 Found 172 hits with Last Name = 'steele' and Initial = 'r'
Found 172 hits with Last Name = 'steele' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598740
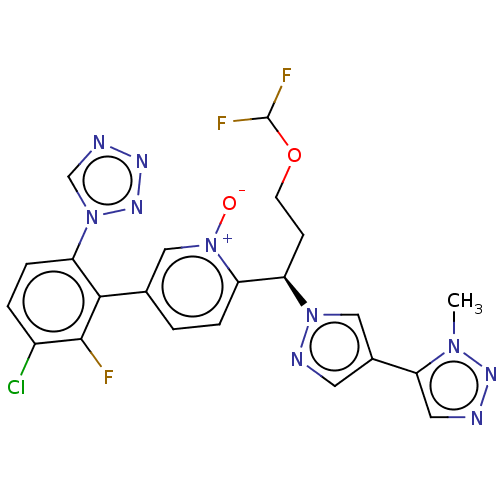
(CHEMBL5175227)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598738
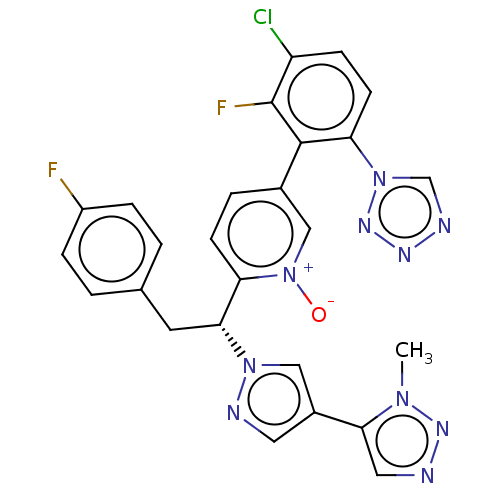
(CHEMBL5204065)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598739

(CHEMBL5188215)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598737
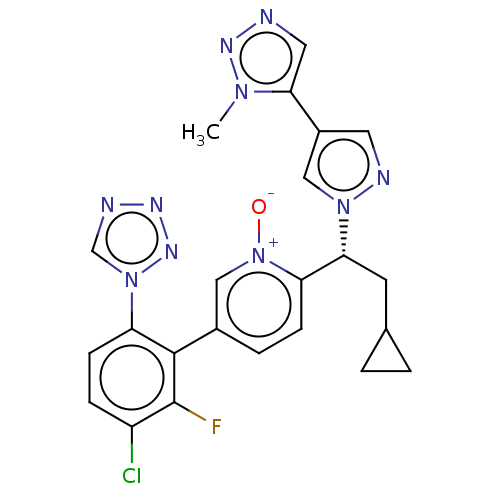
(CHEMBL5205631)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598741

(CHEMBL5204894)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598725

(CHEMBL5185397)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598743
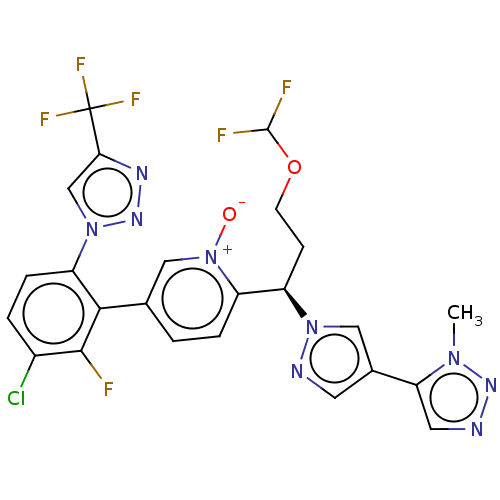
(CHEMBL5178223)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598734
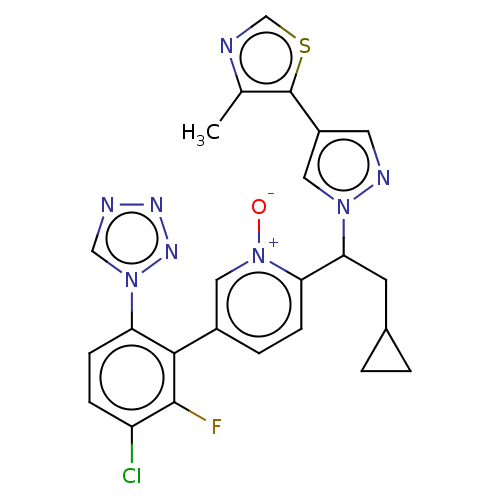
(CHEMBL5197480)Show SMILES Cc1ncsc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598745
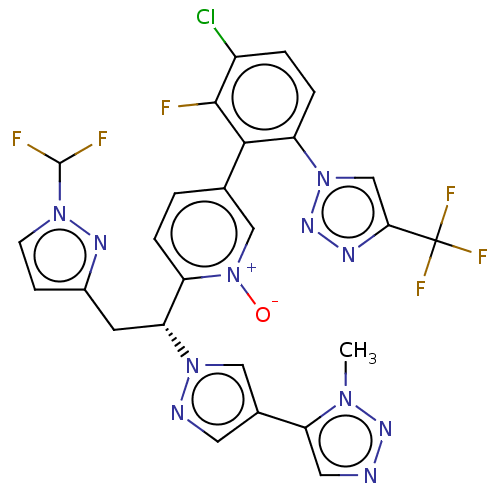
(CHEMBL5198823)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccn(n1)C(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598736

