 Found 987 hits with Last Name = 'sun' and Initial = 'g'
Found 987 hits with Last Name = 'sun' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity against Rat 5-hydroxytryptamine 7 receptor using [3H]-5-HT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25391
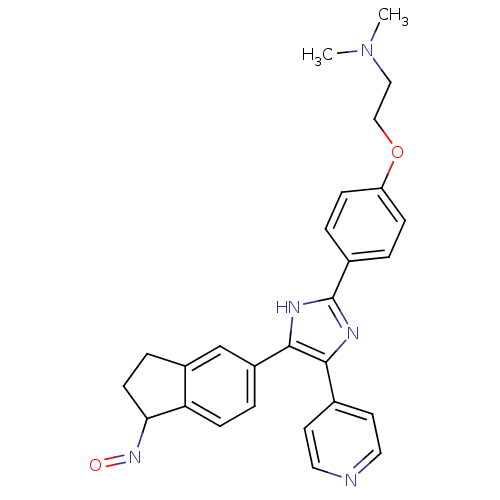
(CHEMBL200622 | SB-590885 | SB590885 | [2-(4-{4-[(1...)Show SMILES CN(C)CCOc1ccc(cc1)-c1nc(c([nH]1)-c1ccc2C(CCc2c1)N=O)-c1ccncc1 Show InChI InChI=1S/C27H27N5O2/c1-32(2)15-16-34-22-7-3-19(4-8-22)27-29-25(18-11-13-28-14-12-18)26(30-27)21-5-9-23-20(17-21)6-10-24(23)31-33/h3-5,7-9,11-14,17,24H,6,10,15-16H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
Bioorg Med Chem 20: 3746-55 (2012)
Article DOI: 10.1016/j.bmc.2012.04.047
BindingDB Entry DOI: 10.7270/Q2QF8TXP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087151
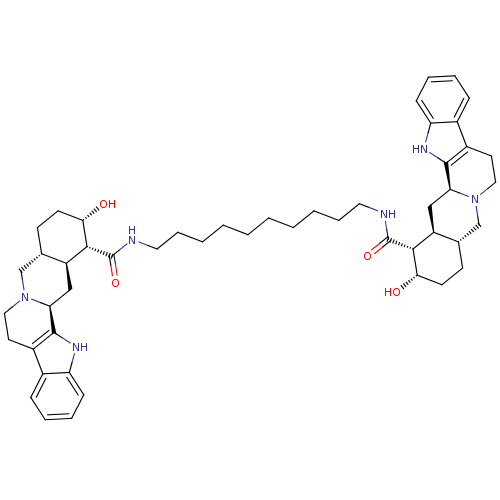
(1N-{10-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C50H68N6O4/c57-43-19-17-31-29-55-25-21-35-33-13-7-9-15-39(33)53-47(35)41(55)27-37(31)45(43)49(59)51-23-11-5-3-1-2-4-6-12-24-52-50(60)46-38-28-42-48-36(34-14-8-10-16-40(34)54-48)22-26-56(42)30-32(38)18-20-44(46)58/h7-10,13-16,31-32,37-38,41-46,53-54,57-58H,1-6,11-12,17-30H2,(H,51,59)(H,52,60)/t31-,32-,37-,38-,41-,42-,43-,44-,45+,46+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087149
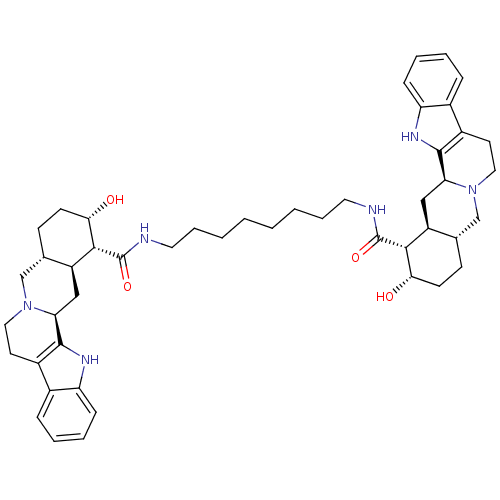
(1N-{8-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C48H64N6O4/c55-41-17-15-29-27-53-23-19-33-31-11-5-7-13-37(31)51-45(33)39(53)25-35(29)43(41)47(57)49-21-9-3-1-2-4-10-22-50-48(58)44-36-26-40-46-34(32-12-6-8-14-38(32)52-46)20-24-54(40)28-30(36)16-18-42(44)56/h5-8,11-14,29-30,35-36,39-44,51-52,55-56H,1-4,9-10,15-28H2,(H,49,57)(H,50,58)/t29-,30-,35-,36-,39-,40-,41-,42-,43+,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087152
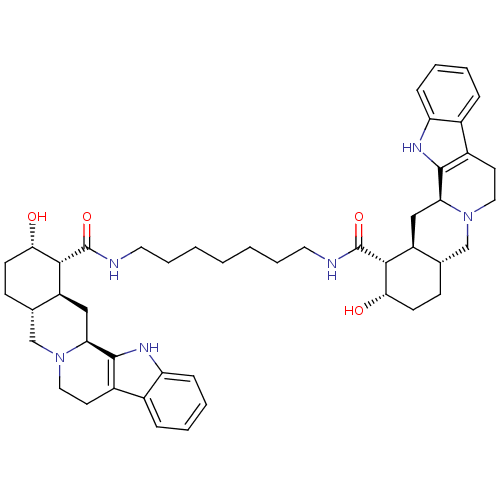
(1N-{7-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C47H62N6O4/c54-40-16-14-28-26-52-22-18-32-30-10-4-6-12-36(30)50-44(32)38(52)24-34(28)42(40)46(56)48-20-8-2-1-3-9-21-49-47(57)43-35-25-39-45-33(31-11-5-7-13-37(31)51-45)19-23-53(39)27-29(35)15-17-41(43)55/h4-7,10-13,28-29,34-35,38-43,50-51,54-55H,1-3,8-9,14-27H2,(H,48,56)(H,49,57)/t28-,29-,34-,35-,38-,39-,40-,41-,42+,43+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350022
