 Found 1919 hits with Last Name = 'tam' and Initial = 'c'
Found 1919 hits with Last Name = 'tam' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442142
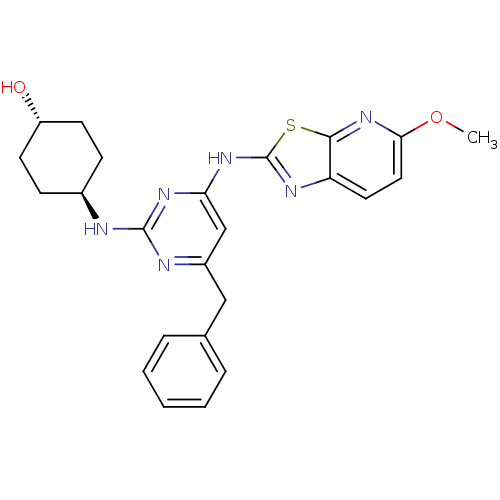
(CHEMBL2441275)Show SMILES COc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2n1 |r,wU:25.26,wD:22.22,(34.29,-5.26,;33.51,-3.93,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C24H26N6O2S/c1-32-21-12-11-19-22(30-21)33-24(27-19)29-20-14-17(13-15-5-3-2-4-6-15)26-23(28-20)25-16-7-9-18(31)10-8-16/h2-6,11-12,14,16,18,31H,7-10,13H2,1H3,(H2,25,26,27,28,29)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442141

(CHEMBL2441276)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3cccnc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.36,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C23H24N6OS/c30-18-10-8-16(9-11-18)25-22-26-17(13-15-5-2-1-3-6-15)14-20(28-22)29-23-27-19-7-4-12-24-21(19)31-23/h1-7,12,14,16,18,30H,8-11,13H2,(H2,25,26,27,28,29)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442143
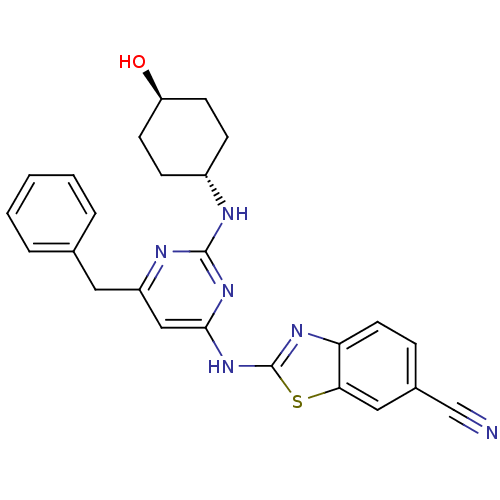
(CHEMBL2441274)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(cc3s2)C#N)n1 |r,wU:1.0,wD:4.7,(23.68,-13.98,;22.35,-14.78,;22.37,-16.32,;21.05,-17.1,;19.71,-16.35,;19.69,-14.81,;21.01,-14.02,;18.39,-17.14,;17.04,-16.39,;15.72,-17.18,;14.38,-16.44,;13.04,-17.23,;11.71,-16.46,;11.71,-14.92,;10.38,-14.14,;9.04,-14.92,;9.04,-16.46,;10.38,-17.22,;14.35,-14.9,;15.67,-14.1,;15.65,-12.56,;16.85,-11.63,;16.82,-10.09,;18.27,-9.58,;18.87,-8.16,;20.4,-7.96,;21.33,-9.19,;20.73,-10.61,;19.2,-10.8,;18.33,-12.08,;22.85,-8.99,;24.37,-8.8,;17.02,-14.86,)| Show InChI InChI=1S/C25H24N6OS/c26-15-17-6-11-21-22(13-17)33-25(29-21)31-23-14-19(12-16-4-2-1-3-5-16)28-24(30-23)27-18-7-9-20(32)10-8-18/h1-6,11,13-14,18,20,32H,7-10,12H2,(H2,27,28,29,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442145