(CHEMBL5208095)Show SMILES Cn1cncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598744
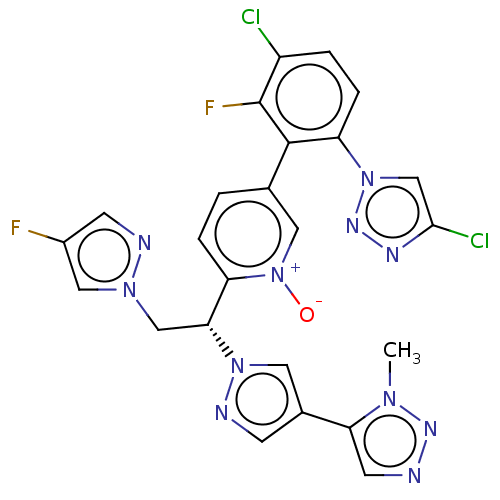
(CHEMBL5190323)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cn1cc(F)cn1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598732
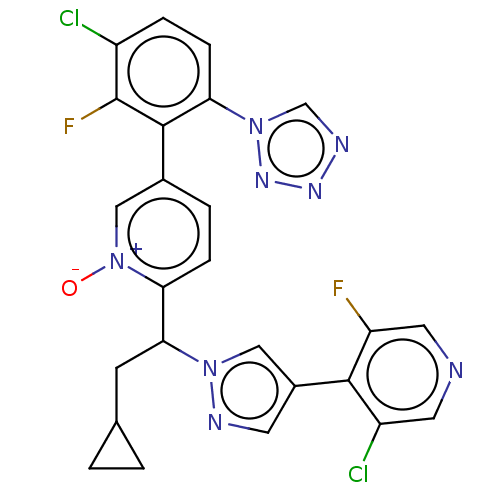
(CHEMBL5192284)Show SMILES [O-][n+]1cc(ccc1C(CC1CC1)n1cc(cn1)-c1c(F)cncc1Cl)-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM10044
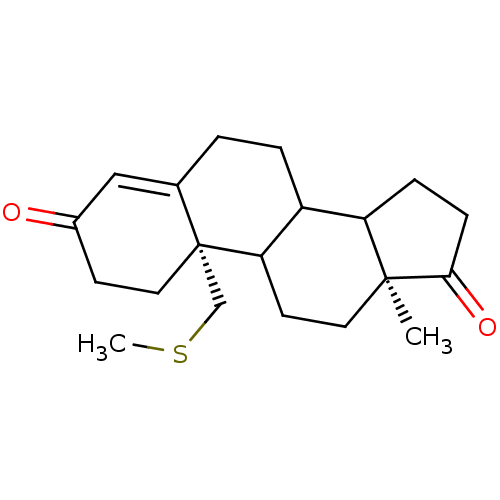
((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...)Show SMILES CSC[C@]12CCC(=O)C=C1CCC1C3CCC(=O)[C@@]3(C)CCC21 |r,c:8| Show InChI InChI=1S/C20H28O2S/c1-19-9-8-17-15(16(19)5-6-18(19)22)4-3-13-11-14(21)7-10-20(13,17)12-23-2/h11,15-17H,3-10,12H2,1-2H3/t15?,16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Aromatase inhibitor potency as iron-binding-related type II difference spectrum |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598742
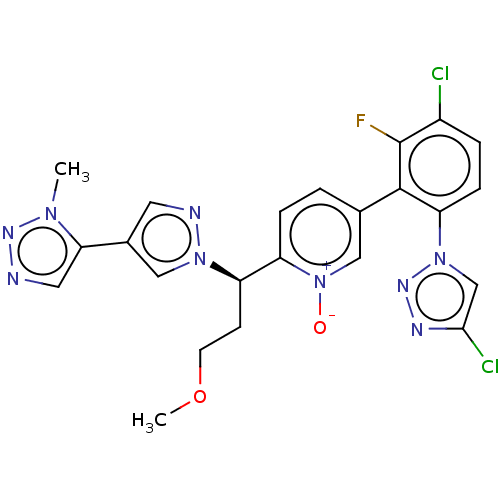
(CHEMBL5182855)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598729
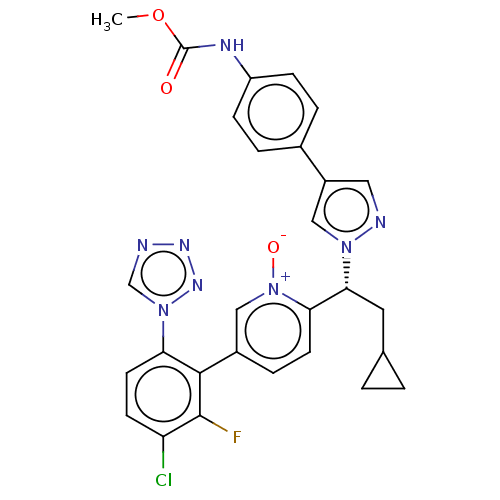
(CHEMBL5195600)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598727
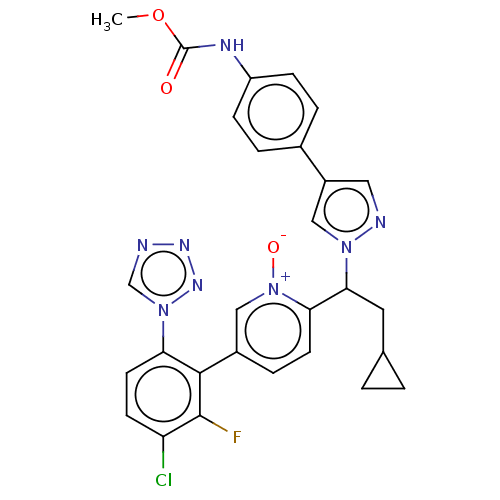
(CHEMBL5198338)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598735
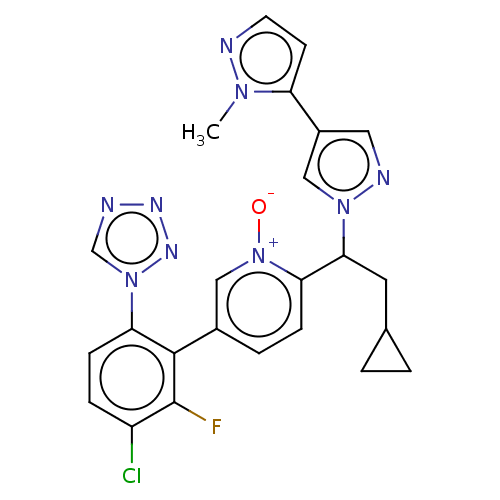
(CHEMBL5193267)Show SMILES Cn1nccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598730

