 Found 83 hits with Last Name = 'stray' and Initial = 'km'
Found 83 hits with Last Name = 'stray' and Initial = 'km' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350205
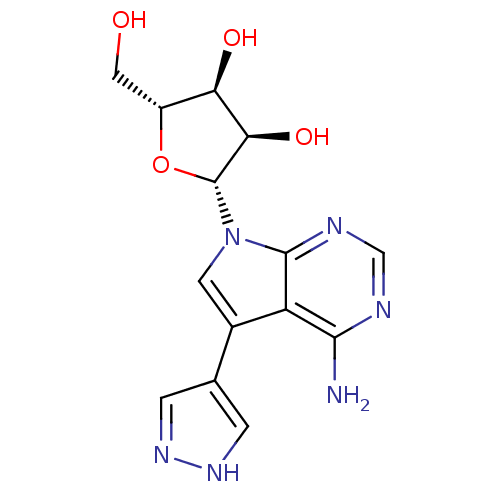
(CHEMBL1814774)Show SMILES Nc1ncnc2n(cc(-c3cn[nH]c3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H16N6O4/c15-12-9-7(6-1-18-19-2-6)3-20(13(9)17-5-16-12)14-11(23)10(22)8(4-21)24-14/h1-3,5,8,10-11,14,21-23H,4H2,(H,18,19)(H2,15,16,17)/t8-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350207

(CHEMBL1814776)Show SMILES Nc1ncnc2n(cc(C#C)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C13H14N4O4/c1-2-6-3-17(12-8(6)11(14)15-5-16-12)13-10(20)9(19)7(4-18)21-13/h1,3,5,7,9-10,13,18-20H,4H2,(H2,14,15,16)/t7-,9-,10-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447466

(CHEMBL3115191)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CC[C@@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C38H46N8O4S2/c1-26(2)36-43-32(23-51-36)21-46(3)37(48)45-34(18-31-19-39-24-41-31)35(47)42-29(16-27-10-6-4-7-11-27)14-15-30(17-28-12-8-5-9-13-28)44-38(49)50-22-33-20-40-25-52-33/h4-13,19-20,23-26,29-30,34H,14-18,21-22H2,1-3H3,(H,39,41)(H,42,47)(H,44,49)(H,45,48)/t29-,30-,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447393
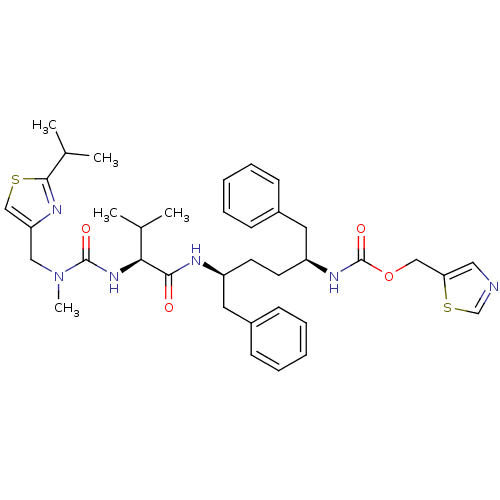
(CHEMBL3114722)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@@H](CC[C@@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-25(2)33(42-36(45)43(5)21-31-23-48-35(40-31)26(3)4)34(44)39-29(18-27-12-8-6-9-13-27)16-17-30(19-28-14-10-7-11-15-28)41-37(46)47-22-32-20-38-24-49-32/h6-15,20,23-26,29-30,33H,16-19,21-22H2,1-5H3,(H,39,44)(H,41,46)(H,42,45)/t29-,30-,33-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447469

(CHEMBL3115185)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](Cc2cn(C)cn2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C39H48N8O4S2/c1-27(2)37-43-33(24-52-37)22-47(4)38(49)45-35(19-32-21-46(3)25-41-32)36(48)42-30(17-28-11-7-5-8-12-28)15-16-31(18-29-13-9-6-10-14-29)44-39(50)51-23-34-20-40-26-53-34/h5-14,20-21,24-27,30-31,35H,15-19,22-23H2,1-4H3,(H,42,48)(H,44,50)(H,45,49)/t30-,31-,35+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447472

(CHEMBL3115177)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C36H47N7O4S2/c1-25(2)34-40-30(23-48-34)21-43(3)35(45)42-32(16-17-37)33(44)39-28(18-26-10-6-4-7-11-26)14-15-29(19-27-12-8-5-9-13-27)41-36(46)47-22-31-20-38-24-49-31/h4-13,20,23-25,28-29,32H,14-19,21-22,37H2,1-3H3,(H,39,44)(H,41,46)(H,42,45)/t28-,29-,32+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447467

