

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research Curated by PDSP Ki Database | Br J Pharmacol 115: 622-8 (1995) Article DOI: 10.1111/j.1476-5381.1995.tb14977.x BindingDB Entry DOI: 10.7270/Q2BR8QP0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156449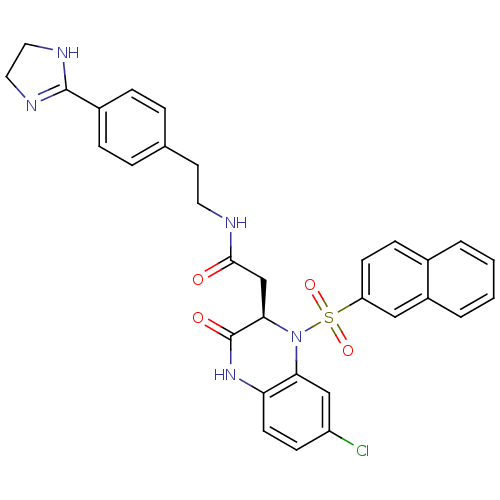 (2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086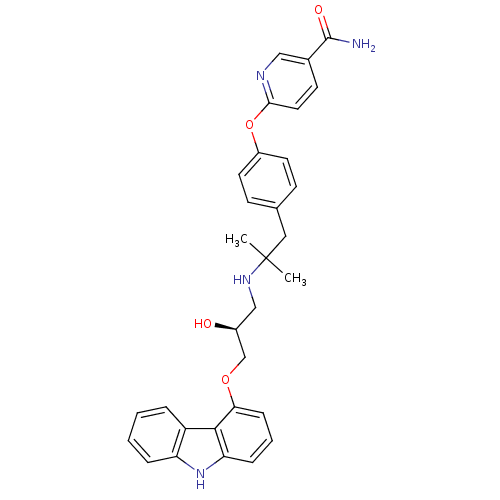 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM542562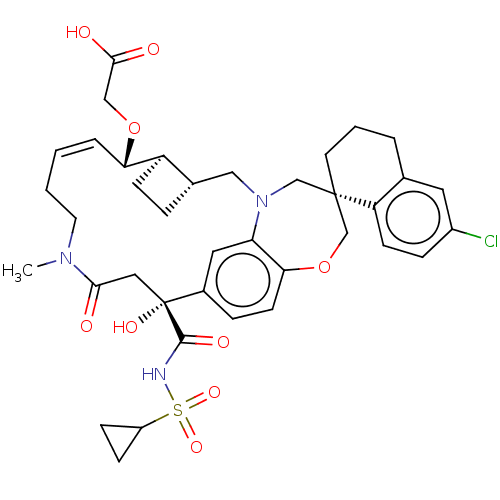 ((1S,3′R,6′R,7′S,8′E,15R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition of the Mcl-1/Bim interaction was measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. The recombinan... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V40ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1C (Gallus gallus) | BDBM50470712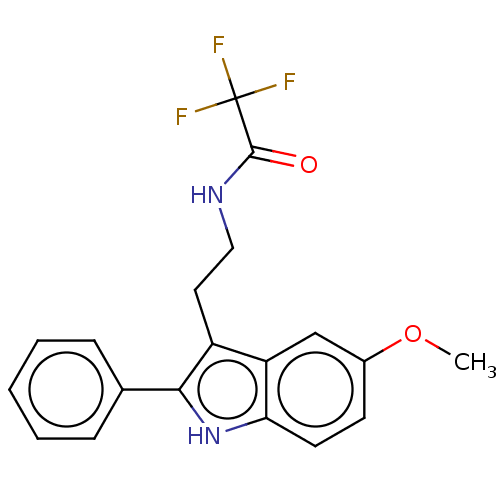 (CHEMBL417988) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay | J Med Chem 38: 1132-9 (1995) Article DOI: 10.1021/jm00007a010 BindingDB Entry DOI: 10.7270/Q2HM5C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156446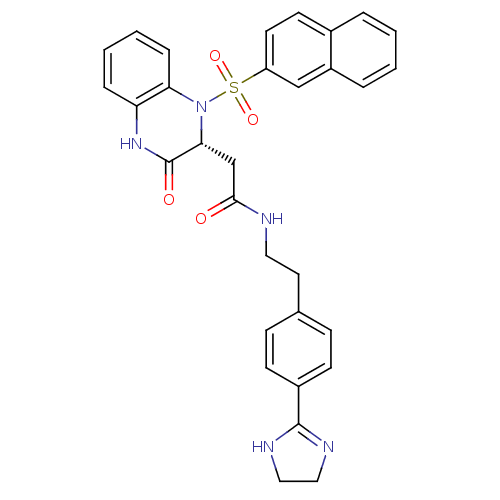 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to rhesus monkey Bradykinin receptor B1 | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350090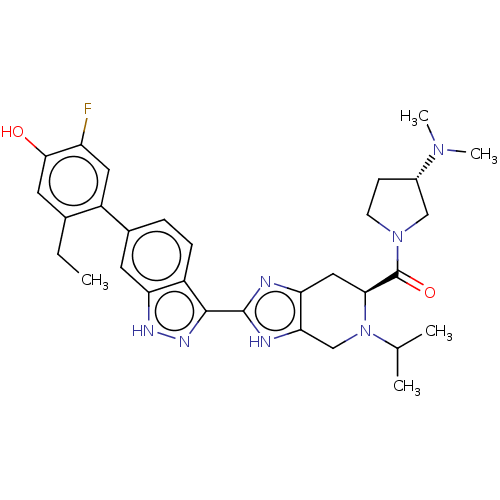 (((S)-3-(dimethylamino)pyrrolidin-1-yl)((S)-5-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20C50QQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156446 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo rec... | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156451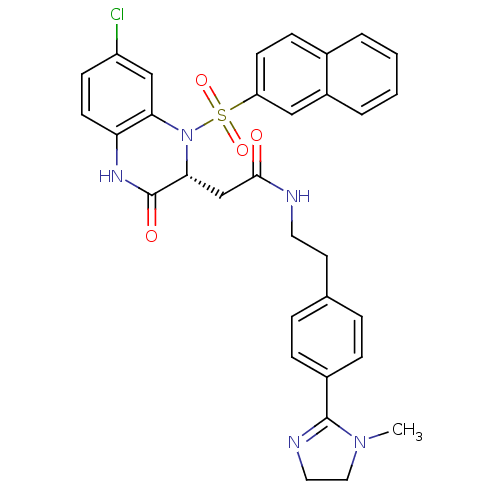 (2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350089 (((S)-2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20C50QQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350089 (((S)-2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THERAVANCE