 Found 46 hits with Last Name = 'stefanova' and Initial = 'i'
Found 46 hits with Last Name = 'stefanova' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50040294
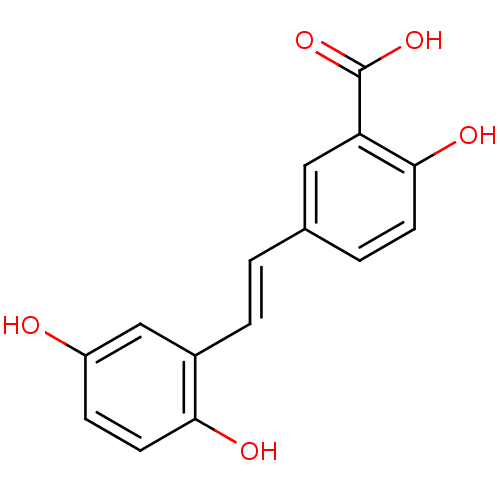
(5-[2-(2,5-Dihydroxy-phenyl)-vinyl]-2-hydroxy-benzo...)Show InChI InChI=1S/C15H12O5/c16-11-4-6-13(17)10(8-11)3-1-9-2-5-14(18)12(7-9)15(19)20/h1-8,16-18H,(H,19,20)/b3-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of p56lck autophosphorylation |
J Med Chem 36: 3010-4 (1993)
BindingDB Entry DOI: 10.7270/Q2XK8DNC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50040294

(5-[2-(2,5-Dihydroxy-phenyl)-vinyl]-2-hydroxy-benzo...)Show InChI InChI=1S/C15H12O5/c16-11-4-6-13(17)10(8-11)3-1-9-2-5-14(18)12(7-9)15(19)20/h1-8,16-18H,(H,19,20)/b3-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of protein-tyrosine kinase p56lck |
J Med Chem 36: 3015-20 (1993)
BindingDB Entry DOI: 10.7270/Q2V123VX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50029222
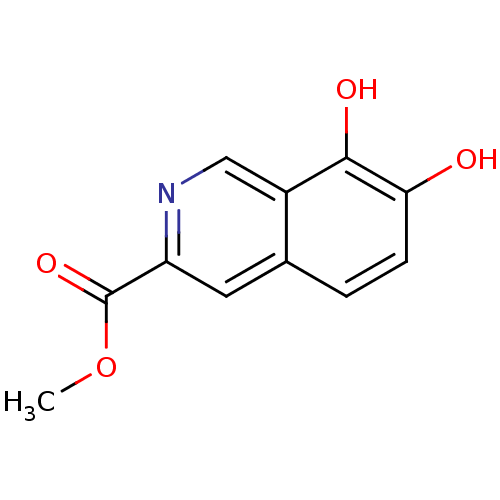
(7,8-Dihydroxy-isoquinoline-3-carboxylic acid methy...)Show InChI InChI=1S/C11H9NO4/c1-16-11(15)8-4-6-2-3-9(13)10(14)7(6)5-12-8/h2-5,13-14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046762
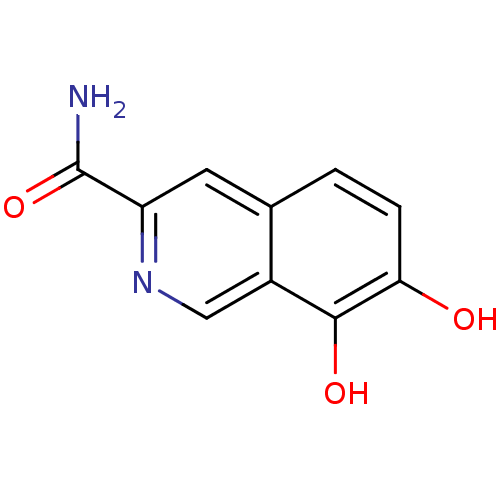
(7,8-Dihydroxy-isoquinoline-3-carboxylic acid amide...)Show InChI InChI=1S/C10H8N2O3/c11-10(15)7-3-5-1-2-8(13)9(14)6(5)4-12-7/h1-4,13-14H,(H2,11,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046762