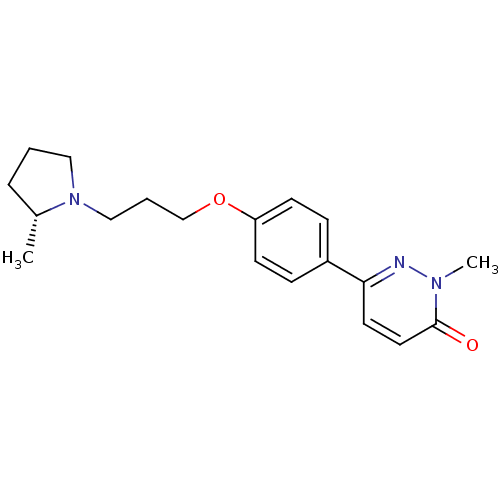
(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 21: 5543-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.094
BindingDB Entry DOI: 10.7270/Q2QF8TW7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50087029
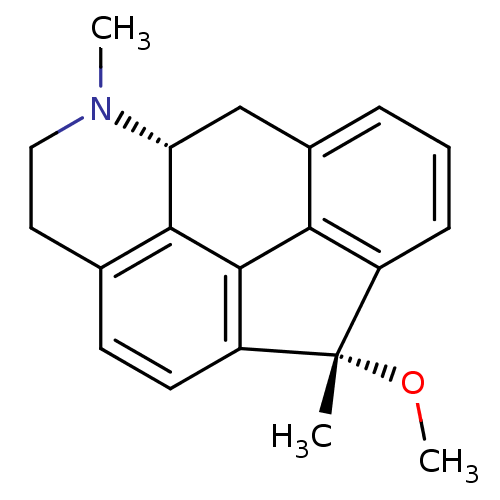
((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...)Show SMILES CO[C@@]1(C)c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C20H21NO/c1-20(22-3)14-6-4-5-13-11-16-18-12(9-10-21(16)2)7-8-15(20)19(18)17(13)14/h4-8,16H,9-11H2,1-3H3/t16-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-HT from over-expressed rat 5-hydroxytryptamine 7 receptor |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50087033

((1R,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...)Show SMILES CO[C@]1(C)c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C20H21NO/c1-20(22-3)14-6-4-5-13-11-16-18-12(9-10-21(16)2)7-8-15(20)19(18)17(13)14/h4-8,16H,9-11H2,1-3H3/t16-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-HT from over-expressed rat 5-hydroxytryptamine 7 receptor |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087145
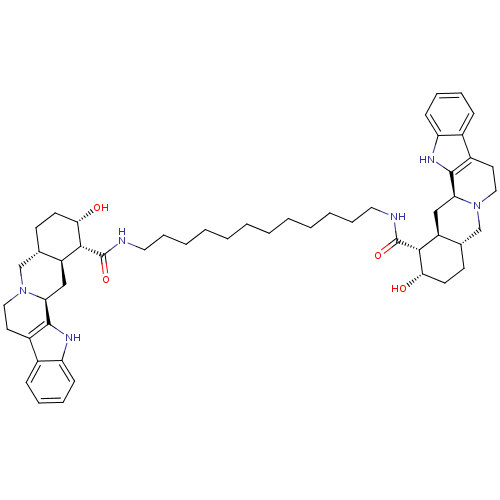
(1N-{12-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C52H72N6O4/c59-45-21-19-33-31-57-27-23-37-35-15-9-11-17-41(35)55-49(37)43(57)29-39(33)47(45)51(61)53-25-13-7-5-3-1-2-4-6-8-14-26-54-52(62)48-40-30-44-50-38(36-16-10-12-18-42(36)56-50)24-28-58(44)32-34(40)20-22-46(48)60/h9-12,15-18,33-34,39-40,43-48,55-56,59-60H,1-8,13-14,19-32H2,(H,53,61)(H,54,62)/t33-,34-,39-,40-,43-,44-,45-,46-,47+,48+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087148
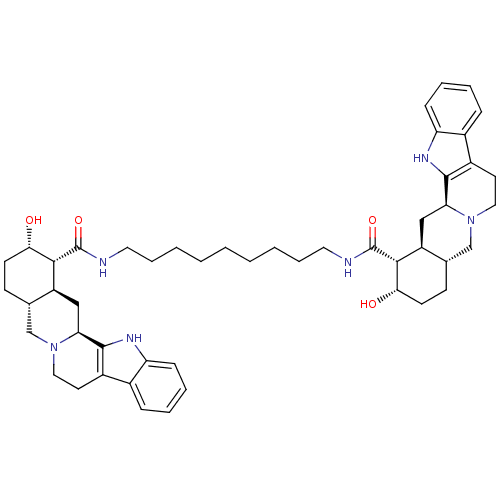
(1N-{9-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C49H66N6O4/c56-42-18-16-30-28-54-24-20-34-32-12-6-8-14-38(32)52-46(34)40(54)26-36(30)44(42)48(58)50-22-10-4-2-1-3-5-11-23-51-49(59)45-37-27-41-47-35(33-13-7-9-15-39(33)53-47)21-25-55(41)29-31(37)17-19-43(45)57/h6-9,12-15,30-31,36-37,40-45,52-53,56-57H,1-5,10-11,16-29H2,(H,50,58)(H,51,59)/t30-,31-,36-,37-,40-,41-,42-,43-,44+,45+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50016777