(CHEMBL5204289)Show SMILES Cc1nc(N)ccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598731
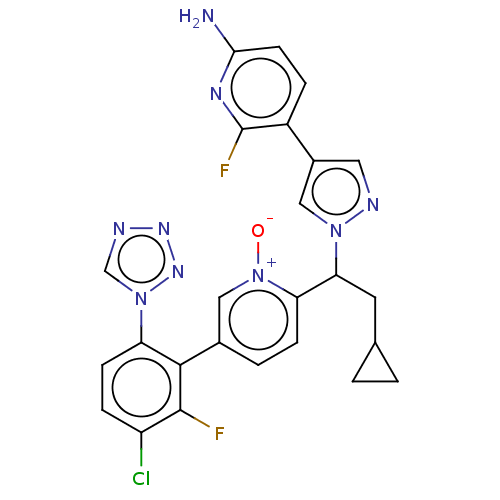
(CHEMBL5198972)Show SMILES Nc1ccc(-c2cnn(c2)C(CC2CC2)c2ccc(c[n+]2[O-])-c2c(F)c(Cl)ccc2-n2cnnn2)c(F)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598733
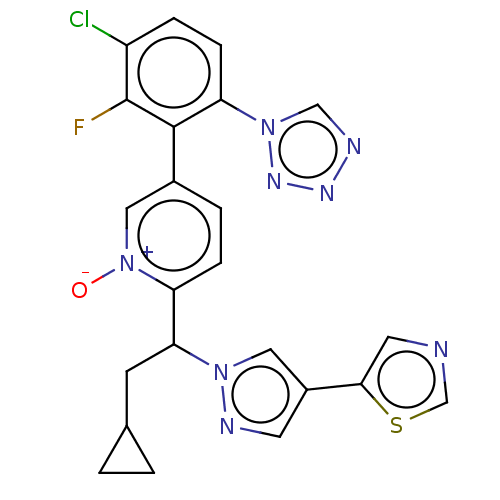
(CHEMBL5188316)Show SMILES [O-][n+]1cc(ccc1C(CC1CC1)n1cc(cn1)-c1cncs1)-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598726
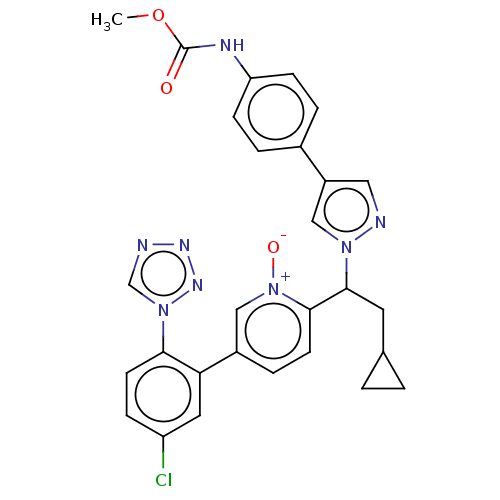
(CHEMBL5171252)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303032
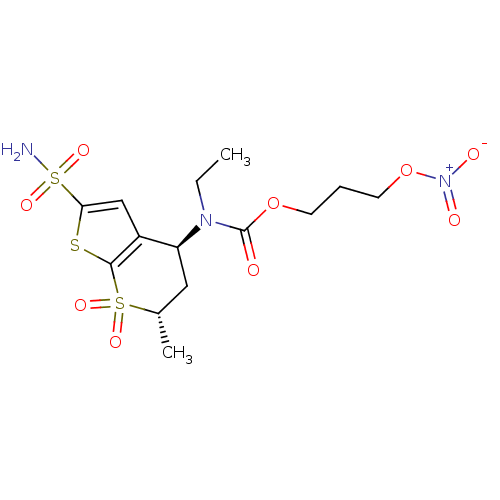
(CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C14H21N3O9S3/c1-3-16(14(18)25-5-4-6-26-17(19)20)11-7-9(2)28(21,22)13-10(11)8-12(27-13)29(15,23)24/h8-9,11H,3-7H2,1-2H3,(H2,15,23,24)/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303030
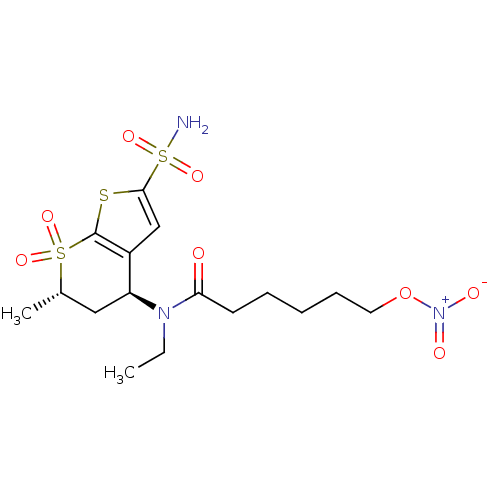