BIOPHARMA R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10208040 (2019) BindingDB Entry DOI: 10.7270/Q20867FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350090 (((S)-3-(dimethylamino)pyrrolidin-1-yl)((S)-5-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THERAVANCE BIOPHARMA R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10208040 (2019) BindingDB Entry DOI: 10.7270/Q20867FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333120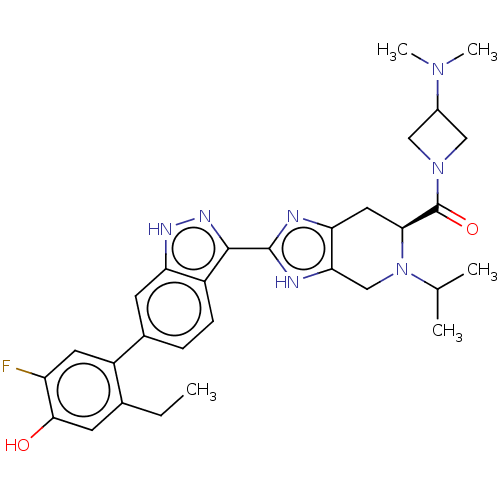 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THERAVANCE BIOPHARMA R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10196393 (2019) BindingDB Entry DOI: 10.7270/Q2ST7RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333120 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10550118 (2020) BindingDB Entry DOI: 10.7270/Q25D8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350089 (((S)-2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10519153 (2019) BindingDB Entry DOI: 10.7270/Q2DB8482 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350090 (((S)-3-(dimethylamino)pyrrolidin-1-yl)((S)-5-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10519153 (2019) BindingDB Entry DOI: 10.7270/Q2DB8482 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333120 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10954237 (2021) BindingDB Entry DOI: 10.7270/Q2571G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350089 (((S)-2-(6-(2-ethyl-5-fluoro-4-hydroxyphenyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | Citation and Details BindingDB Entry DOI: 10.7270/Q2000588 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350090 (((S)-3-(dimethylamino)pyrrolidin-1-yl)((S)-5-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | Citation and Details BindingDB Entry DOI: 10.7270/Q2000588 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333120 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 