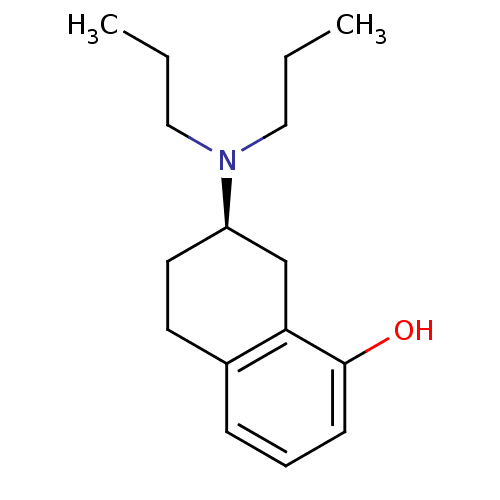
(((R)-8-Hydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity was measured on cloned Human 5-hydroxytryptamine 1A receptor which is labeled by [3H]-8-OH-DPAT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353165
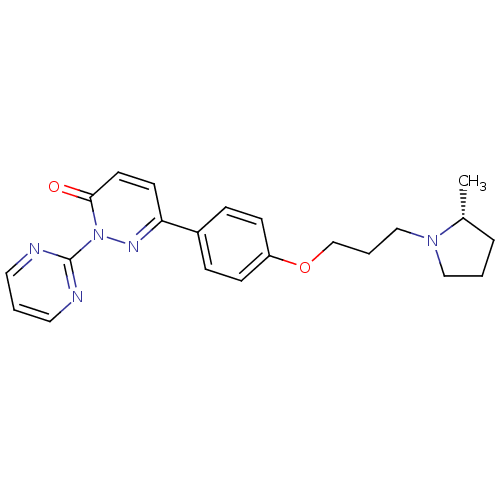
(CHEMBL1829472)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ncccn1 |r| Show InChI InChI=1S/C22H25N5O2/c1-17-5-2-14-26(17)15-4-16-29-19-8-6-18(7-9-19)20-10-11-21(28)27(25-20)22-23-12-3-13-24-22/h3,6-13,17H,2,4-5,14-16H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350022

(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087147
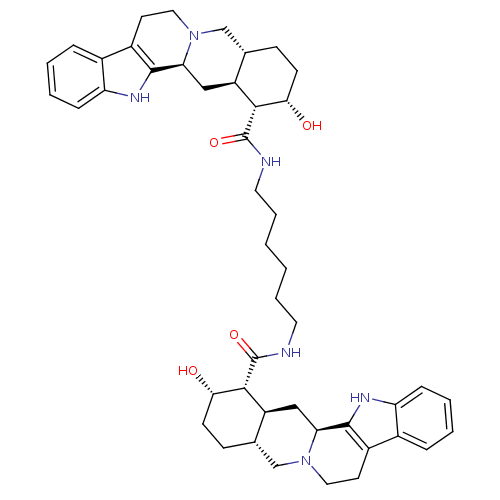
(1N-{6-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C46H60N6O4/c53-39-15-13-27-25-51-21-17-31-29-9-3-5-11-35(29)49-43(31)37(51)23-33(27)41(39)45(55)47-19-7-1-2-8-20-48-46(56)42-34-24-38-44-32(30-10-4-6-12-36(30)50-44)18-22-52(38)26-28(34)14-16-40(42)54/h3-6,9-12,27-28,33-34,37-42,49-50,53-54H,1-2,7-8,13-26H2,(H,47,55)(H,48,56)/t27-,28-,33-,34-,37-,38-,39-,40-,41+,42+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361051
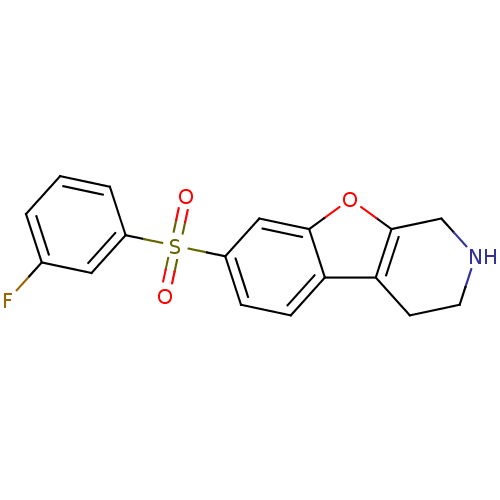
(CHEMBL1935590)Show InChI InChI=1S/C17H14FNO3S/c18-11-2-1-3-12(8-11)23(20,21)13-4-5-14-15-6-7-19-10-17(15)22-16(14)9-13/h1-5,8-9,19H,6-7,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50087154
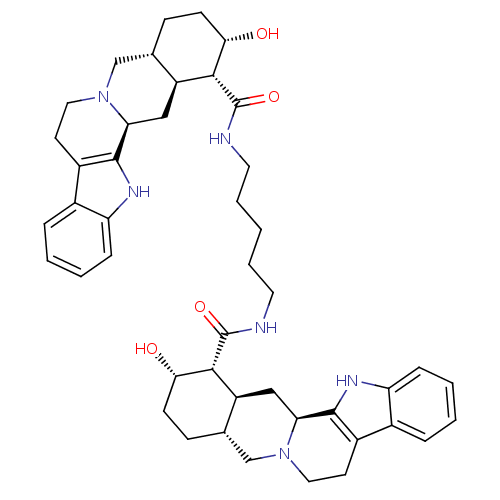
(1N-{5-[2-hydroxy-(1R,2S,4aR,13bS,14aS)-1,2,3,4,4a,...)Show SMILES O[C@H]1CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@@H]2[C@H]1C(=O)NCCCCCNC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 Show InChI InChI=1S/C45H58N6O4/c52-38-14-12-26-24-50-20-16-30-28-8-2-4-10-34(28)48-42(30)36(50)22-32(26)40(38)44(54)46-18-6-1-7-19-47-45(55)41-33-23-37-43-31(29-9-3-5-11-35(29)49-43)17-21-51(37)25-27(33)13-15-39(41)53/h2-5,8-11,26-27,32-33,36-41,48-49,52-53H,1,6-7,12-25H2,(H,46,54)(H,47,55)/t26-,27-,32-,33-,36-,37-,38-,39-,40+,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-2A adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50087037