(7,8-Dihydroxy-isoquinoline-3-carboxylic acid amide...)Show InChI InChI=1S/C10H8N2O3/c11-10(15)7-3-5-1-2-8(13)9(14)6(5)4-12-7/h1-4,13-14H,(H2,11,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50280317
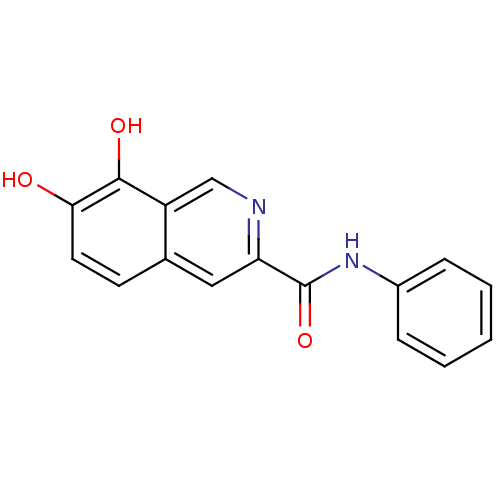
(7,8-Dihydroxy-isoquinoline-3-carboxylic acid pheny...)Show InChI InChI=1S/C16H12N2O3/c19-14-7-6-10-8-13(17-9-12(10)15(14)20)16(21)18-11-4-2-1-3-5-11/h1-9,19-20H,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50038203
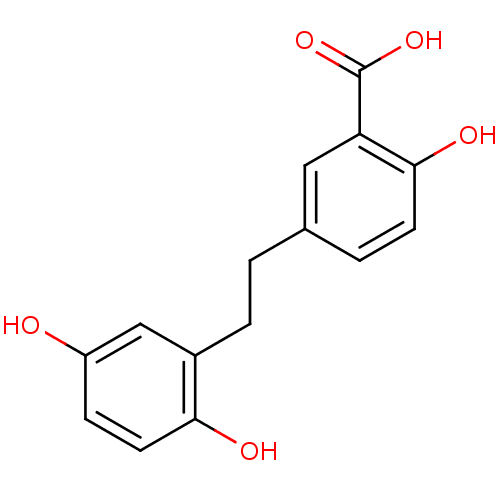
(5-[2-(2,5-Dihydroxy-phenyl)-ethyl]-2-hydroxy-benzo...)Show InChI InChI=1S/C15H14O5/c16-11-4-6-13(17)10(8-11)3-1-9-2-5-14(18)12(7-9)15(19)20/h2,4-8,16-18H,1,3H2,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of p56lck autophosphorylation |
J Med Chem 36: 3010-4 (1993)
BindingDB Entry DOI: 10.7270/Q2XK8DNC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50029199
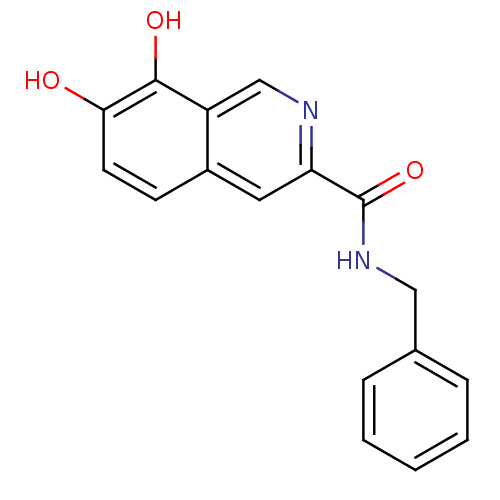
(7,8-Dihydroxy-isoquinoline-3-carboxylic acid benzy...)Show InChI InChI=1S/C17H14N2O3/c20-15-7-6-12-8-14(18-10-13(12)16(15)21)17(22)19-9-11-4-2-1-3-5-11/h1-8,10,20-21H,9H2,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50040296
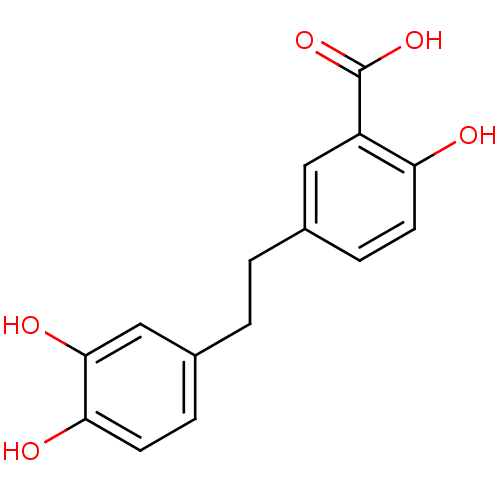
(5-[2-(3,4-Dihydroxy-phenyl)-ethyl]-2-hydroxy-benzo...)Show InChI InChI=1S/C15H14O5/c16-12-5-3-9(7-11(12)15(19)20)1-2-10-4-6-13(17)14(18)8-10/h3-8,16-18H,1-2H2,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of p56lck autophosphorylation |
J Med Chem 36: 3010-4 (1993)
BindingDB Entry DOI: 10.7270/Q2XK8DNC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029199