1: A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BG2S7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM542561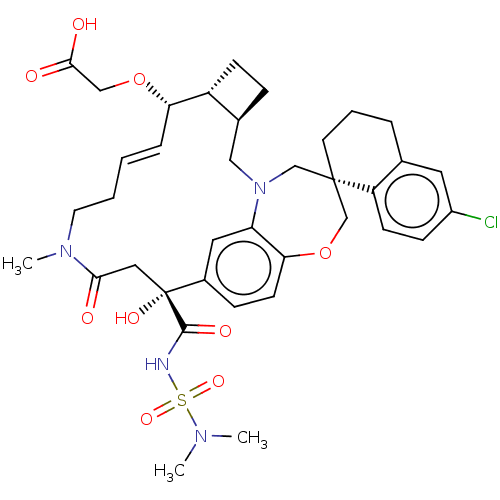 ((1S,3′R,6′R,7′S,8′E,15R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition of the Mcl-1/Bim interaction was measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. The recombinan... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V40ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50379086 (CHEMBL2012521 | CHEMBL2012522 | LY-377604) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting | ACS Med Chem Lett 2: 583-586 (2011) Article DOI: 10.1021/ml200071k BindingDB Entry DOI: 10.7270/Q20R9QDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455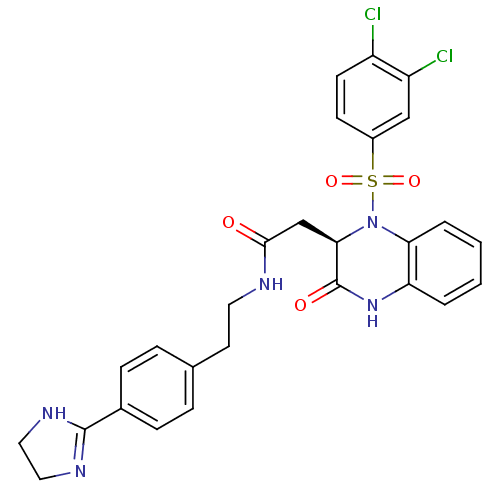 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM573151 (US11453668, Example 8-15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 1: A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BG2S7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM333122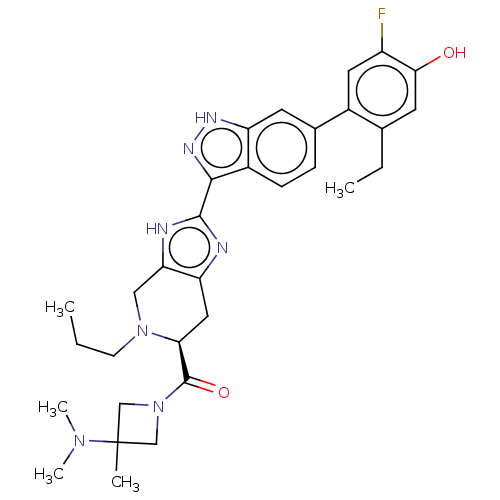 ((S)-(3-(dimethylamino)-3-methylazetidin-1-yl)(2-(6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THERAVANCE BIOPHARMA R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10196393 (2019) BindingDB Entry DOI: 10.7270/Q2ST7RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350094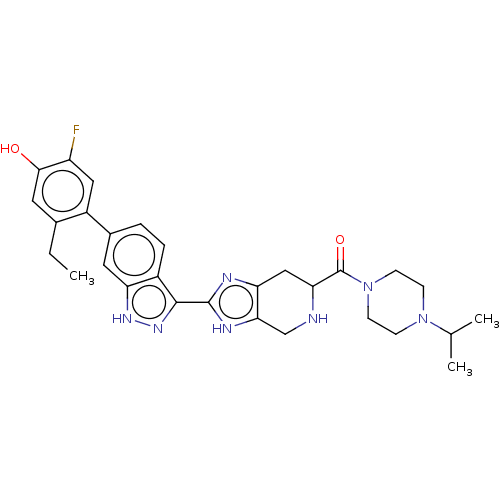 (US10208040, Example 1-2 | US10519153, Example 1-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THERAVANCE BIOPHARMA R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10208040 (2019) BindingDB Entry DOI: 10.7270/Q20867FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350098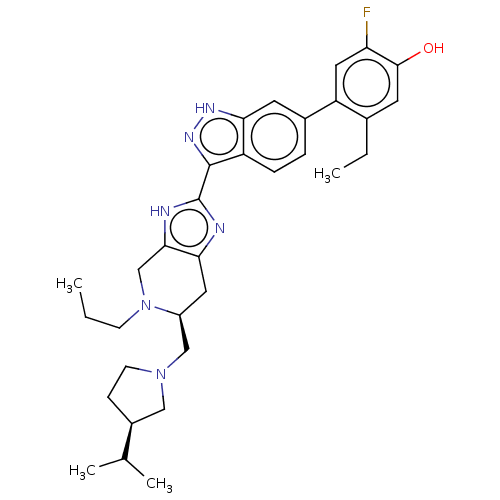 (US10208040, Example 9-21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THERAVANCE BIOPHARMA R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10208040 (2019) BindingDB Entry DOI: 