(6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)CCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C16H25N3O8S3/c1-3-18(14(20)7-5-4-6-8-27-19(21)22)13-9-11(2)29(23,24)16-12(13)10-15(28-16)30(17,25)26/h10-11,13H,3-9H2,1-2H3,(H2,17,25,26)/t11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303031
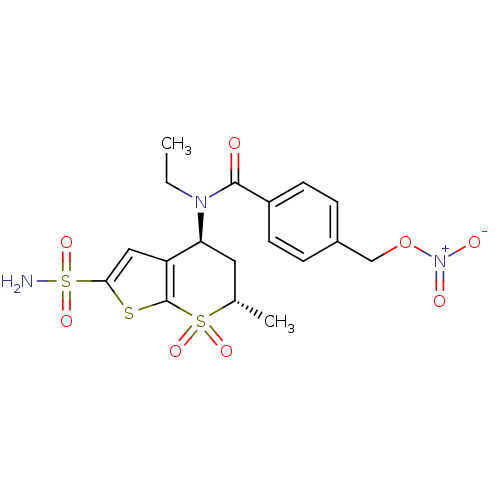
(CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)c1ccc(CO[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C18H21N3O8S3/c1-3-20(17(22)13-6-4-12(5-7-13)10-29-21(23)24)15-8-11(2)31(25,26)18-14(15)9-16(30-18)32(19,27)28/h4-7,9,11,15H,3,8,10H2,1-2H3,(H2,19,27,28)/t11-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598728
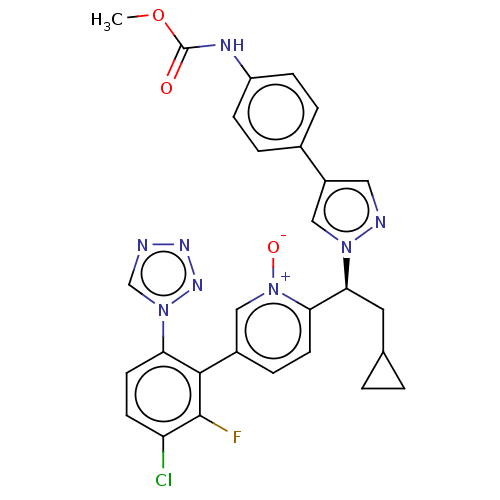
(CHEMBL5180591)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)[C@@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303034
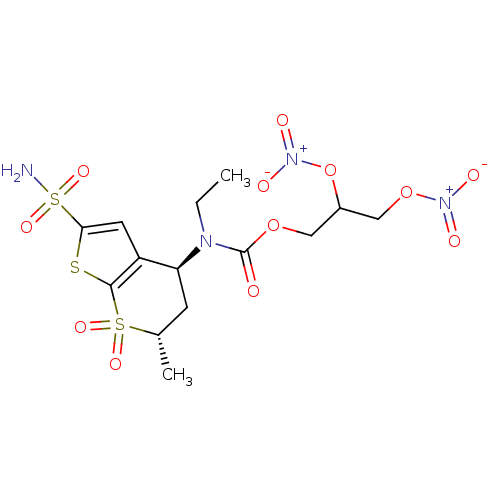
(CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O |r| Show InChI InChI=1S/C14H20N4O12S3/c1-3-16(14(19)28-6-9(30-18(22)23)7-29-17(20)21)11-4-8(2)32(24,25)13-10(11)5-12(31-13)33(15,26)27/h5,8-9,11H,3-4,6-7H2,1-2H3,(H2,15,26,27)/t8-,9?,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303031

(CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)c1ccc(CO[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C18H21N3O8S3/c1-3-20(17(22)13-6-4-12(5-7-13)10-29-21(23)24)15-8-11(2)31(25,26)18-14(15)9-16(30-18)32(19,27)28/h4-7,9,11,15H,3,8,10H2,1-2H3,(H2,19,27,28)/t11-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50303033
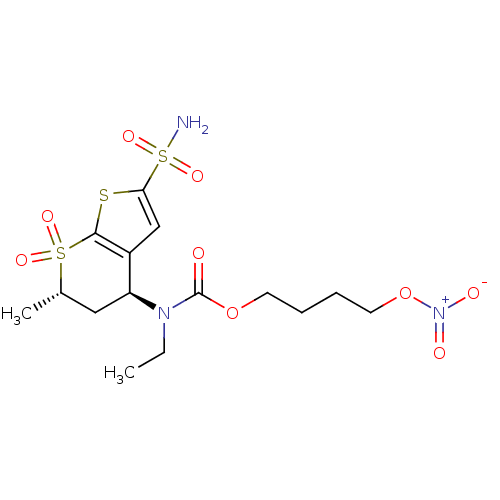
(CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C15H23N3O9S3/c1-3-17(15(19)26-6-4-5-7-27-18(20)21)12-8-10(2)29(22,23)14-11(12)9-13(28-14)30(16,24)25/h9-10,12H,3-8H2,1-2H3,(H2,16,24,25)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303032

(CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C14H21N3O9S3/c1-3-16(14(18)25-5-4-6-26-17(19)20)11-7-9(2)28(21,22)13-10(11)8-12(27-13)29(15,23)24/h8-9,11H,3-7H2,1-2H3,(H2,15,23,24)/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303033

(CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C15H23N3O9S3/c1-3-17(15(19)26-6-4-5-7-27-18(20)21)12-8-10(2)29(22,23)14-11(12)9-13(28-14)30(16,24)25/h9-10,12H,3-8H2,1-2H3,(H2,16,24,25)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303032

(CHEMBL584939 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C14H21N3O9S3/c1-3-16(14(18)25-5-4-6-26-17(19)20)11-7-9(2)28(21,22)13-10(11)8-12(27-13)29(15,23)24/h8-9,11H,3-7H2,1-2H3,(H2,15,23,24)/t9-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Competitive inhibition of human placental Cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303031

(CHEMBL570894 | N-Ethyl-N-((4S,6S)-6-methyl-7,7-dio...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)c1ccc(CO[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C18H21N3O8S3/c1-3-20(17(22)13-6-4-12(5-7-13)10-29-21(23)24)15-8-11(2)31(25,26)18-14(15)9-16(30-18)32(19,27)28/h4-7,9,11,15H,3,8,10H2,1-2H3,(H2,19,27,28)/t11-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303033

(CHEMBL585756 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C15H23N3O9S3/c1-3-17(15(19)26-6-4-5-7-27-18(20)21)12-8-10(2)29(22,23)14-11(12)9-13(28-14)30(16,24)25/h9-10,12H,3-8H2,1-2H3,(H2,16,24,25)/t10-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 705 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303034

(CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O |r| Show InChI InChI=1S/C14H20N4O12S3/c1-3-16(14(19)28-6-9(30-18(22)23)7-29-17(20)21)11-4-8(2)32(24,25)13-10(11)5-12(31-13)33(15,26)27/h5,8-9,11H,3-4,6-7H2,1-2H3,(H2,15,26,27)/t8-,9?,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50303030

(6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)CCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C16H25N3O8S3/c1-3-18(14(20)7-5-4-6-8-27-19(21)22)13-9-11(2)29(23,24)16-12(13)10-15(28-16)30(17,25)26/h10-11,13H,3-9H2,1-2H3,(H2,17,25,26)/t11-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303034

(CHEMBL571337 | Ethyl-((4S,6S)-6-methyl-7,7-dioxo-2...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)OCC(CO[N+]([O-])=O)O[N+]([O-])=O |r| Show InChI InChI=1S/C14H20N4O12S3/c1-3-16(14(19)28-6-9(30-18(22)23)7-29-17(20)21)11-4-8(2)32(24,25)13-10(11)5-12(31-13)33(15,26)27/h5,8-9,11H,3-4,6-7H2,1-2H3,(H2,15,26,27)/t8-,9?,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50303030