(CHEMBL3115188)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCNC(C)=O)C(=O)N[C@@H](CC[C@@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C38H49N7O5S2/c1-26(2)36-42-32(24-51-36)22-45(4)37(48)44-34(17-18-40-27(3)46)35(47)41-30(19-28-11-7-5-8-12-28)15-16-31(20-29-13-9-6-10-14-29)43-38(49)50-23-33-21-39-25-52-33/h5-14,21,24-26,30-31,34H,15-20,22-23H2,1-4H3,(H,40,46)(H,41,47)(H,43,49)(H,44,48)/t30-,31-,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447391
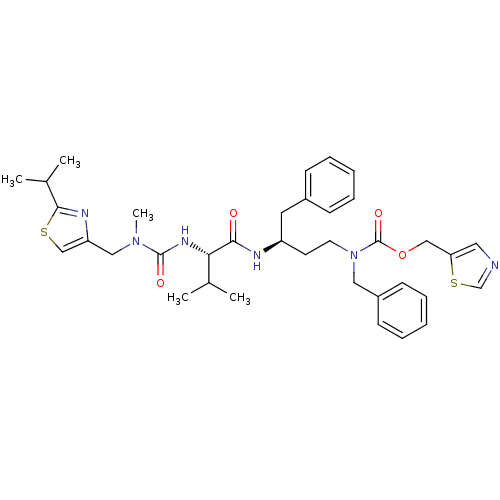
(CHEMBL3114730)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](CCN(Cc1ccccc1)C(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C36H46N6O4S2/c1-25(2)32(40-35(44)41(5)21-30-23-47-34(39-30)26(3)4)33(43)38-29(18-27-12-8-6-9-13-27)16-17-42(20-28-14-10-7-11-15-28)36(45)46-22-31-19-37-24-48-31/h6-15,19,23-26,29,32H,16-18,20-22H2,1-5H3,(H,38,43)(H,40,44)/t29-,32+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447392
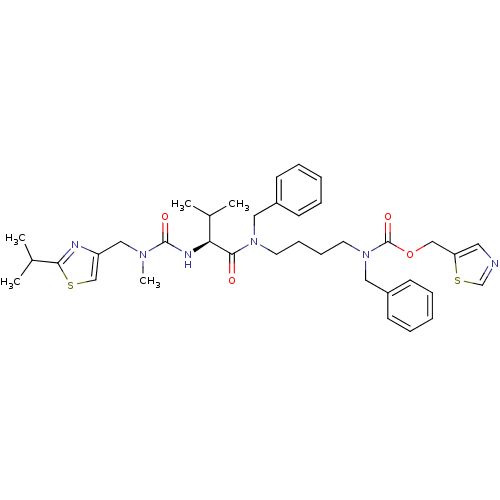
(CHEMBL3114724)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N(CCCCN(Cc1ccccc1)C(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-27(2)33(40-36(45)41(5)23-31-25-48-34(39-31)28(3)4)35(44)42(21-29-14-8-6-9-15-29)18-12-13-19-43(22-30-16-10-7-11-17-30)37(46)47-24-32-20-38-26-49-32/h6-11,14-17,20,25-28,33H,12-13,18-19,21-24H2,1-5H3,(H,40,45)/t33-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447468

(CHEMBL3115187)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC[C@@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C35H44N6O5S2/c1-24(2)33-38-29(22-47-33)19-41(3)34(44)40-31(20-42)32(43)37-27(16-25-10-6-4-7-11-25)14-15-28(17-26-12-8-5-9-13-26)39-35(45)46-21-30-18-36-23-48-30/h4-13,18,22-24,27-28,31,42H,14-17,19-21H2,1-3H3,(H,37,43)(H,39,45)(H,40,44)/t27-,28-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447390
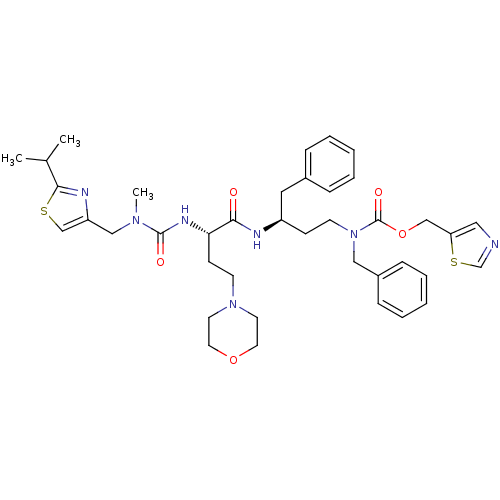
(CHEMBL3114731)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CCN(Cc2ccccc2)C(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C39H51N7O5S2/c1-29(2)37-42-33(27-52-37)25-44(3)38(48)43-35(15-16-45-18-20-50-21-19-45)36(47)41-32(22-30-10-6-4-7-11-30)14-17-46(24-31-12-8-5-9-13-31)39(49)51-26-34-23-40-28-53-34/h4-13,23,27-29,32,35H,14-22,24-26H2,1-3H3,(H,41,47)(H,43,48)/t32-,35+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447470