(7,8-Dihydroxy-isoquinoline-3-carboxylic acid benzy...)Show InChI InChI=1S/C17H14N2O3/c20-15-7-6-12-8-14(18-10-13(12)16(15)21)17(22)19-9-11-4-2-1-3-5-11/h1-8,10,20-21H,9H2,(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029221
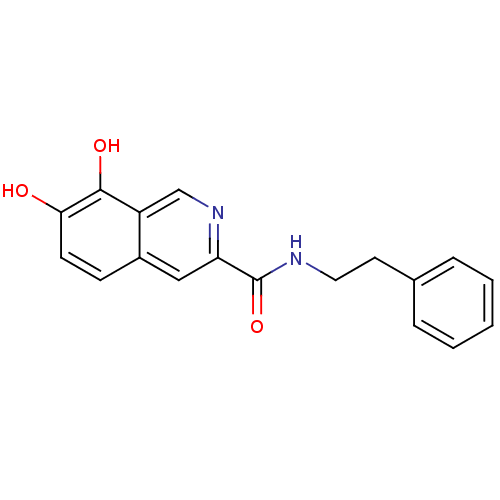
(7,8-Dihydroxy-isoquinoline-3-carboxylic acid phene...)Show InChI InChI=1S/C18H16N2O3/c21-16-7-6-13-10-15(20-11-14(13)17(16)22)18(23)19-9-8-12-4-2-1-3-5-12/h1-7,10-11,21-22H,8-9H2,(H,19,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029201

(6,7-Dihydroxy-isoquinoline-3-carboxylic acid benzy...)Show InChI InChI=1S/C17H14N2O3/c20-15-7-12-6-14(18-10-13(12)8-16(15)21)17(22)19-9-11-4-2-1-3-5-11/h1-8,10,20-21H,9H2,(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50280318
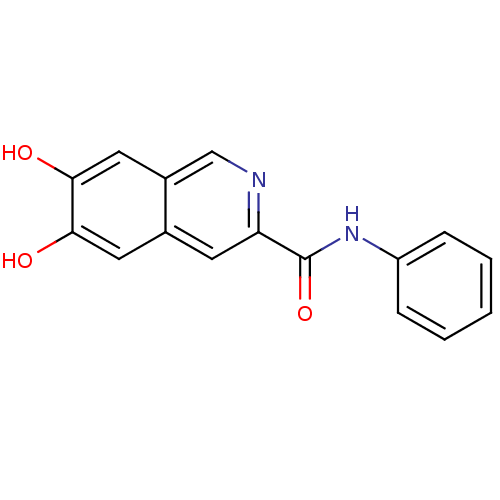
(6,7-Dihydroxy-isoquinoline-3-carboxylic acid pheny...)Show InChI InChI=1S/C16H12N2O3/c19-14-7-10-6-13(17-9-11(10)8-15(14)20)16(21)18-12-4-2-1-3-5-12/h1-9,19-20H,(H,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50280317

(7,8-Dihydroxy-isoquinoline-3-carboxylic acid pheny...)Show InChI InChI=1S/C16H12N2O3/c19-14-7-6-10-8-13(17-9-12(10)15(14)20)16(21)18-11-4-2-1-3-5-11/h1-9,19-20H,(H,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50038202

(5-((2,5-dihydroxybenzyl)(2-hydroxybenzyl)amino)-2-...)Show SMILES OC(=O)c1cc(ccc1O)N(Cc1ccccc1O)Cc1cc(O)ccc1O Show InChI InChI=1S/C21H19NO6/c23-16-6-8-19(25)14(9-16)12-22(11-13-3-1-2-4-18(13)24)15-5-7-20(26)17(10-15)21(27)28/h1-10,23-26H,11-12H2,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of p56lck autophosphorylation |
J Med Chem 36: 3010-4 (1993)
BindingDB Entry DOI: 10.7270/Q2XK8DNC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50038199
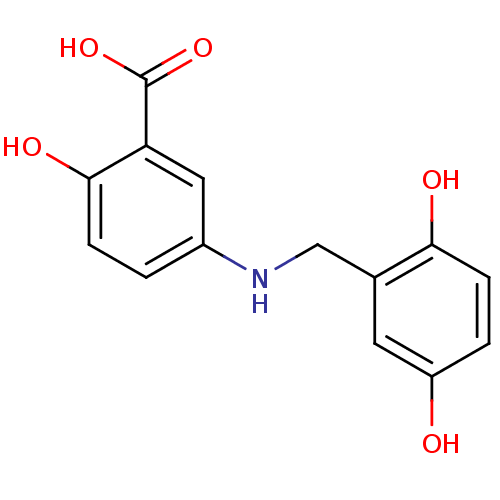
(5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C14H13NO5/c16-10-2-4-12(17)8(5-10)7-15-9-1-3-13(18)11(6-9)14(19)20/h1-6,15-18H,7H2,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of protein-tyrosine kinase p56lck |
J Med Chem 36: 3015-20 (1993)
BindingDB Entry DOI: 10.7270/Q2V123VX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50038199