(6-Nitrooxy-hexanoic acid ethyl-((4S,6S)-6-methyl-7...)Show SMILES CCN([C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O)C(=O)CCCCCO[N+]([O-])=O |r| Show InChI InChI=1S/C16H25N3O8S3/c1-3-18(14(20)7-5-4-6-8-27-19(21)22)13-9-11(2)29(23,24)16-12(13)10-15(28-16)30(17,25)26/h10-11,13H,3-9H2,1-2H3,(H2,17,25,26)/t11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NicOx Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 by CO2 hydrase stopped flow assay |
Bioorg Med Chem Lett 19: 6565-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.036
BindingDB Entry DOI: 10.7270/Q2X92C80 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Aromatase inhibitor potency as iron-binding-related type II difference spectrum |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Apolipoprotein B-100
(Homo sapiens (Human)) | BDBM50107813

((S)-methyl 5-(6-methyl-4'-(trifluoromethyl)bipheny...)Show SMILES COC(=O)N[C@H]1Cc2ccc(NC(=O)c3cccc(C)c3-c3ccc(cc3)C(F)(F)F)cc2C1 |r| Show InChI InChI=1S/C26H23F3N2O3/c1-15-4-3-5-22(23(15)16-6-9-19(10-7-16)26(27,28)29)24(32)30-20-11-8-17-12-21(14-18(17)13-20)31-25(33)34-2/h3-11,13,21H,12,14H2,1-2H3,(H,30,32)(H,31,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch
Curated by ChEMBL
| Assay Description
Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM |
J Med Chem 44: 4677-87 (2001)
BindingDB Entry DOI: 10.7270/Q23F4NXH |
More data for this
Ligand-Target Pair | |
Apolipoprotein B-100
(Homo sapiens (Human)) | BDBM50107808
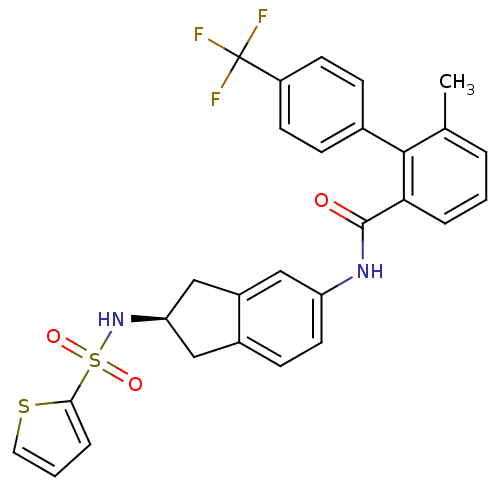
(6-Methyl-4'-trifluoromethyl-biphenyl-2-carboxylic ...)Show SMILES Cc1cccc(C(=O)Nc2ccc3C[C@H](Cc3c2)NS(=O)(=O)c2cccs2)c1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H23F3N2O3S2/c1-17-4-2-5-24(26(17)18-7-10-21(11-8-18)28(29,30)31)27(34)32-22-12-9-19-14-23(16-20(19)15-22)33-38(35,36)25-6-3-13-37-25/h2-13,15,23,33H,14,16H2,1H3,(H,32,34)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch
Curated by ChEMBL
| Assay Description
Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM |
J Med Chem 44: 4677-87 (2001)
BindingDB Entry DOI: 10.7270/Q23F4NXH |
More data for this
Ligand-Target Pair | |