(CHEMBL3115180)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447392

(CHEMBL3114724)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N(CCCCN(Cc1ccccc1)C(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-27(2)33(40-36(45)41(5)23-31-25-48-34(39-31)28(3)4)35(44)42(21-29-14-8-6-9-15-29)18-12-13-19-43(22-30-16-10-7-11-17-30)37(46)47-24-32-20-38-26-49-32/h6-11,14-17,20,25-28,33H,12-13,18-19,21-24H2,1-5H3,(H,40,45)/t33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350199
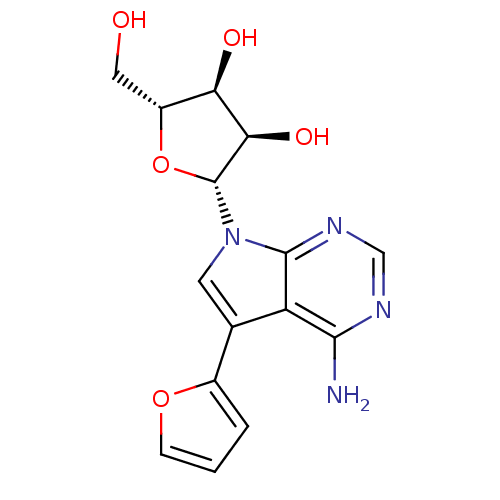
(CHEMBL1814767)Show SMILES Nc1ncnc2n(cc(-c3ccco3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H16N4O5/c16-13-10-7(8-2-1-3-23-8)4-19(14(10)18-6-17-13)15-12(22)11(21)9(5-20)24-15/h1-4,6,9,11-12,15,20-22H,5H2,(H2,16,17,18)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350195
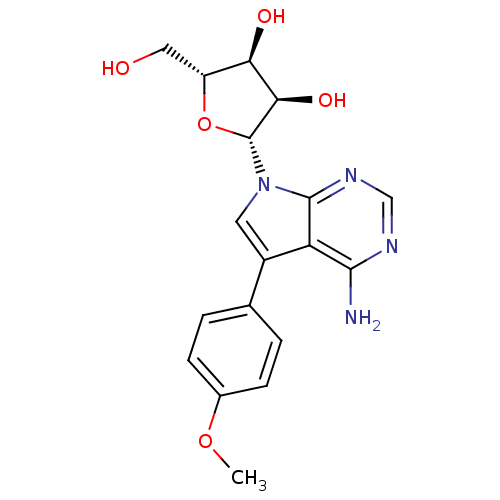
(CHEMBL1814763)Show SMILES COc1ccc(cc1)-c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C18H20N4O5/c1-26-10-4-2-9(3-5-10)11-6-22(17-13(11)16(19)20-8-21-17)18-15(25)14(24)12(7-23)27-18/h2-6,8,12,14-15,18,23-25H,7H2,1H3,(H2,19,20,21)/t12-,14-,15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447466

(CHEMBL3115191)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CC[C@@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C38H46N8O4S2/c1-26(2)36-43-32(23-51-36)21-46(3)37(48)45-34(18-31-19-39-24-41-31)35(47)42-29(16-27-10-6-4-7-11-27)14-15-30(17-28-12-8-5-9-13-28)44-38(49)50-22-33-20-40-25-52-33/h4-13,19-20,23-26,29-30,34H,14-18,21-22H2,1-3H3,(H,39,41)(H,42,47)(H,44,49)(H,45,48)/t29-,30-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447469

(CHEMBL3115185)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](Cc2cn(C)cn2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C39H48N8O4S2/c1-27(2)37-43-33(24-52-37)22-47(4)38(49)45-35(19-32-21-46(3)25-41-32)36(48)42-30(17-28-11-7-5-8-12-28)15-16-31(18-29-13-9-6-10-14-29)44-39(50)51-23-34-20-40-26-53-34/h5-14,20-21,24-27,30-31,35H,15-19,22-23H2,1-4H3,(H,42,48)(H,44,50)(H,45,49)/t30-,31-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447388
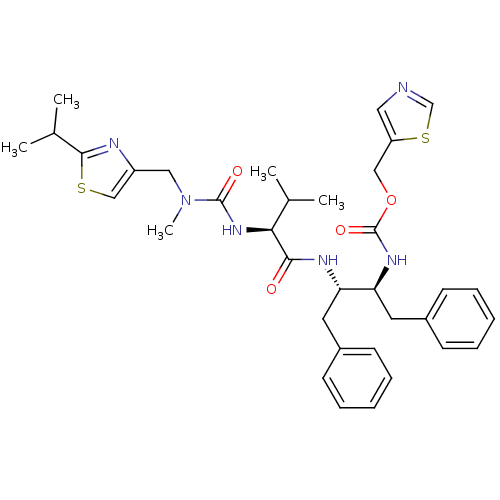
(CHEMBL3114738)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C35H44N6O4S2/c1-23(2)31(40-34(43)41(5)19-27-21-46-33(37-27)24(3)4)32(42)38-29(16-25-12-8-6-9-13-25)30(17-26-14-10-7-11-15-26)39-35(44)45-20-28-18-36-22-47-28/h6-15,18,21-24,29-31H,16-17,19-20H2,1-5H3,(H,38,42)(H,39,44)(H,40,43)/t29-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447395
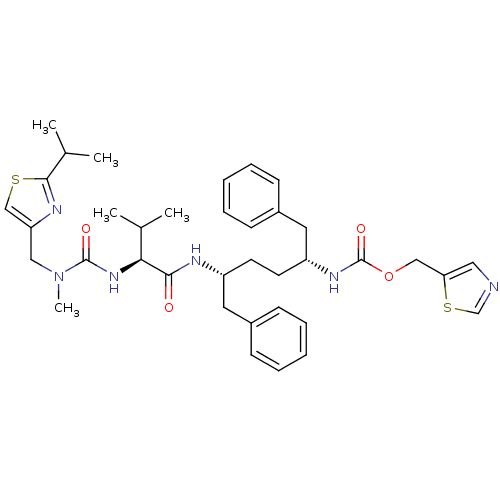
(CHEMBL3114713)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](CC[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-25(2)33(42-36(45)43(5)21-31-23-48-35(40-31)26(3)4)34(44)39-29(18-27-12-8-6-9-13-27)16-17-30(19-28-14-10-7-11-15-28)41-37(46)47-22-32-20-38-24-49-32/h6-15,20,23-26,29-30,33H,16-19,21-22H2,1-5H3,(H,39,44)(H,41,46)(H,42,45)/t29-,30-,33+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447470