(5-(2,5-Dihydroxy-benzylamino)-2-hydroxy-benzoic ac...)Show InChI InChI=1S/C14H13NO5/c16-10-2-4-12(17)8(5-10)7-15-9-1-3-13(18)11(6-9)14(19)20/h1-6,15-18H,7H2,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of p56lck autophosphorylation |
J Med Chem 36: 3010-4 (1993)
BindingDB Entry DOI: 10.7270/Q2XK8DNC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4301
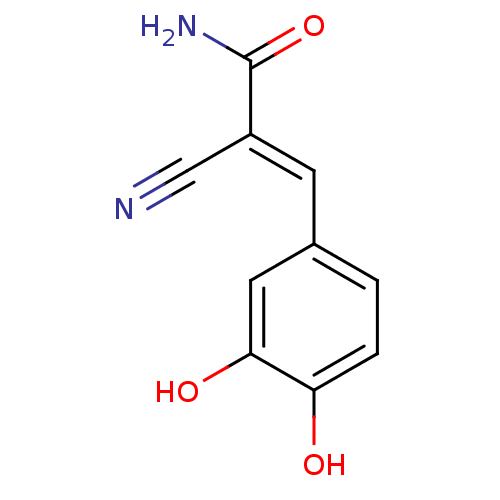
((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM4301

((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50029224

(5,6-Dihydroxy-naphthalene-2-carboxylic acid methyl...)Show InChI InChI=1S/C12H10O4/c1-16-12(15)8-2-4-9-7(6-8)3-5-10(13)11(9)14/h2-6,13-14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046763
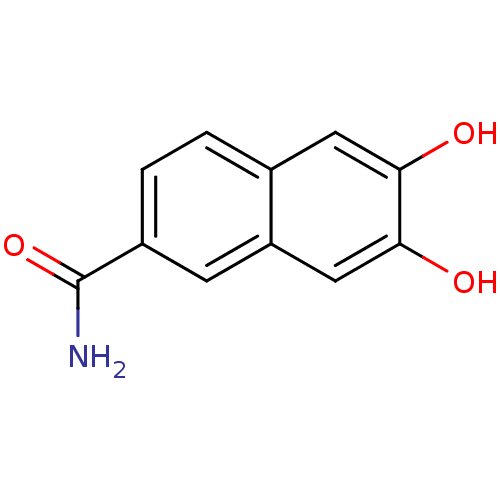
(6,7-Dihydroxy-naphthalene-2-carboxylic acid amide ...)Show InChI InChI=1S/C11H9NO3/c12-11(15)7-2-1-6-4-9(13)10(14)5-8(6)3-7/h1-5,13-14H,(H2,12,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50029221