Apolipoprotein B-100
(Homo sapiens (Human)) | BDBM50107774
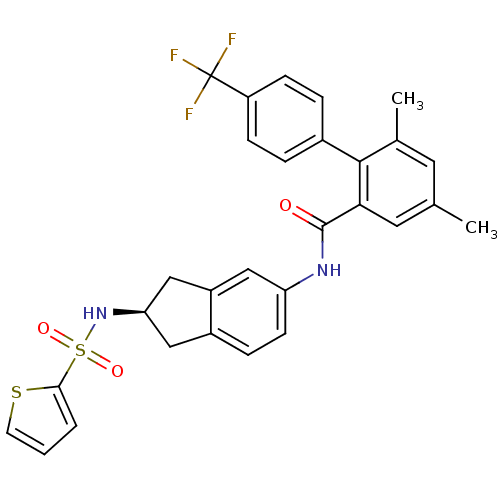
(4,6-Dimethyl-4'-trifluoromethyl-biphenyl-2-carboxy...)Show SMILES Cc1cc(C)c(-c2ccc(cc2)C(F)(F)F)c(c1)C(=O)Nc1ccc2C[C@H](Cc2c1)NS(=O)(=O)c1cccs1 Show InChI InChI=1S/C29H25F3N2O3S2/c1-17-12-18(2)27(19-5-8-22(9-6-19)29(30,31)32)25(13-17)28(35)33-23-10-7-20-14-24(16-21(20)15-23)34-39(36,37)26-4-3-11-38-26/h3-13,15,24,34H,14,16H2,1-2H3,(H,33,35)/t24-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch
Curated by ChEMBL
| Assay Description
Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM |
J Med Chem 44: 4677-87 (2001)
BindingDB Entry DOI: 10.7270/Q23F4NXH |
More data for this
Ligand-Target Pair | |
Apolipoprotein B-100
(Homo sapiens (Human)) | BDBM50107809
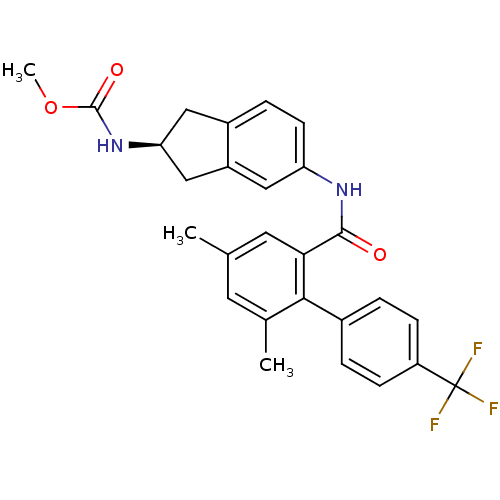
(CHEMBL143284 | {5-[(4,6-Dimethyl-4'-trifluoromethy...)Show SMILES COC(=O)N[C@H]1Cc2ccc(NC(=O)c3cc(C)cc(C)c3-c3ccc(cc3)C(F)(F)F)cc2C1 Show InChI InChI=1S/C27H25F3N2O3/c1-15-10-16(2)24(17-4-7-20(8-5-17)27(28,29)30)23(11-15)25(33)31-21-9-6-18-12-22(14-19(18)13-21)32-26(34)35-3/h4-11,13,22H,12,14H2,1-3H3,(H,31,33)(H,32,34)/t22-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch
Curated by ChEMBL
| Assay Description
Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM |
J Med Chem 44: 4677-87 (2001)
BindingDB Entry DOI: 10.7270/Q23F4NXH |
More data for this
Ligand-Target Pair | |
Apolipoprotein B-100
(Homo sapiens (Human)) | BDBM50107797
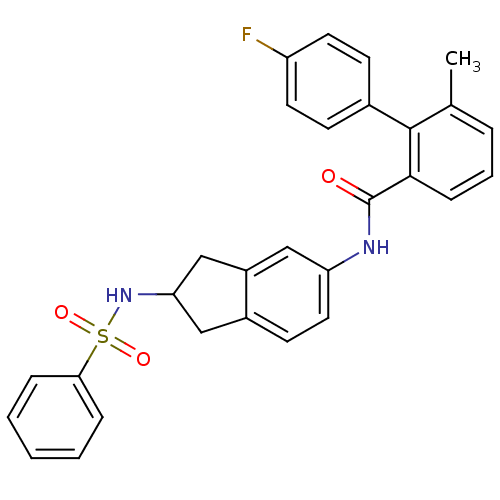
(4'-Fluoro-6-methyl-biphenyl-2-carboxylic acid (2-b...)Show SMILES Cc1cccc(C(=O)Nc2ccc3CC(Cc3c2)NS(=O)(=O)c2ccccc2)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H25FN2O3S/c1-19-6-5-9-27(28(19)20-10-13-23(30)14-11-20)29(33)31-24-15-12-21-16-25(18-22(21)17-24)32-36(34,35)26-7-3-2-4-8-26/h2-15,17,25,32H,16,18H2,1H3,(H,31,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch
Curated by ChEMBL
| Assay Description
Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM |
J Med Chem 44: 4677-87 (2001)
BindingDB Entry DOI: 10.7270/Q23F4NXH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data