(1-Ethoxy-1-ethyl-6-methyl-1,5,5a,6,7,8-hexahydro-6...)Show SMILES CCO[C@]1(CC)c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C22H25NO/c1-4-22(24-5-2)16-8-6-7-15-13-18-20-14(11-12-23(18)3)9-10-17(22)21(20)19(15)16/h6-10,18H,4-5,11-13H2,1-3H3/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity was measured on cloned human 5-hydroxytryptamine 1A receptor which is labeled by [3H]-8-OH-DPAT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity against human alpha 2b-adrenergic receptor expressed stably in CHO cells using [3H]rauwolscine as radioligand |
Bioorg Med Chem Lett 10: 627-30 (2000)
BindingDB Entry DOI: 10.7270/Q2WM1DXB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350021
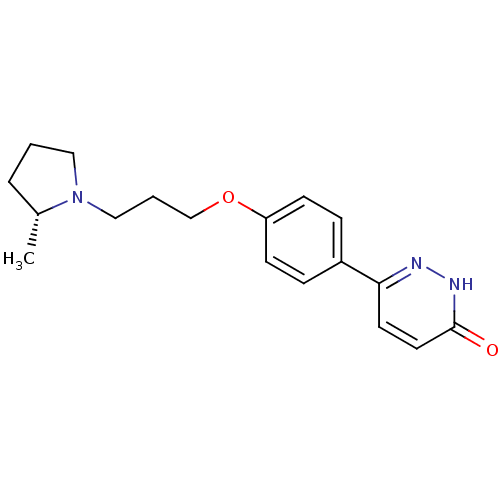
(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361055
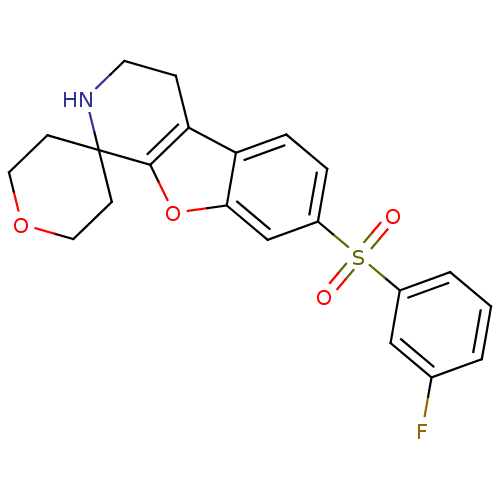
(CHEMBL1935599)Show SMILES Fc1cccc(c1)S(=O)(=O)c1ccc2c3CCNC4(CCOCC4)c3oc2c1 Show InChI InChI=1S/C21H20FNO4S/c22-14-2-1-3-15(12-14)28(24,25)16-4-5-17-18-6-9-23-21(7-10-26-11-8-21)20(18)27-19(17)13-16/h1-5,12-13,23H,6-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353181
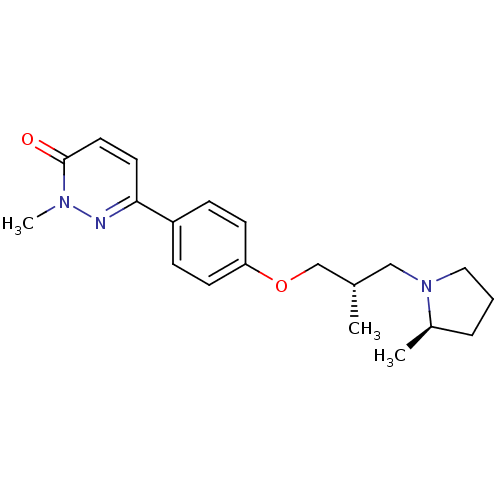
(CHEMBL1829485)Show SMILES C[C@H](COc1ccc(cc1)-c1ccc(=O)n(C)n1)CN1CCC[C@H]1C |r| Show InChI InChI=1S/C20H27N3O2/c1-15(13-23-12-4-5-16(23)2)14-25-18-8-6-17(7-9-18)19-10-11-20(24)22(3)21-19/h6-11,15-16H,4-5,12-14H2,1-3H3/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350030
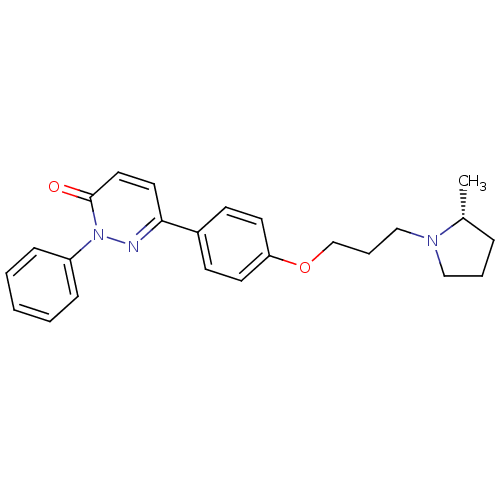
(CHEMBL1813060)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccccc1 |r| Show InChI InChI=1S/C24H27N3O2/c1-19-7-5-16-26(19)17-6-18-29-22-12-10-20(11-13-22)23-14-15-24(28)27(25-23)21-8-3-2-4-9-21/h2-4,8-15,19H,5-7,16-18H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361052
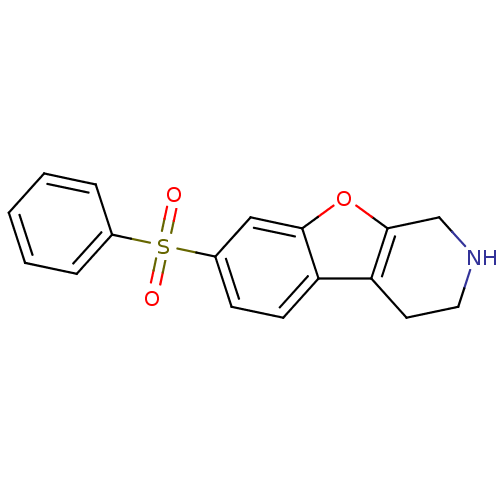
(CHEMBL1935589 | CHEMBL1949758)Show InChI InChI=1S/C17H15NO3S/c19-22(20,12-4-2-1-3-5-12)13-6-7-14-15-8-9-18-11-17(15)21-16(14)10-13/h1-7,10,18H,8-9,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
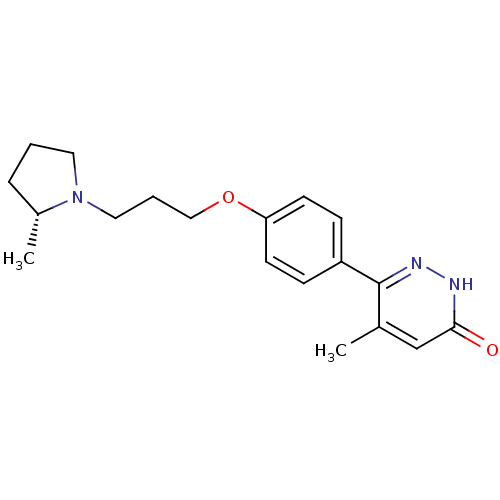
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361058
(CHEMBL1935594)Show SMILES CC1(C)NCCc2c1oc1cc(ccc21)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C19H18FNO3S/c1-19(2)18-16(8-9-21-19)15-7-6-14(11-17(15)24-18)25(22,23)13-5-3-4-12(20)10-13/h3-7,10-11,21H,8-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