(CHEMBL2441271)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(Cl)cc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;33.51,-3.74,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H24ClN5OS/c25-16-6-11-20-21(13-16)32-24(28-20)30-22-14-18(12-15-4-2-1-3-5-15)27-23(29-22)26-17-7-9-19(31)10-8-17/h1-6,11,13-14,17,19,31H,7-10,12H2,(H2,26,27,28,29,30)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442146
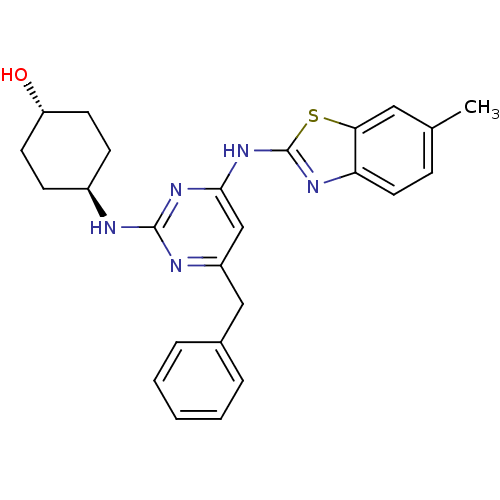
(CHEMBL2441270)Show SMILES Cc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:24.25,wD:21.21,(33.51,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C25H27N5OS/c1-16-7-12-21-22(13-16)32-25(28-21)30-23-15-19(14-17-5-3-2-4-6-17)27-24(29-23)26-18-8-10-20(31)11-9-18/h2-7,12-13,15,18,20,31H,8-11,14H2,1H3,(H2,26,27,28,29,30)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50442149

(CHEMBL2441267)Show SMILES Cc1cnc(Nc2cc(Cc3ccccc3)nc(N[C@H]3CC[C@H](O)CC3)n2)s1 |r,wU:22.23,wD:19.19,(57.53,-16.46,;56.01,-16.27,;55.28,-14.93,;53.76,-15.22,;53.57,-16.74,;52.23,-17.49,;52.2,-19.03,;50.85,-19.77,;50.82,-21.31,;49.48,-22.06,;48.11,-21.34,;46.81,-22.18,;45.44,-21.47,;45.37,-19.93,;46.67,-19.1,;48.04,-19.8,;52.14,-22.11,;53.49,-21.36,;54.81,-22.16,;54.78,-23.7,;53.43,-24.44,;53.4,-25.98,;54.73,-26.78,;54.7,-28.32,;56.07,-26.03,;56.1,-24.49,;53.52,-19.82,;54.97,-17.41,)| Show InChI InChI=1S/C21H25N5OS/c1-14-13-22-21(28-14)26-19-12-17(11-15-5-3-2-4-6-15)24-20(25-19)23-16-7-9-18(27)10-8-16/h2-6,12-13,16,18,27H,7-11H2,1H3,(H2,22,23,24,25,26)/t16-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B (unknown origin) using 5FAM-PKAtide as substrate after 120 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442139
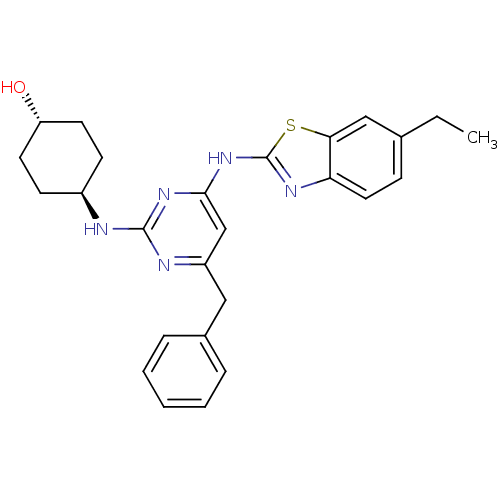