(CHEMBL3115180)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50447392

(CHEMBL3114724)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N(CCCCN(Cc1ccccc1)C(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-27(2)33(40-36(45)41(5)23-31-25-48-34(39-31)28(3)4)35(44)42(21-29-14-8-6-9-15-29)18-12-13-19-43(22-30-16-10-7-11-17-30)37(46)47-24-32-20-38-26-49-32/h6-11,14-17,20,25-28,33H,12-13,18-19,21-24H2,1-5H3,(H,40,45)/t33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50447469

(CHEMBL3115185)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](Cc2cn(C)cn2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C39H48N8O4S2/c1-27(2)37-43-33(24-52-37)22-47(4)38(49)45-35(19-32-21-46(3)25-41-32)36(48)42-30(17-28-11-7-5-8-12-28)15-16-31(18-29-13-9-6-10-14-29)44-39(50)51-23-34-20-40-26-53-34/h5-14,20-21,24-27,30-31,35H,15-19,22-23H2,1-4H3,(H,42,48)(H,44,50)(H,45,49)/t30-,31-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50447466

(CHEMBL3115191)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CC[C@@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C38H46N8O4S2/c1-26(2)36-43-32(23-51-36)21-46(3)37(48)45-34(18-31-19-39-24-41-31)35(47)42-29(16-27-10-6-4-7-11-27)14-15-30(17-28-12-8-5-9-13-28)44-38(49)50-22-33-20-40-25-52-33/h4-13,19-20,23-26,29-30,34H,14-18,21-22H2,1-3H3,(H,39,41)(H,42,47)(H,44,49)(H,45,48)/t29-,30-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350200
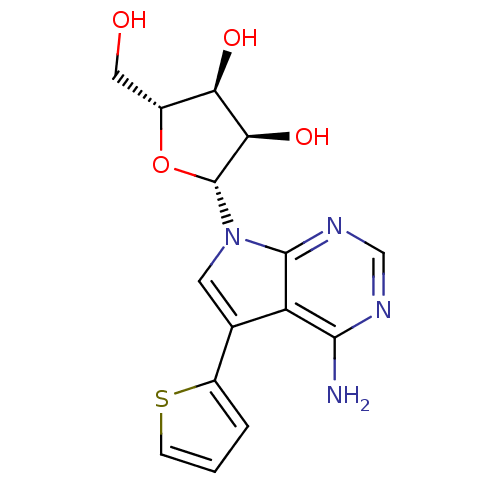
(CHEMBL1814768)Show SMILES Nc1ncnc2n(cc(-c3cccs3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H16N4O4S/c16-13-10-7(9-2-1-3-24-9)4-19(14(10)18-6-17-13)15-12(22)11(21)8(5-20)23-15/h1-4,6,8,11-12,15,20-22H,5H2,(H2,16,17,18)/t8-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350202
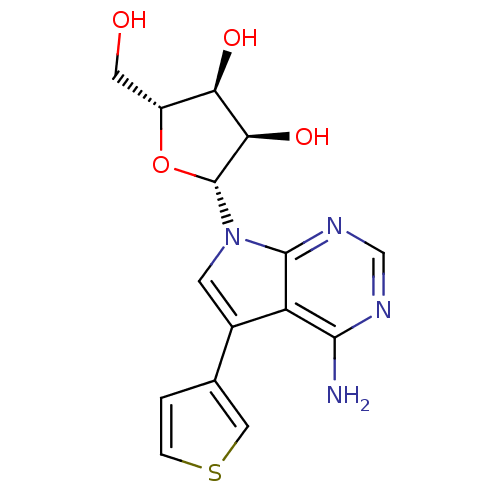
(CHEMBL1814770)Show SMILES Nc1ncnc2n(cc(-c3ccsc3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H16N4O4S/c16-13-10-8(7-1-2-24-5-7)3-19(14(10)18-6-17-13)15-12(22)11(21)9(4-20)23-15/h1-3,5-6,9,11-12,15,20-22H,4H2,(H2,16,17,18)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350194
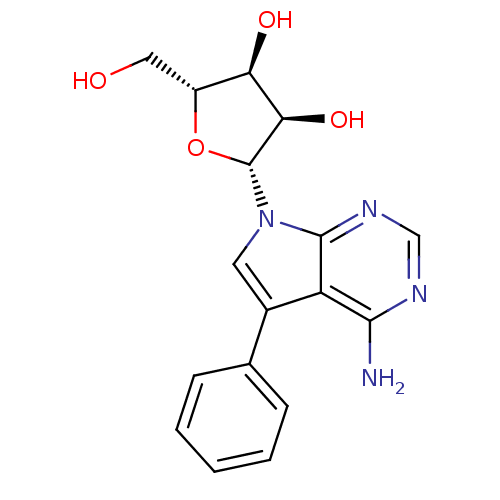
(CHEMBL1814762)Show SMILES Nc1ncnc2n(cc(-c3ccccc3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H18N4O4/c18-15-12-10(9-4-2-1-3-5-9)6-21(16(12)20-8-19-15)17-14(24)13(23)11(7-22)25-17/h1-6,8,11,13-14,17,22-24H,7H2,(H2,18,19,20)/t11-,13-,14-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447393