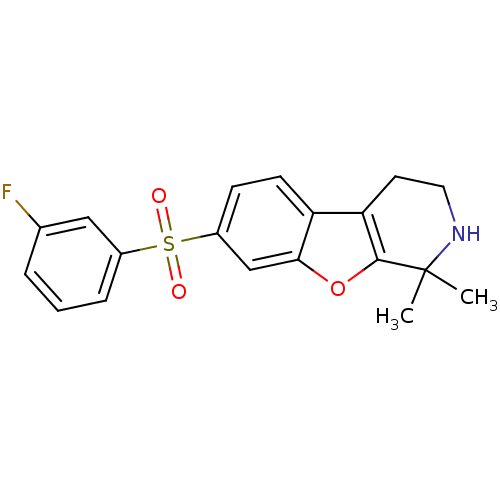
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353167
(CHEMBL1829475)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1n[nH]c(=O)cc1C |r| Show InChI InChI=1S/C19H25N3O2/c1-14-13-18(23)20-21-19(14)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,13,15H,3-5,10-12H2,1-2H3,(H,20,23)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50352798
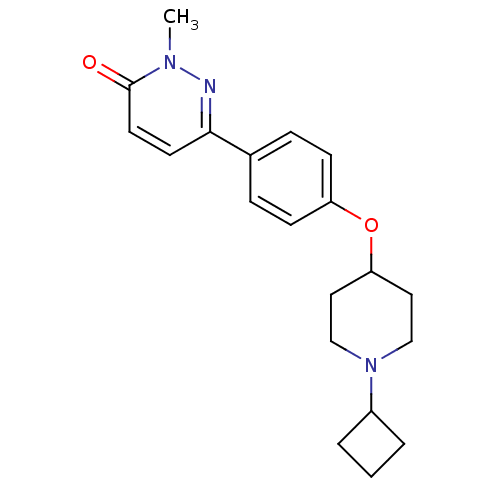
(CHEMBL1823402)Show InChI InChI=1S/C20H25N3O2/c1-22-20(24)10-9-19(21-22)15-5-7-17(8-6-15)25-18-11-13-23(14-12-18)16-3-2-4-16/h5-10,16,18H,2-4,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 21: 5543-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.094
BindingDB Entry DOI: 10.7270/Q2QF8TW7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353164
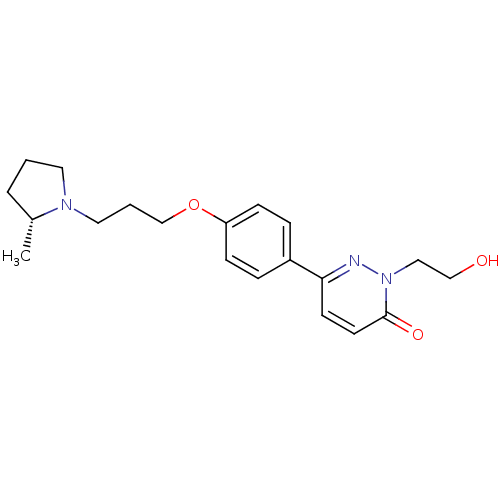
(CHEMBL1829469)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(CCO)n1 |r| Show InChI InChI=1S/C20H27N3O3/c1-16-4-2-11-22(16)12-3-15-26-18-7-5-17(6-8-18)19-9-10-20(25)23(21-19)13-14-24/h5-10,16,24H,2-4,11-15H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50352799
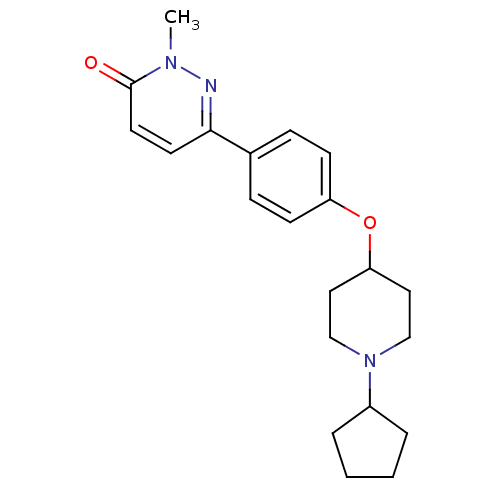
(CHEMBL1823403)Show InChI InChI=1S/C21H27N3O2/c1-23-21(25)11-10-20(22-23)16-6-8-18(9-7-16)26-19-12-14-24(15-13-19)17-4-2-3-5-17/h6-11,17,19H,2-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 21: 5543-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.094
BindingDB Entry DOI: 10.7270/Q2QF8TW7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352798

(CHEMBL1823402)Show InChI InChI=1S/C20H25N3O2/c1-22-20(24)10-9-19(21-22)15-5-7-17(8-6-15)25-18-11-13-23(14-12-18)16-3-2-4-16/h5-10,16,18H,2-4,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NAMH from rat histamine H3 receptor |
Bioorg Med Chem Lett 21: 5543-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.094
BindingDB Entry DOI: 10.7270/Q2QF8TW7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50087027