(CHEMBL2441273)Show SMILES CCc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:25.26,wD:22.22,(34.44,-4.96,;33.51,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C26H29N5OS/c1-2-17-8-13-22-23(15-17)33-26(29-22)31-24-16-20(14-18-6-4-3-5-7-18)28-25(30-24)27-19-9-11-21(32)12-10-19/h3-8,13,15-16,19,21,32H,2,9-12,14H2,1H3,(H2,27,28,29,30,31)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50055575
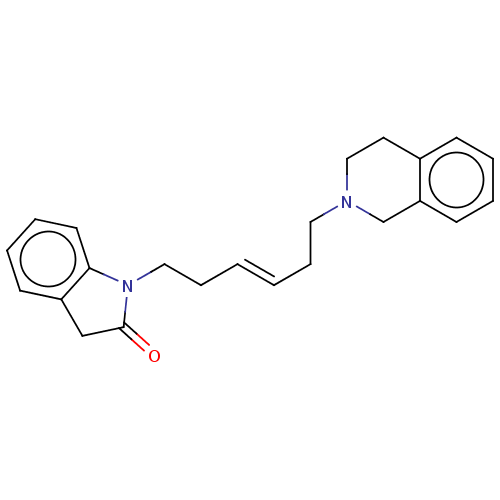
(CHEMBL3321794)Show InChI InChI=1S/C23H26N2O/c26-23-17-20-10-5-6-12-22(20)25(23)15-8-2-1-7-14-24-16-13-19-9-3-4-11-21(19)18-24/h1-6,9-12H,7-8,13-18H2/b2-1+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT7 expressed in HEK-293 cells after 120 mins by scintillation spectrometry |
J Med Chem 57: 6879-84 (2014)
Article DOI: 10.1021/jm500880c
BindingDB Entry DOI: 10.7270/Q2J104TB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442147
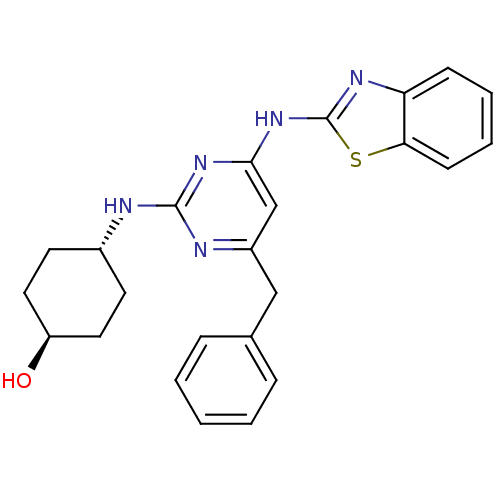
(CHEMBL2441269)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccccc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H25N5OS/c30-19-12-10-17(11-13-19)25-23-26-18(14-16-6-2-1-3-7-16)15-22(28-23)29-24-27-20-8-4-5-9-21(20)31-24/h1-9,15,17,19,30H,10-14H2,(H2,25,26,27,28,29)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50123790

(3-{[4-Aminomethyl-2-(1-carboxy-ethyl)-phenylcarbam...)Show SMILES C[C@@H](C(O)=O)c1cc(CN)ccc1NC(=O)C[C@@H]1CCc2cc(Cl)cc3[nH]c(C(O)=O)c1c23 Show InChI InChI=1S/C24H24ClN3O5/c1-11(23(30)31)16-6-12(10-26)2-5-17(16)27-19(29)8-14-4-3-13-7-15(25)9-18-20(13)21(14)22(28-18)24(32)33/h2,5-7,9,11,14,28H,3-4,8,10,26H2,1H3,(H,27,29)(H,30,31)(H,32,33)/t11-,14+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity for glycine site of N-methyl-D-aspartate glutamate receptor determined by displacement of [3H]- DCKA (5,7-dichlorokynurenic acid) in... |
J Med Chem 46: 691-701 (2003)
Article DOI: 10.1021/jm020239l