(CHEMBL3114722)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@@H](CC[C@@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-25(2)33(42-36(45)43(5)21-31-23-48-35(40-31)26(3)4)34(44)39-29(18-27-12-8-6-9-13-27)16-17-30(19-28-14-10-7-11-15-28)41-37(46)47-22-32-20-38-24-49-32/h6-15,20,23-26,29-30,33H,16-19,21-22H2,1-5H3,(H,39,44)(H,41,46)(H,42,45)/t29-,30-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350198

(CHEMBL1814766)Show SMILES Nc1ncnc2n(cc(-c3ccc4ccccc4c3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H20N4O4/c22-19-16-14(13-6-5-11-3-1-2-4-12(11)7-13)8-25(20(16)24-10-23-19)21-18(28)17(27)15(9-26)29-21/h1-8,10,15,17-18,21,26-28H,9H2,(H2,22,23,24)/t15-,17-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350201
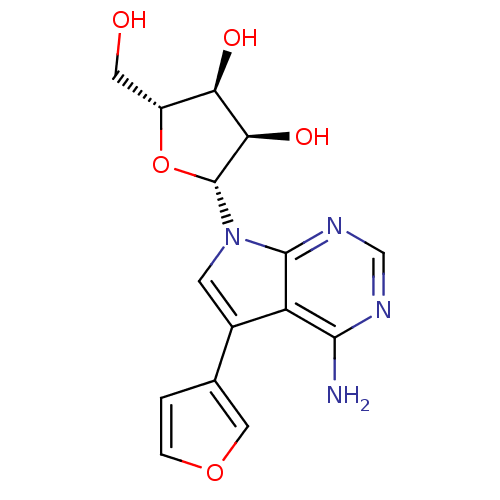
(CHEMBL1814769)Show SMILES Nc1ncnc2n(cc(-c3ccoc3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H16N4O5/c16-13-10-8(7-1-2-23-5-7)3-19(14(10)18-6-17-13)15-12(22)11(21)9(4-20)24-15/h1-3,5-6,9,11-12,15,20-22H,4H2,(H2,16,17,18)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350209
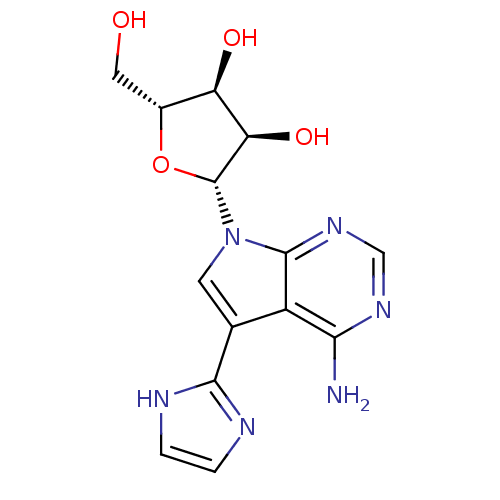
(CHEMBL1814780)Show SMILES Nc1ncnc2n(cc(-c3ncc[nH]3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H16N6O4/c15-11-8-6(12-16-1-2-17-12)3-20(13(8)19-5-18-11)14-10(23)9(22)7(4-21)24-14/h1-3,5,7,9-10,14,21-23H,4H2,(H,16,17)(H2,15,18,19)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50447468