(7,8-Dihydroxy-isoquinoline-3-carboxylic acid phene...)Show InChI InChI=1S/C18H16N2O3/c21-16-7-6-13-10-15(20-11-14(13)17(16)22)18(23)19-9-8-12-4-2-1-3-5-12/h1-7,10-11,21-22H,8-9H2,(H,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046063
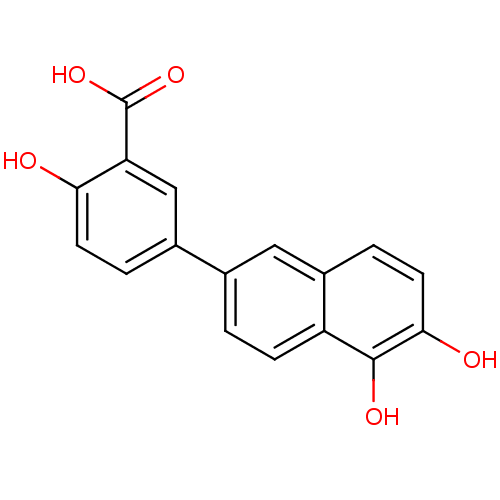
(5-(5,6-Dihydroxy-naphthalen-2-yl)-2-hydroxy-benzoi...)Show InChI InChI=1S/C17H12O5/c18-14-5-2-10(8-13(14)17(21)22)9-1-4-12-11(7-9)3-6-15(19)16(12)20/h1-8,18-20H,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of protein-tyrosine kinase p56lck |
J Med Chem 36: 3015-20 (1993)
BindingDB Entry DOI: 10.7270/Q2V123VX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046060
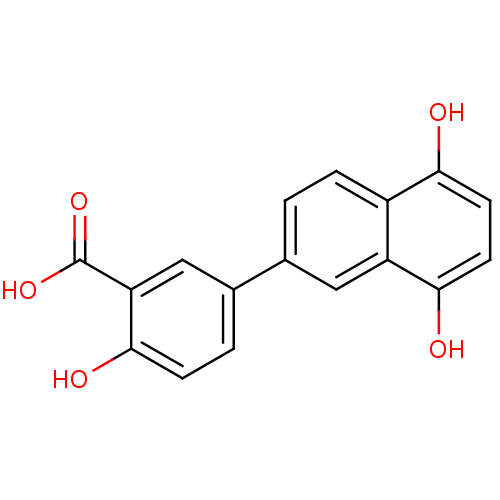
(5-(5,8-Dihydroxy-naphthalen-2-yl)-2-hydroxy-benzoi...)Show InChI InChI=1S/C17H12O5/c18-14-5-6-15(19)12-7-9(1-3-11(12)14)10-2-4-16(20)13(8-10)17(21)22/h1-8,18-20H,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of protein-tyrosine kinase p56lck |
J Med Chem 36: 3015-20 (1993)
BindingDB Entry DOI: 10.7270/Q2V123VX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50040295
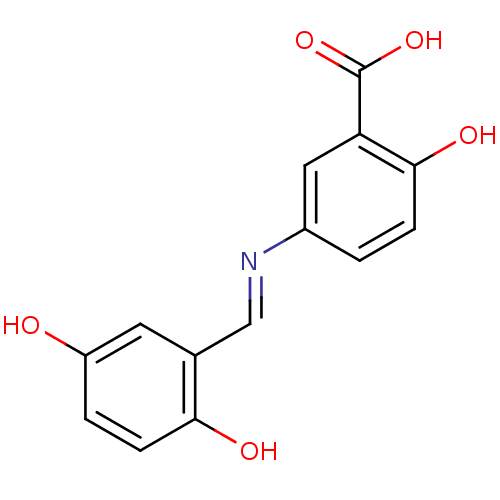
(5-[(2,5-Dihydroxy-benzylidene)-amino]-2-hydroxy-be...)Show InChI InChI=1S/C14H11NO5/c16-10-2-4-12(17)8(5-10)7-15-9-1-3-13(18)11(6-9)14(19)20/h1-7,16-18H,(H,19,20)/b15-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of p56lck autophosphorylation |
J Med Chem 36: 3010-4 (1993)
BindingDB Entry DOI: 10.7270/Q2XK8DNC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50046762