(1-Ethoxy-1-ethyl-6-methyl-1,5,5a,6,7,8-hexahydro-6...)Show SMILES CCO[C@@]1(CC)c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C22H25NO/c1-4-22(24-5-2)16-8-6-7-15-13-18-20-14(11-12-23(18)3)9-10-17(22)21(20)19(15)16/h6-10,18H,4-5,11-13H2,1-3H3/t18-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-HT from over-expressed rat 5-hydroxytryptamine 7 receptor |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353163
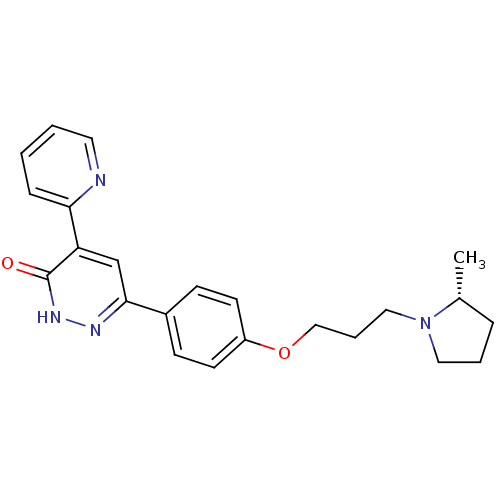
(CHEMBL1829474)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cc(-c2ccccn2)c(=O)[nH]n1 |r| Show InChI InChI=1S/C23H26N4O2/c1-17-6-4-13-27(17)14-5-15-29-19-10-8-18(9-11-19)22-16-20(23(28)26-25-22)21-7-2-3-12-24-21/h2-3,7-12,16-17H,4-6,13-15H2,1H3,(H,26,28)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50087025
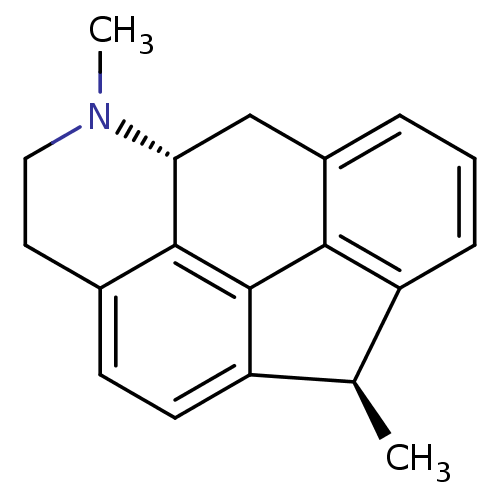
((1R,5aR)-1,6-dimethyl-1,5,5a,6,7,8-hexahydro-6-aza...)Show SMILES C[C@@H]1c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C19H19N/c1-11-14-5-3-4-13-10-16-18-12(8-9-20(16)2)6-7-15(11)19(18)17(13)14/h3-7,11,16H,8-10H2,1-2H3/t11-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity against Rat 5-hydroxytryptamine 7 receptor using [3H]-5-HT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353168
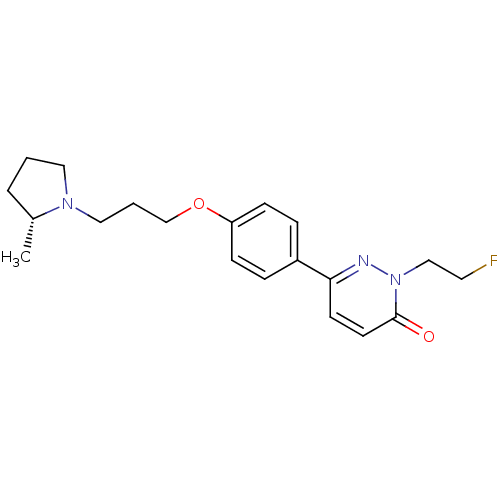
(CHEMBL1829337)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(CCF)n1 |r| Show InChI InChI=1S/C20H26FN3O2/c1-16-4-2-12-23(16)13-3-15-26-18-7-5-17(6-8-18)19-9-10-20(25)24(22-19)14-11-21/h5-10,16H,2-4,11-15H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353166

(CHEMBL1829473)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cc(C)c(=O)[nH]n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-14-13-18(20-21-19(14)23)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,13,15H,3-5,10-12H2,1-2H3,(H,21,23)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50087031

((1S,5aR)-1,6-dimethyl-1,5,5a,6,7,8-hexahydro-6-aza...)Show SMILES CN1CCc2ccc3c4-c5c(cccc5C[C@@H]1c24)[C@]3(C)O Show InChI InChI=1S/C19H19NO/c1-19(21)13-5-3-4-12-10-15-17-11(8-9-20(15)2)6-7-14(19)18(17)16(12)13/h3-7,15,21H,8-10H2,1-2H3/t15-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-HT from over-expressed rat 5-hydroxytryptamine 7 receptor |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24198
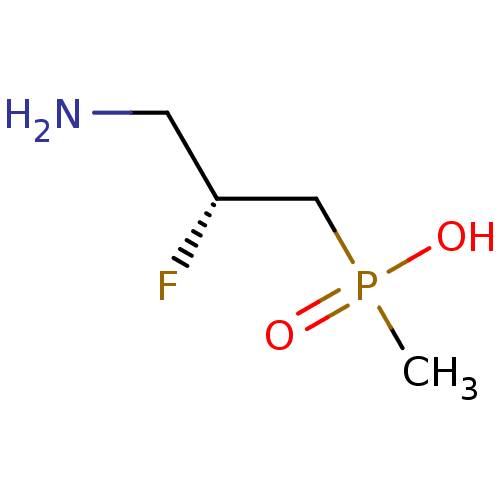