BindingDB Entry DOI: 10.7270/Q2G44PP3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442140
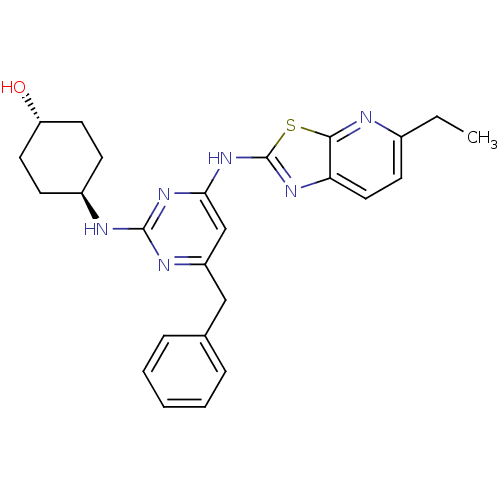
(CHEMBL2441277)Show SMILES CCc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2n1 |r,wU:25.26,wD:22.22,(34.44,-4.96,;33.5,-3.74,;31.98,-3.93,;31.04,-2.71,;29.52,-2.9,;28.92,-4.33,;27.47,-4.83,;27.5,-6.37,;26.29,-7.31,;26.32,-8.85,;25,-9.64,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.36,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;26.37,-11.93,;27.69,-11.14,;29.03,-11.88,;30.36,-11.09,;31.7,-11.84,;33.02,-11.06,;33,-9.52,;34.33,-8.73,;31.66,-8.77,;30.34,-9.56,;27.67,-9.6,;28.98,-6.82,;29.86,-5.55,;31.38,-5.35,)| Show InChI InChI=1S/C25H28N6OS/c1-2-17-10-13-21-23(26-17)33-25(29-21)31-22-15-19(14-16-6-4-3-5-7-16)28-24(30-22)27-18-8-11-20(32)12-9-18/h3-7,10,13,15,18,20,32H,2,8-9,11-12,14H2,1H3,(H2,27,28,29,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442149

(CHEMBL2441267)Show SMILES Cc1cnc(Nc2cc(Cc3ccccc3)nc(N[C@H]3CC[C@H](O)CC3)n2)s1 |r,wU:22.23,wD:19.19,(57.53,-16.46,;56.01,-16.27,;55.28,-14.93,;53.76,-15.22,;53.57,-16.74,;52.23,-17.49,;52.2,-19.03,;50.85,-19.77,;50.82,-21.31,;49.48,-22.06,;48.11,-21.34,;46.81,-22.18,;45.44,-21.47,;45.37,-19.93,;46.67,-19.1,;48.04,-19.8,;52.14,-22.11,;53.49,-21.36,;54.81,-22.16,;54.78,-23.7,;53.43,-24.44,;53.4,-25.98,;54.73,-26.78,;54.7,-28.32,;56.07,-26.03,;56.1,-24.49,;53.52,-19.82,;54.97,-17.41,)| Show InChI InChI=1S/C21H25N5OS/c1-14-13-22-21(28-14)26-19-12-17(11-15-5-3-2-4-6-15)24-20(25-19)23-16-7-9-18(27)10-8-16/h2-6,12-13,16,18,27H,7-11H2,1H3,(H2,22,23,24,25,26)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50055574
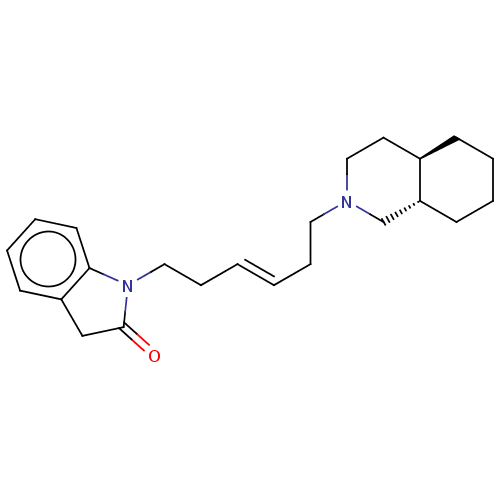