10.7270/Q20867FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350094 (US10208040, Example 1-2 | US10519153, Example 1-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10519153 (2019) BindingDB Entry DOI: 10.7270/Q2DB8482 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM425122 (US10519153, Example 9-21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10519153 (2019) BindingDB Entry DOI: 10.7270/Q2DB8482 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM431908 (US10550118, Example 8-15 | US10954237, Example 8-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10550118 (2020) BindingDB Entry DOI: 10.7270/Q25D8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM431908 (US10550118, Example 8-15 | US10954237, Example 8-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10954237 (2021) BindingDB Entry DOI: 10.7270/Q2571G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM539139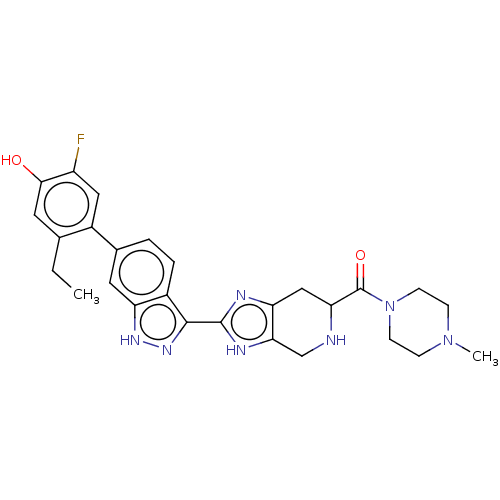 (US11254669, Example 1-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | Citation and Details BindingDB Entry DOI: 10.7270/Q2000588 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM539143 (US11254669, Example 9-21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | Citation and Details BindingDB Entry DOI: 10.7270/Q2000588 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50388901 (CHEMBL2063237) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... | ACS Med Chem Lett 3: 397-401 (2012) Article DOI: 10.1021/ml3000325 BindingDB Entry DOI: 10.7270/Q22N53BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM604898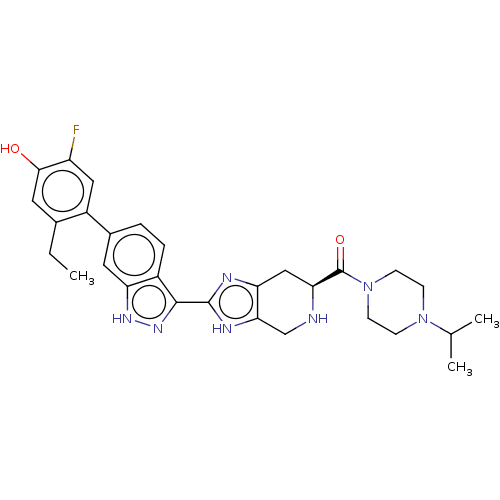 (US11667637, Example 1-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20C50QQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM604911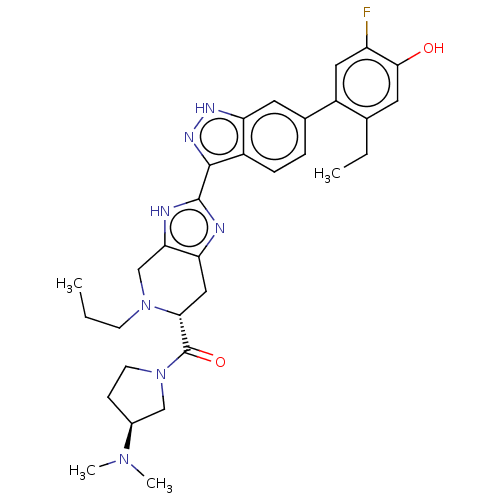 (US11667637, Example 9-21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20C50QQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM542679 ((1 S,3′R,6′R,7′S, 8′E,15&#...