(7,8-Dihydroxy-isoquinoline-3-carboxylic acid amide...)Show InChI InChI=1S/C10H8N2O3/c11-10(15)7-3-5-1-2-8(13)9(14)6(5)4-12-7/h1-4,13-14H,(H2,11,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046758
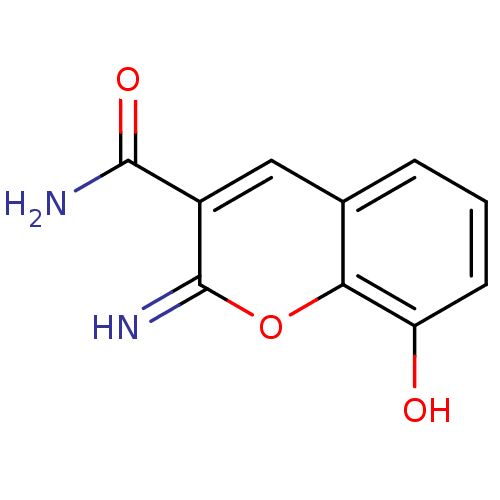
(8-Hydroxy-2-imino-2H-chromene-3-carboxylic acid am...)Show InChI InChI=1S/C10H8N2O3/c11-9(14)6-4-5-2-1-3-7(13)8(5)15-10(6)12/h1-4,12-13H,(H2,11,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50046760
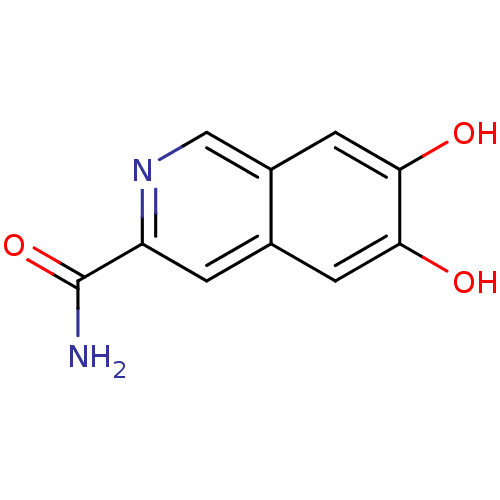
(6,7-Dihydroxy-isoquinoline-3-carboxylic acid amide...)Show InChI InChI=1S/C10H8N2O3/c11-10(15)7-1-5-2-8(13)9(14)3-6(5)4-12-7/h1-4,13-14H,(H2,11,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor (EGFR) |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046761
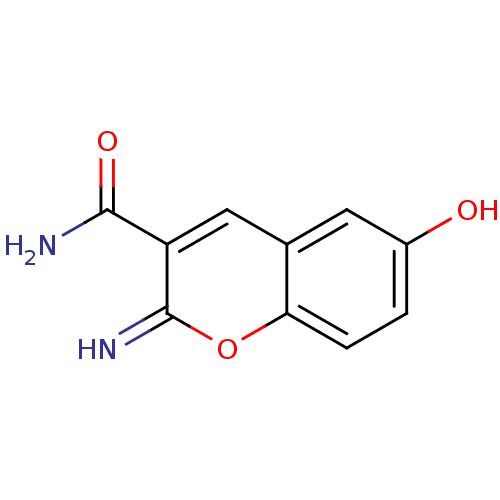
(6-Hydroxy-2-imino-2H-chromene-3-carboxylic acid am...)Show InChI InChI=1S/C10H8N2O3/c11-9(14)7-4-5-3-6(13)1-2-8(5)15-10(7)12/h1-4,12-13H,(H2,11,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046061
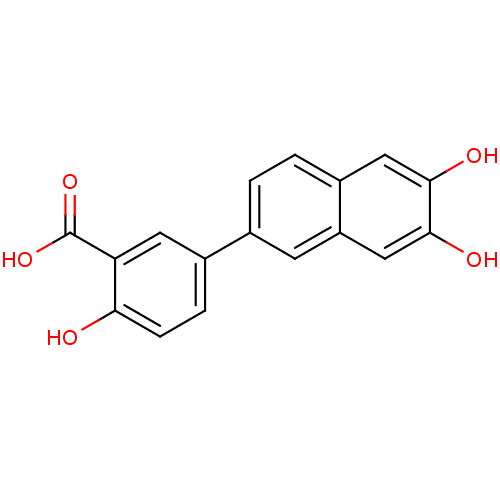
(5-(6,7-Dihydroxy-naphthalen-2-yl)-2-hydroxy-benzoi...)Show InChI InChI=1S/C17H12O5/c18-14-4-3-10(6-13(14)17(21)22)9-1-2-11-7-15(19)16(20)8-12(11)5-9/h1-8,18-20H,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of protein-tyrosine kinase p56lck |
J Med Chem 36: 3015-20 (1993)
BindingDB Entry DOI: 10.7270/Q2V123VX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046062
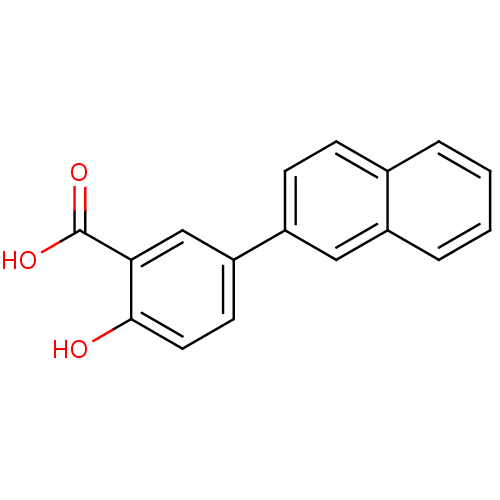
(2-Hydroxy-5-naphthalen-2-yl-benzoic acid | CHEMBL1...)Show InChI InChI=1S/C17H12O3/c18-16-8-7-14(10-15(16)17(19)20)13-6-5-11-3-1-2-4-12(11)9-13/h1-10,18H,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health
Curated by ChEMBL
| Assay Description
Inhibition of protein-tyrosine kinase p56lck |
J Med Chem 36: 3015-20 (1993)
BindingDB Entry DOI: 10.7270/Q2V123VX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046755