(CHEMBL3321798)Show SMILES [H][C@]12CCCC[C@]1([H])CN(CC\C=C\CCN1C(=O)Cc3ccccc13)CC2 |r| Show InChI InChI=1S/C23H32N2O/c26-23-17-20-10-5-6-12-22(20)25(23)15-8-2-1-7-14-24-16-13-19-9-3-4-11-21(19)18-24/h1-2,5-6,10,12,19,21H,3-4,7-9,11,13-18H2/b2-1+/t19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT7 expressed in HEK-293 cells after 120 mins by scintillation spectrometry |
J Med Chem 57: 6879-84 (2014)
Article DOI: 10.1021/jm500880c
BindingDB Entry DOI: 10.7270/Q2J104TB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50448771
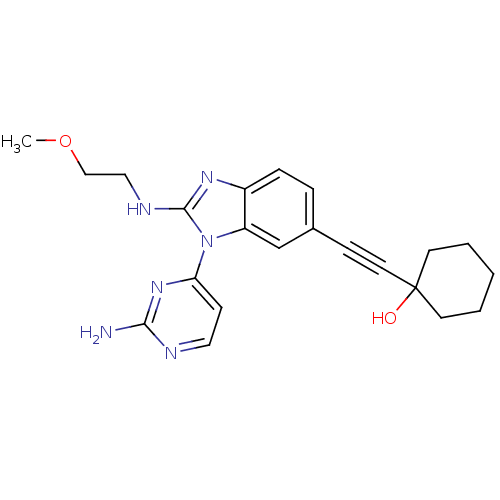
(CHEMBL3128042)Show SMILES COCCNc1nc2ccc(cc2n1-c1ccnc(N)n1)C#CC1(O)CCCCC1 Show InChI InChI=1S/C22H26N6O2/c1-30-14-13-25-21-26-17-6-5-16(7-11-22(29)9-3-2-4-10-22)15-18(17)28(21)19-8-12-24-20(23)27-19/h5-6,8,12,15,29H,2-4,9-10,13-14H2,1H3,(H,25,26)(H2,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST-tagged recombinant human PAK4 kinase domain expressed in Escherichia coli using KKRNRRLSVA as substrate preincubat... |
J Med Chem 57: 1033-45 (2014)
Article DOI: 10.1021/jm401768t
BindingDB Entry DOI: 10.7270/Q2F47QNW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442150
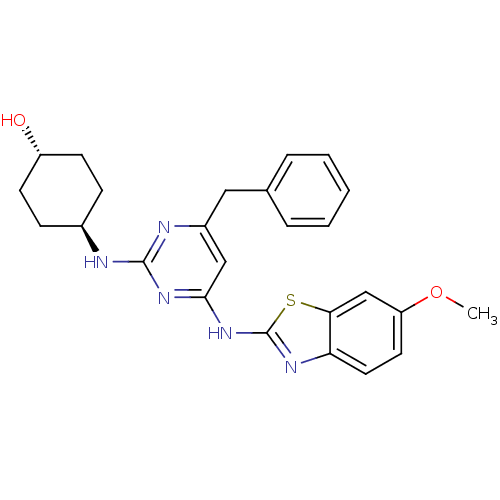
(CHEMBL2441112)Show SMILES COc1ccc2nc(Nc3cc(Cc4ccccc4)nc(N[C@H]4CC[C@H](O)CC4)n3)sc2c1 |r,wU:25.26,wD:22.22,(39.46,-21.76,;38.71,-20.41,;37.17,-20.38,;36.42,-19.04,;34.89,-19.01,;34.11,-20.32,;32.6,-20.62,;32.41,-22.14,;31.06,-22.88,;31.04,-24.42,;29.69,-25.17,;29.66,-26.7,;28.32,-27.45,;26.94,-26.74,;25.65,-27.57,;24.28,-26.86,;24.21,-25.33,;25.51,-24.5,;26.87,-25.2,;30.98,-27.5,;32.32,-26.75,;33.65,-27.55,;33.62,-29.09,;32.27,-29.83,;32.24,-31.37,;33.56,-32.17,;33.53,-33.71,;34.9,-31.42,;34.93,-29.88,;32.35,-25.21,;33.8,-22.8,;34.85,-21.67,;36.38,-21.7,)| Show InChI InChI=1S/C25H27N5O2S/c1-32-20-11-12-21-22(15-20)33-25(28-21)30-23-14-18(13-16-5-3-2-4-6-16)27-24(29-23)26-17-7-9-19(31)10-8-17/h2-6,11-12,14-15,17,19,31H,7-10,13H2,1H3,(H2,26,27,28,29,30)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM82517
