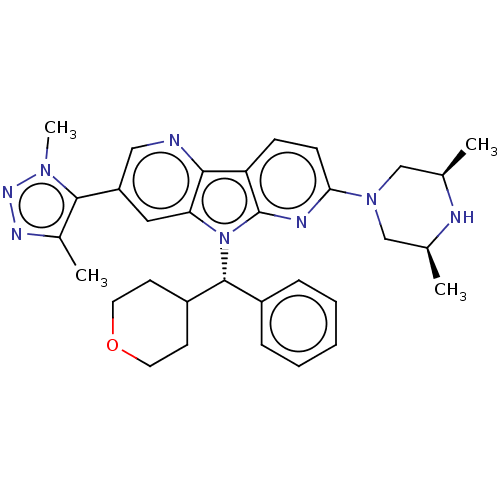 (11-[(3R,5S)-3,5-Dimethylpiperazin-1-yl]-5-[4-(2H3)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331441 (2-{10-Fluoro-5-[4-(2H3)methyl-1-methyl-1H-1,2,3-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331446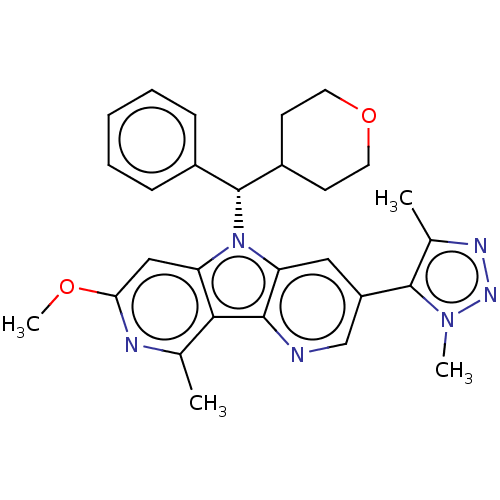 (5-(Dimethyl-1H-1,2,3-triazol-5-yl)-11-methoxy-13-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331447 (2-{5-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331451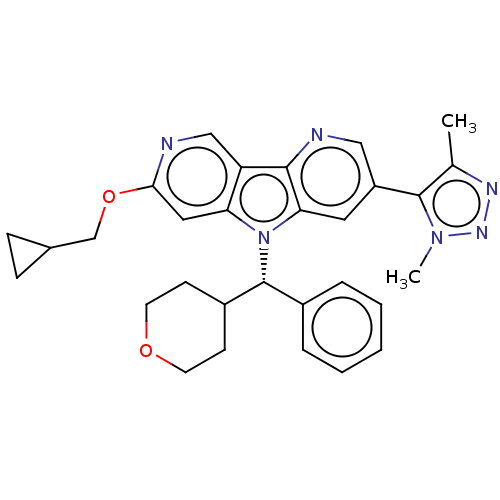 (11-(Cyclopropylmethoxy)-5-[4-(2H3)methyl-1-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331505 (2-{8-[(4,4-Difluorocyclohexyl)(phenyl)methyl]-13-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331295 (13-Ethoxy-5-[4-(2H3)methyl-1-methyl-1H-1,2,3-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331299 (N-(2,2-Difluoroethyl)-5-[4-(2H3)methyl-1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331300 (5-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl]-8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331329 (5,11-Bis(dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331334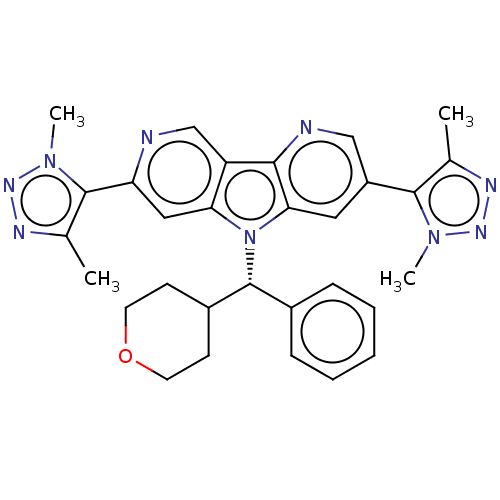 (5,11-Bis[4-(2H3)methyl-1-methyl-1H-1,2,3-triazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331340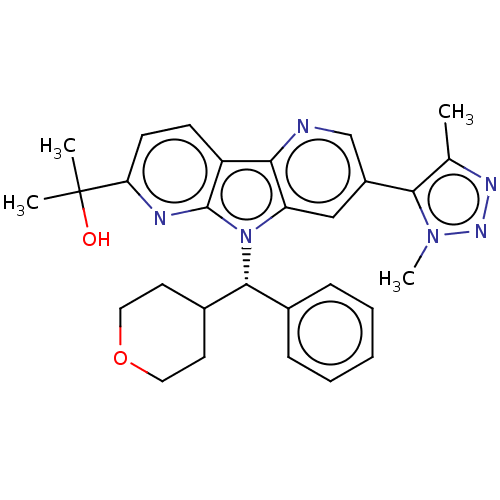 (2-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331340 (2-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331344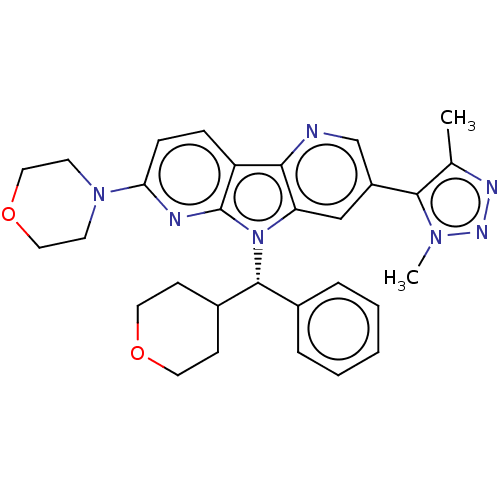 (5-(Dimethyl-1H-1,2,3-triazol-5-yl)-11-(morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331345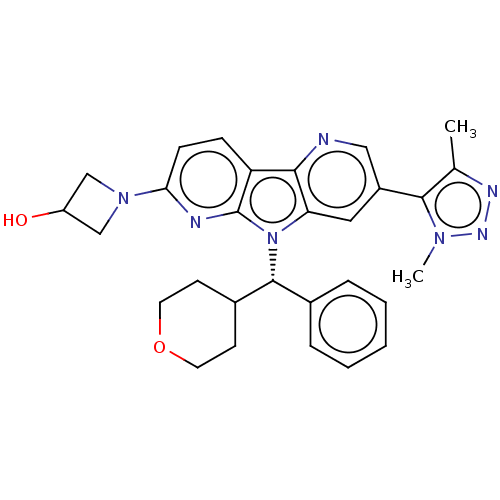 (1-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331346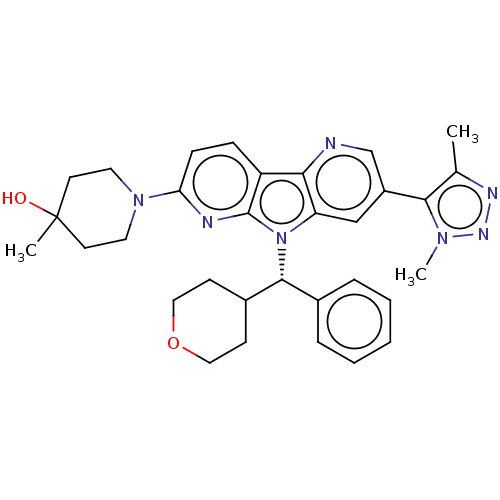 (1-[5-(Dimethyl-1H-1,2,3-triazol-5-yl)-8-[(S)-oxan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331347 (5-(Dimethyl-1H-1,2,3-triazol-5-yl)-N,N-dimethyl-8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [1-477]/[333-460]/[44-168]/[44-460] (Homo sapiens (Human)) | BDBM331354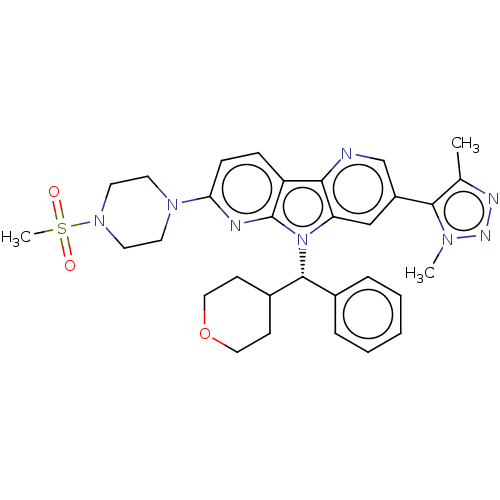 (5-(Dimethyl-1H-1,2,3-triazol-5-yl)-11-(4-methanesu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The binding of compounds to bromodomain BRD4 (44-168), BRD4 (333-460), and BRD4 (1-477 or 44-460) was assessed using a time resolved fluorescent reso... | US Patent US9725449 (2017) BindingDB Entry DOI: 10.7270/Q2P55QM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 700 total ) | Next | Last >> |