(2-Cyano-3-(3,5-dihydroxy-phenyl)-acrylamide | CHEM...)Show InChI InChI=1S/C10H8N2O3/c11-5-7(10(12)15)1-6-2-8(13)4-9(14)3-6/h1-4,13-14H,(H2,12,15)/b7-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50280318

(6,7-Dihydroxy-isoquinoline-3-carboxylic acid pheny...)Show InChI InChI=1S/C16H12N2O3/c19-14-7-10-6-13(17-9-11(10)8-15(14)20)16(21)18-12-4-2-1-3-5-12/h1-9,19-20H,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046754

(6,7-Dihydroxy-quinoline-3-carboxylic acid amide | ...)Show InChI InChI=1S/C10H8N2O3/c11-10(15)6-1-5-2-8(13)9(14)3-7(5)12-4-6/h1-4,13-14H,(H2,11,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50029220
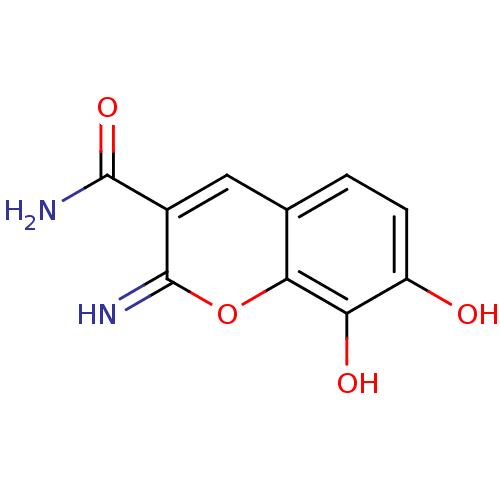
(7,8-Dihydroxy-2-imino-2H-chromene-3-carboxylic aci...)Show InChI InChI=1S/C10H8N2O4/c11-9(15)5-3-4-1-2-6(13)7(14)8(4)16-10(5)12/h1-3,12-14H,(H2,11,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM4355

((2E)-2-cyano-3-(4-hydroxyphenyl)prop-2-enamide | C...)Show InChI InChI=1S/C10H8N2O2/c11-6-8(10(12)14)5-7-1-3-9(13)4-2-7/h1-5,13H,(H2,12,14)/b8-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50029201

(6,7-Dihydroxy-isoquinoline-3-carboxylic acid benzy...)Show InChI InChI=1S/C17H14N2O3/c20-15-7-12-6-14(18-10-13(12)8-16(15)21)17(22)19-9-11-4-2-1-3-5-11/h1-8,10,20-21H,9H2,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046756
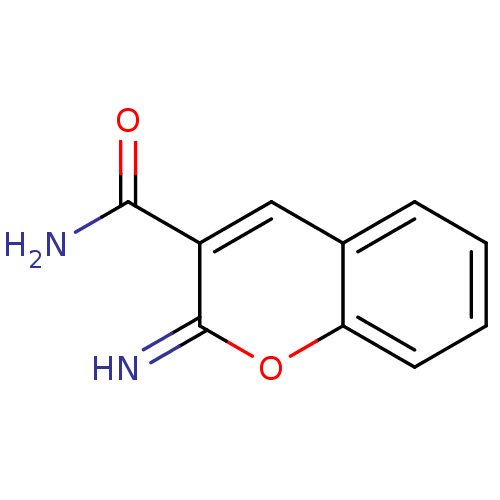
(2-Imino-2H-chromene-3-carboxylic acid amide | CHEM...)Show InChI InChI=1S/C10H8N2O2/c11-9(13)7-5-6-3-1-2-4-8(6)14-10(7)12/h1-5,12H,(H2,11,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046753
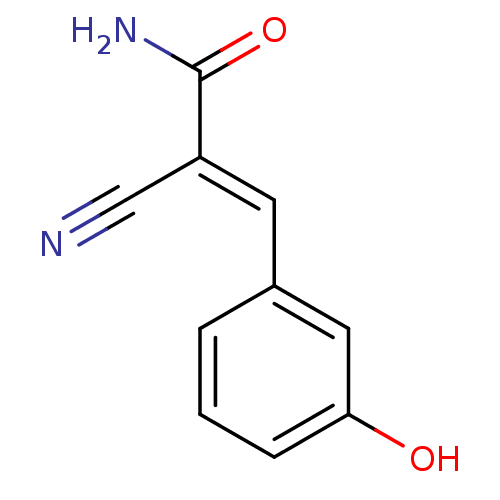
(2-Cyano-3-(3-hydroxy-phenyl)-acrylamide | CHEMBL13...)Show InChI InChI=1S/C10H8N2O2/c11-6-8(10(12)14)4-7-2-1-3-9(13)5-7/h1-5,13H,(H2,12,14)/b8-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046753