(3-aminopropylphosphinic derivative, (R)-8 | [(2R)-...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8)/t4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | -47.3 | n/a | n/a | 14 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353169
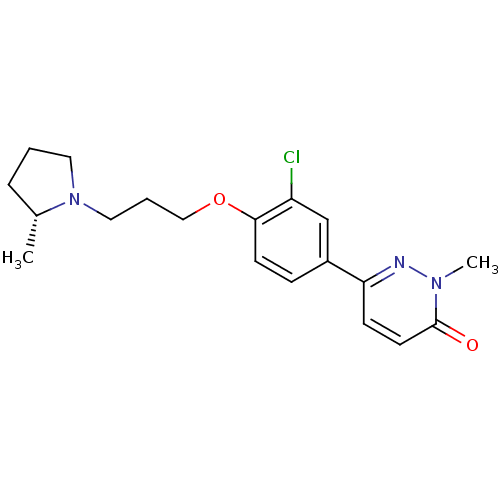
(CHEMBL1829479)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1Cl)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H24ClN3O2/c1-14-5-3-10-23(14)11-4-12-25-18-8-6-15(13-16(18)20)17-7-9-19(24)22(2)21-17/h6-9,13-14H,3-5,10-12H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361053
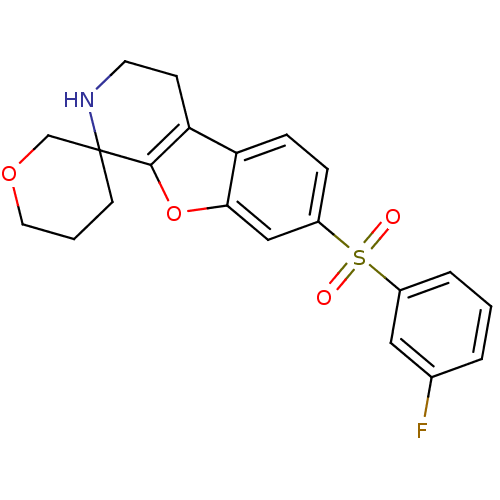
(CHEMBL1935601)Show SMILES Fc1cccc(c1)S(=O)(=O)c1ccc2c3CCNC4(CCCOC4)c3oc2c1 Show InChI InChI=1S/C21H20FNO4S/c22-14-3-1-4-15(11-14)28(24,25)16-5-6-17-18-7-9-23-21(8-2-10-26-13-21)20(18)27-19(17)12-16/h1,3-6,11-12,23H,2,7-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50353181

(CHEMBL1829485)Show SMILES C[C@H](COc1ccc(cc1)-c1ccc(=O)n(C)n1)CN1CCC[C@H]1C |r| Show InChI InChI=1S/C20H27N3O2/c1-15(13-23-12-4-5-16(23)2)14-25-18-8-6-17(7-9-18)19-10-11-20(24)22(3)21-19/h6-11,15-16H,4-5,12-14H2,1-3H3/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50087025

((1R,5aR)-1,6-dimethyl-1,5,5a,6,7,8-hexahydro-6-aza...)Show SMILES C[C@@H]1c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C19H19N/c1-11-14-5-3-4-13-10-16-18-12(8-9-20(16)2)6-7-15(11)19(18)17(13)14/h3-7,11,16H,8-10H2,1-2H3/t11-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity was measured on cloned Human 5-hydroxytryptamine 1A receptor which is labeled by [3H]-8-OH-DPAT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24195
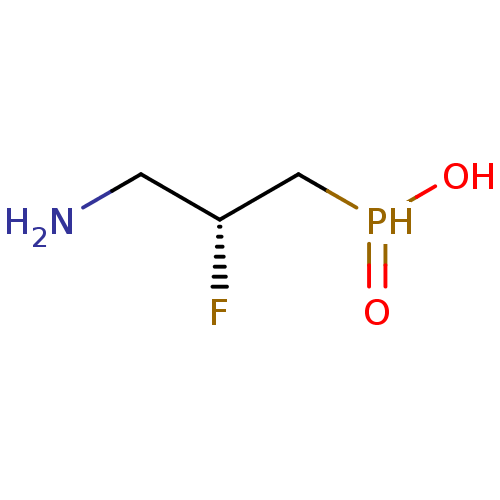
(3-aminopropylphosphinic derivative, (R)-7 | AZD335...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7)/t3-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | -46.9 | n/a | n/a | 8.64 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50365043

(CHEMBL1951055)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCOCC1 |r| Show InChI InChI=1S/C20H30N2O3/c1-17-4-2-9-22(17)10-3-13-25-19-7-5-18(6-8-19)20(23)16-21-11-14-24-15-12-21/h5-8,17H,2-4,9-16H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 22: 1546-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.004