(CHEMBL3115187)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC[C@@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C35H44N6O5S2/c1-24(2)33-38-29(22-47-33)19-41(3)34(44)40-31(20-42)32(43)37-27(16-25-10-6-4-7-11-25)14-15-28(17-26-12-8-5-9-13-26)39-35(45)46-21-30-18-36-23-48-30/h4-13,18,22-24,27-28,31,42H,14-17,19-21H2,1-3H3,(H,37,43)(H,39,45)(H,40,44)/t27-,28-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350204
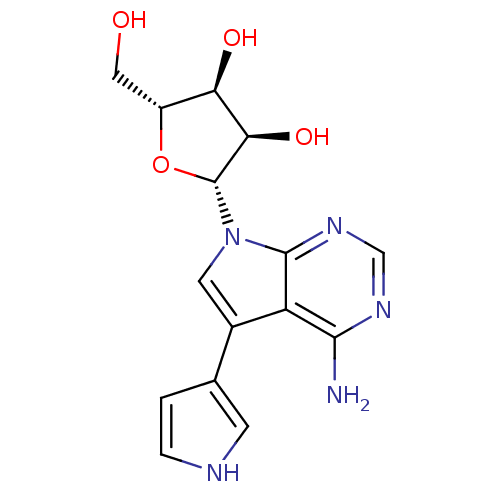
(CHEMBL1814773)Show SMILES Nc1ncnc2n(cc(-c3cc[nH]c3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H17N5O4/c16-13-10-8(7-1-2-17-3-7)4-20(14(10)19-6-18-13)15-12(23)11(22)9(5-21)24-15/h1-4,6,9,11-12,15,17,21-23H,5H2,(H2,16,18,19)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447394
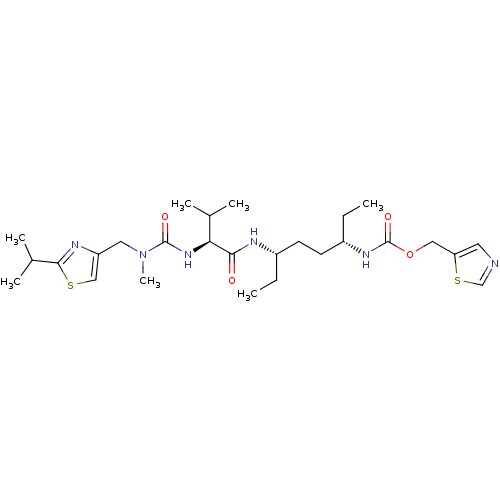
(CHEMBL3114719)Show SMILES CC[C@@H](CC[C@H](CC)NC(=O)[C@@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C27H44N6O4S2/c1-8-19(10-11-20(9-2)31-27(36)37-14-22-12-28-16-39-22)29-24(34)23(17(3)4)32-26(35)33(7)13-21-15-38-25(30-21)18(5)6/h12,15-20,23H,8-11,13-14H2,1-7H3,(H,29,34)(H,31,36)(H,32,35)/t19-,20-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447390

(CHEMBL3114731)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CCN(Cc2ccccc2)C(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C39H51N7O5S2/c1-29(2)37-42-33(27-52-37)25-44(3)38(48)43-35(15-16-45-18-20-50-21-19-45)36(47)41-32(22-30-10-6-4-7-11-30)14-17-46(24-31-12-8-5-9-13-31)39(49)51-26-34-23-40-28-53-34/h4-13,23,27-29,32,35H,14-22,24-26H2,1-3H3,(H,41,47)(H,43,48)/t32-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50447395

(CHEMBL3114713)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](CC[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-25(2)33(42-36(45)43(5)21-31-23-48-35(40-31)26(3)4)34(44)39-29(18-27-12-8-6-9-13-27)16-17-30(19-28-14-10-7-11-15-28)41-37(46)47-22-32-20-38-24-49-32/h6-15,20,23-26,29-30,33H,16-19,21-22H2,1-5H3,(H,39,44)(H,41,46)(H,42,45)/t29-,30-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447394

(CHEMBL3114719)Show SMILES CC[C@@H](CC[C@H](CC)NC(=O)[C@@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C27H44N6O4S2/c1-8-19(10-11-20(9-2)31-27(36)37-14-22-12-28-16-39-22)29-24(34)23(17(3)4)32-26(35)33(7)13-21-15-38-25(30-21)18(5)6/h12,15-20,23H,8-11,13-14H2,1-7H3,(H,29,34)(H,31,36)(H,32,35)/t19-,20-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50447470