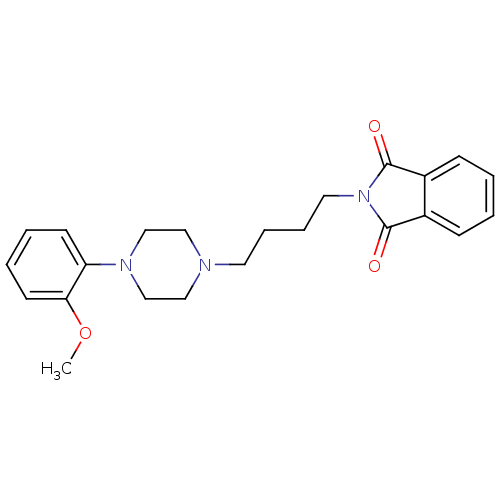
(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5-HT1A expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50442153
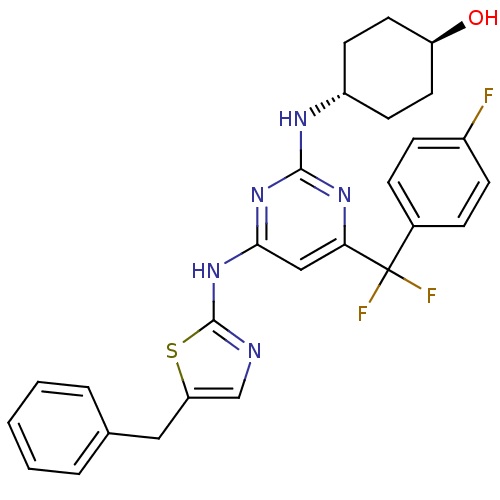
(CHEMBL2441283)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Nc2ncc(Cc3ccccc3)s2)cc(n1)C(F)(F)c1ccc(F)cc1 |r,wU:1.0,wD:4.7,(16.64,-34.58,;16.67,-33.04,;15.34,-32.24,;15.37,-30.7,;16.72,-29.96,;18.04,-30.75,;18.01,-32.29,;16.75,-28.42,;15.43,-27.62,;15.46,-26.08,;14.14,-25.29,;14.17,-23.75,;15.51,-23,;15.7,-21.49,;17.21,-21.19,;17.96,-22.54,;19.49,-22.73,;20.42,-21.5,;21.94,-21.69,;22.87,-20.46,;22.27,-19.04,;20.73,-18.86,;19.81,-20.09,;16.91,-23.66,;12.79,-26.03,;12.76,-27.57,;14.08,-28.37,;11.42,-28.31,;10.23,-29.3,;12.4,-29.5,;10.05,-27.6,;8.75,-28.44,;7.38,-27.73,;7.31,-26.19,;5.94,-25.48,;8.61,-25.36,;9.97,-26.06,)| Show InChI InChI=1S/C27H26F3N5OS/c28-19-8-6-18(7-9-19)27(29,30)23-15-24(34-25(33-23)32-20-10-12-21(36)13-11-20)35-26-31-16-22(37-26)14-17-4-2-1-3-5-17/h1-9,15-16,20-21,36H,10-14H2,(H2,31,32,33,34,35)/t20-,21- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B (unknown origin) using 5FAM-PKAtide as substrate after 120 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442155
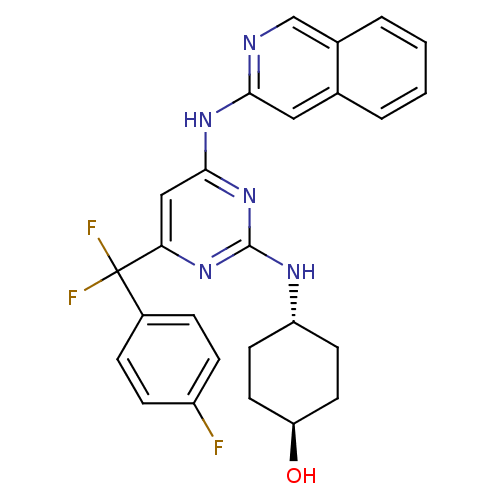
(CHEMBL2441281)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Nc2cc3ccccc3cn2)cc(n1)C(F)(F)c1ccc(F)cc1 |r,wU:1.0,wD:4.7,(43.15,-16.86,;43.18,-15.32,;41.86,-14.53,;41.89,-12.99,;43.24,-12.24,;44.55,-13.04,;44.52,-14.57,;43.27,-10.7,;41.95,-9.91,;41.97,-8.37,;40.65,-7.57,;40.68,-6.03,;41.99,-5.22,;43.35,-5.94,;44.66,-5.12,;46.01,-5.85,;47.32,-5.03,;47.26,-3.49,;45.91,-2.77,;44.61,-3.58,;43.23,-2.86,;41.93,-3.68,;39.3,-8.32,;39.28,-9.86,;40.6,-10.65,;37.93,-10.6,;36.74,-11.58,;38.91,-11.79,;36.57,-9.89,;35.27,-10.72,;33.9,-10.01,;33.83,-8.48,;32.46,-7.77,;35.13,-7.65,;36.49,-8.35,)| Show