(2-Cyano-3-(3-hydroxy-phenyl)-acrylamide | CHEMBL13...)Show InChI InChI=1S/C10H8N2O2/c11-6-8(10(12)14)4-7-2-1-3-9(13)5-7/h1-5,13H,(H2,12,14)/b8-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046760

(6,7-Dihydroxy-isoquinoline-3-carboxylic acid amide...)Show InChI InChI=1S/C10H8N2O3/c11-10(15)7-1-5-2-8(13)9(14)3-6(5)4-12-7/h1-4,13-14H,(H2,11,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046760

(6,7-Dihydroxy-isoquinoline-3-carboxylic acid amide...)Show InChI InChI=1S/C10H8N2O3/c11-10(15)7-1-5-2-8(13)9(14)3-6(5)4-12-7/h1-4,13-14H,(H2,11,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 lck tyrosine kinase |
Bioorg Med Chem Lett 2: 1771-1774 (1992)
Article DOI: 10.1016/S0960-894X(00)80473-4
BindingDB Entry DOI: 10.7270/Q2SF2W33 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50029208

(6,7-Dihydroxy-2-imino-2H-chromene-3-carboxylic aci...)Show InChI InChI=1S/C10H8N2O4/c11-9(15)5-1-4-2-6(13)7(14)3-8(4)16-10(5)12/h1-3,12-14H,(H2,11,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046759
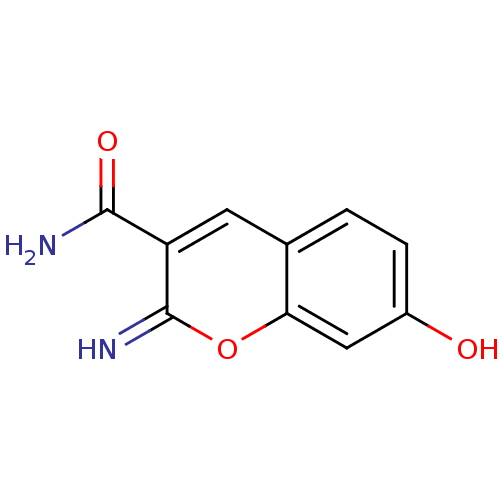
(7-Hydroxy-2-imino-2H-chromene-3-carboxylic acid am...)Show InChI InChI=1S/C10H8N2O3/c11-9(14)7-3-5-1-2-6(13)4-8(5)15-10(7)12/h1-4,12-13H,(H2,11,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50046757
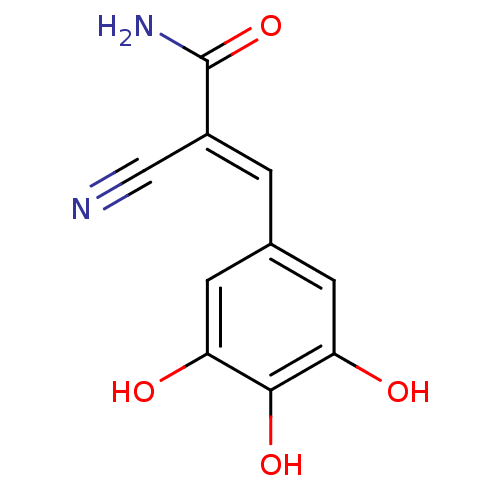
(2-Cyano-3-(3,4,5-trihydroxy-phenyl)-acrylamide | C...)Show InChI InChI=1S/C10H8N2O4/c11-4-6(10(12)16)1-5-2-7(13)9(15)8(14)3-5/h1-3,13-15H,(H2,12,16)/b6-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit autophosphorylation of immunopurified p56Ick |
J Med Chem 36: 425-32 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4GTQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data