(CHEMBL3115180)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50447390

(CHEMBL3114731)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CCN(Cc2ccccc2)C(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C39H51N7O5S2/c1-29(2)37-42-33(27-52-37)25-44(3)38(48)43-35(15-16-45-18-20-50-21-19-45)36(47)41-32(22-30-10-6-4-7-11-30)14-17-46(24-31-12-8-5-9-13-31)39(49)51-26-34-23-40-28-53-34/h4-13,23,27-29,32,35H,14-22,24-26H2,1-3H3,(H,41,47)(H,43,48)/t32-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447389
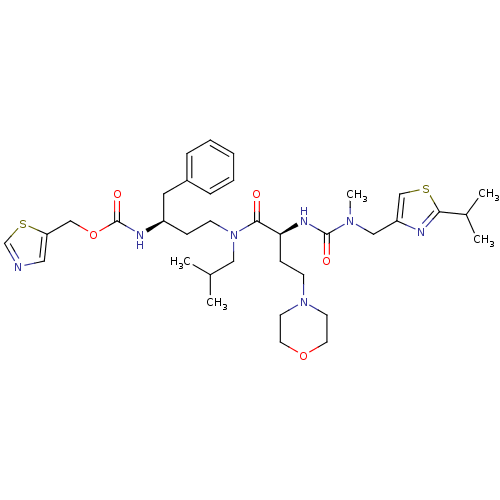
(CHEMBL3114735)Show SMILES CC(C)CN(CC[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)C(=O)[C@H](CCN1CCOCC1)NC(=O)N(C)Cc1csc(n1)C(C)C |r| Show InChI InChI=1S/C36H53N7O5S2/c1-26(2)21-43(14-11-29(19-28-9-7-6-8-10-28)39-36(46)48-23-31-20-37-25-50-31)34(44)32(12-13-42-15-17-47-18-16-42)40-35(45)41(5)22-30-24-49-33(38-30)27(3)4/h6-10,20,24-27,29,32H,11-19,21-23H2,1-5H3,(H,39,46)(H,40,45)/t29-,32+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447388

(CHEMBL3114738)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C35H44N6O4S2/c1-23(2)31(40-34(43)41(5)19-27-21-46-33(37-27)24(3)4)32(42)38-29(16-25-12-8-6-9-13-25)30(17-26-14-10-7-11-15-26)39-35(44)45-20-28-18-36-22-47-28/h6-15,18,21-24,29-31H,16-17,19-20H2,1-5H3,(H,38,42)(H,39,44)(H,40,43)/t29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50447471
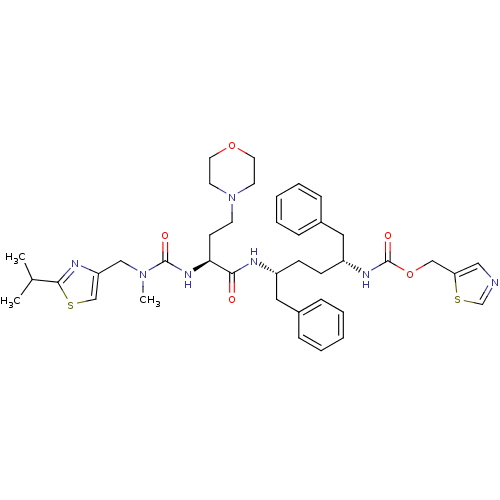
(CHEBI:72291 | Cobicistat)Show SMILES CC(C)c1nc(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)Cc2ccccc2)cs1 |r| Show InChI InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 24: 995-9 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.057
BindingDB Entry DOI: 10.7270/Q2P270M7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50447395

(CHEMBL3114713)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](CC[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-25(2)33(42-36(45)43(5)21-31-23-48-35(40-31)26(3)4)34(44)39-29(18-27-12-8-6-9-13-27)16-17-30(19-28-14-10-7-11-15-28)41-37(46)47-22-32-20-38-24-49-32/h6-15,20,23-26,29-30,33H,16-19,21-22H2,1-5H3,(H,39,44)(H,41,46)(H,42,45)/t29-,30-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350206
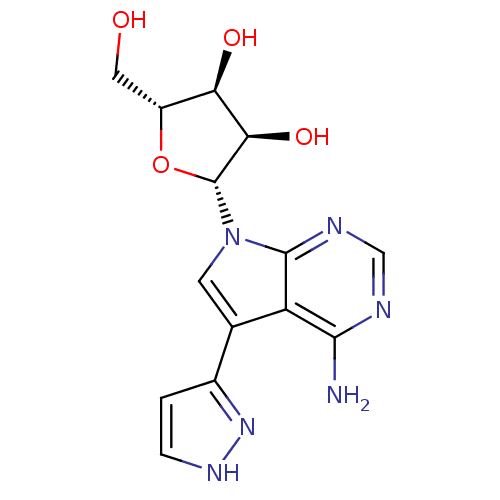
(CHEMBL1814775)Show SMILES Nc1ncnc2n(cc(-c3cc[nH]n3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H16N6O4/c15-12-9-6(7-1-2-18-19-7)3-20(13(9)17-5-16-12)14-11(23)10(22)8(4-21)24-14/h1-3,5,8,10-11,14,21-23H,4H2,(H,18,19)(H2,15,16,17)/t8-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50447393