BindingDB Entry DOI: 10.7270/Q2C24WWN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50087030

((1S,5aR)-1,6-dimethyl-1,5,5a,6,7,8-hexahydro-6-aza...)Show SMILES C[C@H]1c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C19H19N/c1-11-14-5-3-4-13-10-16-18-12(8-9-20(16)2)6-7-15(11)19(18)17(13)14/h3-7,11,16H,8-10H2,1-2H3/t11-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity against Rat 5-hydroxytryptamine 7 receptor using [3H]-5-HT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361050
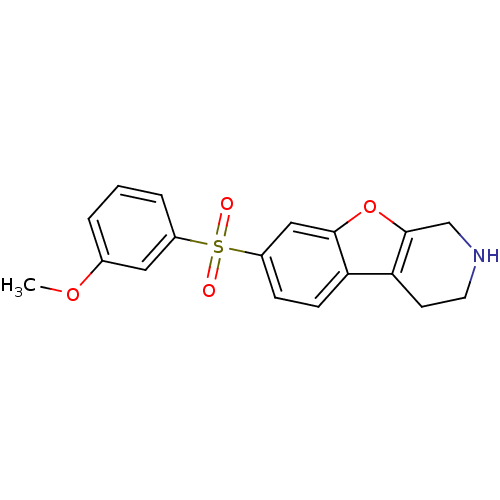
(CHEMBL1935592)Show InChI InChI=1S/C18H17NO4S/c1-22-12-3-2-4-13(9-12)24(20,21)14-5-6-15-16-7-8-19-11-18(16)23-17(15)10-14/h2-6,9-10,19H,7-8,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353170
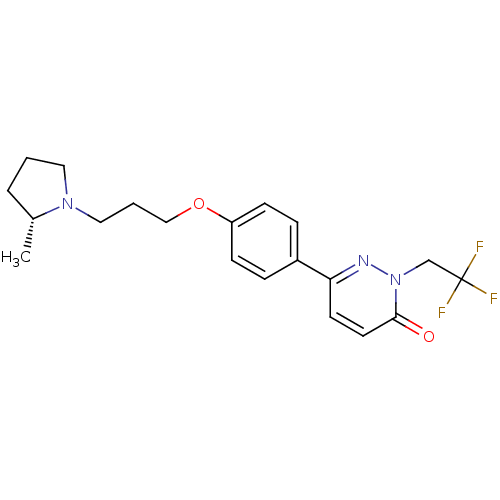
(CHEMBL1829468)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(CC(F)(F)F)n1 |r| Show InChI InChI=1S/C20H24F3N3O2/c1-15-4-2-11-25(15)12-3-13-28-17-7-5-16(6-8-17)18-9-10-19(27)26(24-18)14-20(21,22)23/h5-10,15H,2-4,11-14H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353172
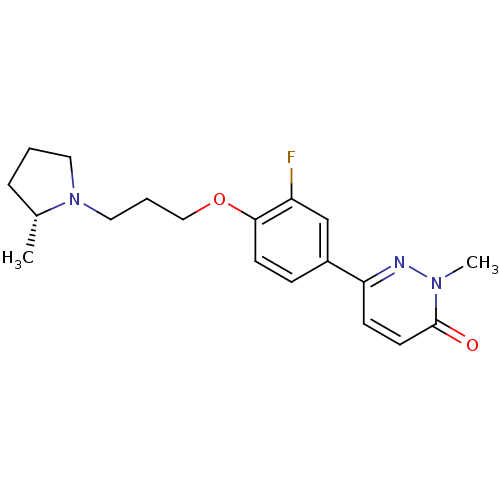
(CHEMBL1829477)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1F)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H24FN3O2/c1-14-5-3-10-23(14)11-4-12-25-18-8-6-15(13-16(18)20)17-7-9-19(24)22(2)21-17/h6-9,13-14H,3-5,10-12H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352799

(CHEMBL1823403)Show InChI InChI=1S/C21H27N3O2/c1-23-21(25)11-10-20(22-23)16-6-8-18(9-7-16)26-19-12-14-24(15-13-19)17-4-2-3-5-17/h6-11,17,19H,2-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NAMH from rat histamine H3 receptor |
Bioorg Med Chem Lett 21: 5543-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.094
BindingDB Entry DOI: 10.7270/Q2QF8TW7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350022

(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NAMH from rat histamine H3 receptor |
Bioorg Med Chem Lett 21: 5543-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.094
BindingDB Entry DOI: 10.7270/Q2QF8TW7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50350022

(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data