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition of the Mcl-1/Bim interaction was measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. The recombinan... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V40ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50388885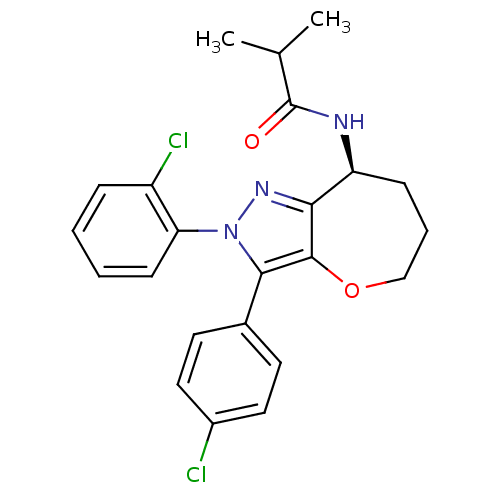 (CHEMBL2063238) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP-55940-induced [35S]GTPgammaS binding incubated for 1... | ACS Med Chem Lett 3: 397-401 (2012) Article DOI: 10.1021/ml3000325 BindingDB Entry DOI: 10.7270/Q22N53BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50514220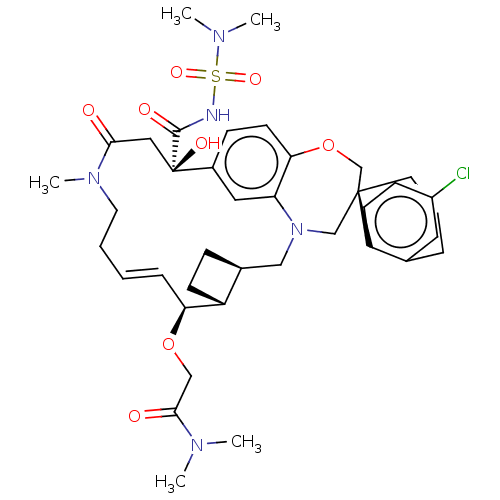 (CHEMBL4535151 | US11274105, Example 188) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... | J Med Chem 62: 10258-10271 (2019) Article DOI: 10.1021/acs.jmedchem.9b01310 BindingDB Entry DOI: 10.7270/Q2TQ64WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM539141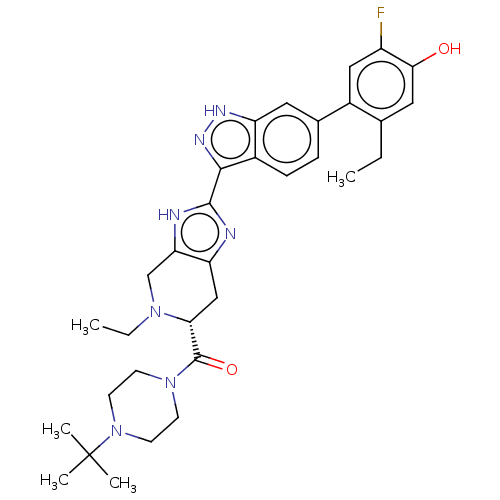 (US11254669, Example 1-37 | US11667637, Example 1-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q20C50QQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM539141 (US11254669, Example 1-37 | US11667637, Example 1-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | Citation and Details BindingDB Entry DOI: 10.7270/Q2000588 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM333120 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10954237 (2021) BindingDB Entry DOI: 10.7270/Q2571G33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM350096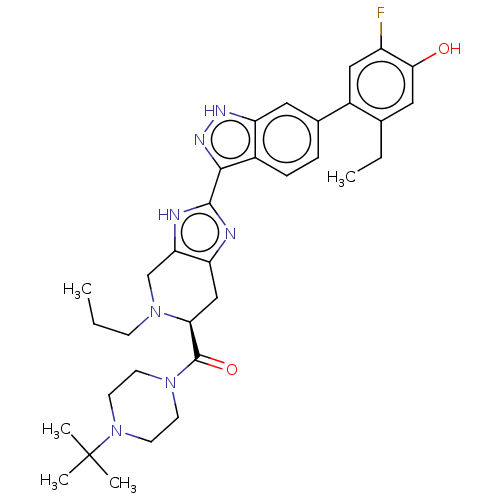 (US10208040, Example 1-37) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THERAVANCE BIOPHARMA R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10208040 (2019) BindingDB Entry DOI: 10.7270/Q20867FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM333120 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THERAVANCE