(CHEMBL3114722)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@@H](CC[C@@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O4S2/c1-25(2)33(42-36(45)43(5)21-31-23-48-35(40-31)26(3)4)34(44)39-29(18-27-12-8-6-9-13-27)16-17-30(19-28-14-10-7-11-15-28)41-37(46)47-22-32-20-38-24-49-32/h6-15,20,23-26,29-30,33H,16-19,21-22H2,1-5H3,(H,39,44)(H,41,46)(H,42,45)/t29-,30-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
Bioorg Med Chem Lett 24: 989-94 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.058
BindingDB Entry DOI: 10.7270/Q2639R75 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350197
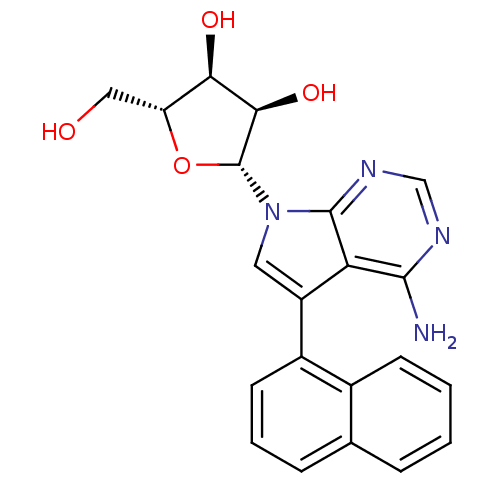
(CHEMBL1814765)Show SMILES Nc1ncnc2n(cc(-c3cccc4ccccc34)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H20N4O4/c22-19-16-14(13-7-3-5-11-4-1-2-6-12(11)13)8-25(20(16)24-10-23-19)21-18(28)17(27)15(9-26)29-21/h1-8,10,15,17-18,21,26-28H,9H2,(H2,22,23,24)/t15-,17-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350208

(CHEMBL1814779)Show SMILES Nc1ncnc2n(cc(-c3c[nH]cn3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H16N6O4/c15-12-9-6(7-1-16-4-17-7)2-20(13(9)19-5-18-12)14-11(23)10(22)8(3-21)24-14/h1-2,4-5,8,10-11,14,21-23H,3H2,(H,16,17)(H2,15,18,19)/t8-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350196
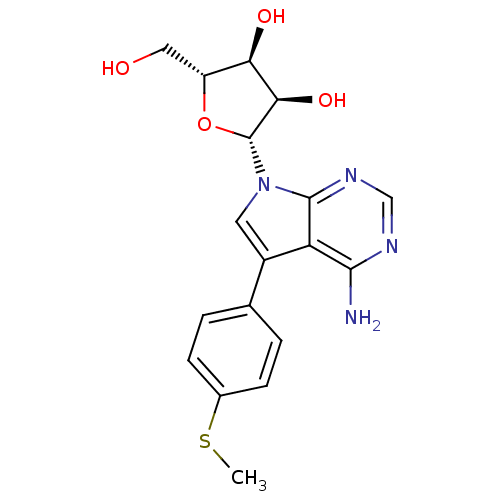
(CHEMBL1814764)Show SMILES CSc1ccc(cc1)-c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C18H20N4O4S/c1-27-10-4-2-9(3-5-10)11-6-22(17-13(11)16(19)20-8-21-17)18-15(25)14(24)12(7-23)26-18/h2-6,8,12,14-15,18,23-25H,7H2,1H3,(H2,19,20,21)/t12-,14-,15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50350203
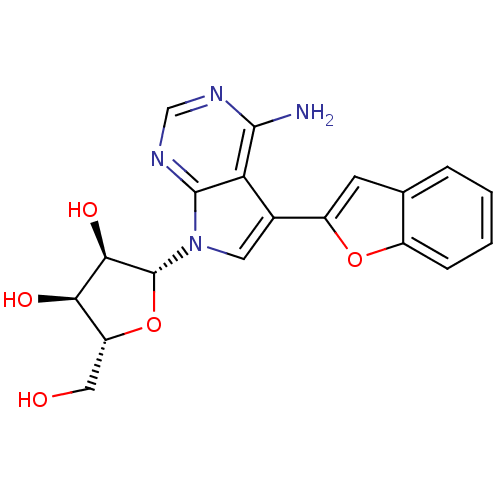
(CHEMBL1814771)Show SMILES Nc1ncnc2n(cc(-c3cc4ccccc4o3)c12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H18N4O5/c20-17-14-10(12-5-9-3-1-2-4-11(9)27-12)6-23(18(14)22-8-21-17)19-16(26)15(25)13(7-24)28-19/h1-6,8,13,15-16,19,24-26H,7H2,(H2,20,21,22)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human ADK using [3H]-adenosine by scintillation counting |
J Med Chem 54: 5498-507 (2011)
Article DOI: 10.1021/jm2005173
BindingDB Entry DOI: 10.7270/Q2BK1CQX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data