BIOPHARMA R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10196393 (2019) BindingDB Entry DOI: 10.7270/Q2ST7RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM425851 (US10519153, Example 1-37) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10519153 (2019) BindingDB Entry DOI: 10.7270/Q2DB8482 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM333120 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7.5, 0.01%... | US Patent US10550118 (2020) BindingDB Entry DOI: 10.7270/Q25D8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM333120 ((S)-(3-(dimethylamino)azetidin-1-yl)(2-(6-(2-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assay 1: A panel of four LanthaScreen JAK biochemical assays (JAK1, 2, 3 and Tyk2) were carried in a common kinase reaction buffer (50 mM HEPES, pH 7... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BG2S7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM542690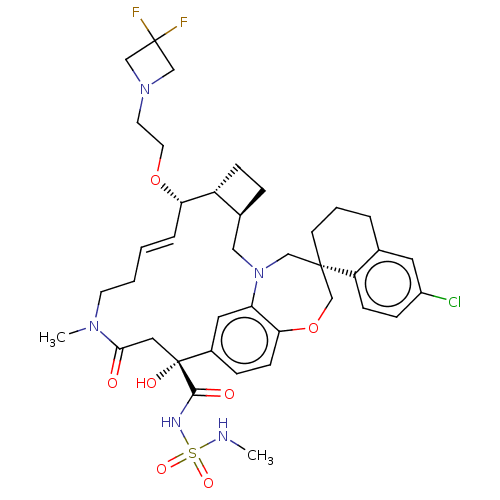 ((1S,3′R,6′R,7′S,8′E,15R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition of the Mcl-1/Bim interaction was measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. The recombinan... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V40ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM542678 ((1S,3′R,6′R,7′S,8′E,15R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition of the Mcl-1/Bim interaction was measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. The recombinan... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V40ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM542691 ((1S,3′R,6′R,7′S,8′E,15R...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition of the Mcl-1/Bim interaction was measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. The recombinan... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V40ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 21489 total ) | Next | Last >> |