InChI InChI=1S/C26H24F3N5O/c27-19-7-5-18(6-8-19)26(28,29)22-14-24(33-23-13-16-3-1-2-4-17(16)15-30-23)34-25(32-22)31-20-9-11-21(35)12-10-20/h1-8,13-15,20-21,35H,9-12H2,(H2,30,31,32,33,34)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442160
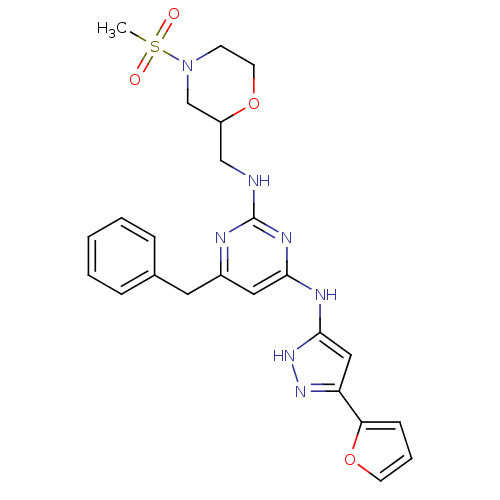
(CHEMBL2441279)Show SMILES CS(=O)(=O)N1CCOC(CNc2nc(Cc3ccccc3)cc(Nc3cc(n[nH]3)-c3ccco3)n2)C1 Show InChI InChI=1S/C24H27N7O4S/c1-36(32,33)31-9-11-34-19(16-31)15-25-24-26-18(12-17-6-3-2-4-7-17)13-22(28-24)27-23-14-20(29-30-23)21-8-5-10-35-21/h2-8,10,13-14,19H,9,11-12,15-16H2,1H3,(H3,25,26,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50267832

(1-[4-(3,4-Dihydroisoquinolin-2(1H)-yl)butyl]-1,3-d...)Show InChI InChI=1S/C21H24N2O/c24-21-15-18-8-3-4-10-20(18)23(21)13-6-5-12-22-14-11-17-7-1-2-9-19(17)16-22/h1-4,7-10H,5-6,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT7 expressed in HEK-293 cells after 120 mins by scintillation spectrometry |
J Med Chem 57: 6879-84 (2014)
Article DOI: 10.1021/jm500880c
BindingDB Entry DOI: 10.7270/Q2J104TB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50564775
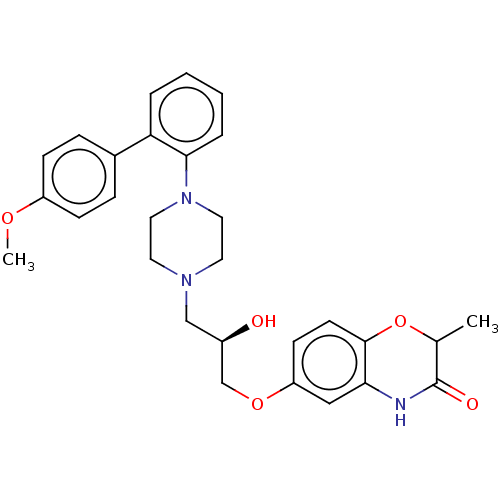
(CHEMBL4795056)Show SMILES COc1ccc(cc1)-c1ccccc1N1CCN(C[C@@H](O)COc2ccc3OC(C)C(=O)Nc3c2)CC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5-HT1A expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50032623
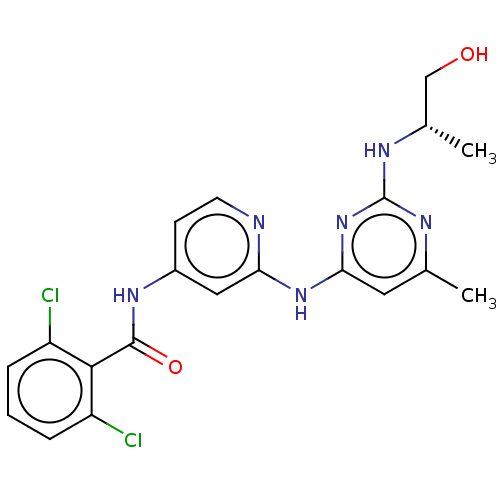
(CHEMBL3354183)Show SMILES C[C@@H](CO)Nc1nc(C)cc(Nc2cc(NC(=O)c3c(Cl)cccc3Cl)ccn2)n1 |r| Show InChI InChI=1S/C20H20Cl2N6O2/c1-11-8-17(28-20(24-11)25-12(2)10-29)27-16-9-13(6-7-23-16)26-19(30)18-14(21)4-3-5-15(18)22/h3-9,12,29H,10H2,1-2H3,(H3,23,24,25,26,27,28,30)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50123790

(3-{[4-Aminomethyl-2-(1-carboxy-ethyl)-phenylcarbam...)Show SMILES C[C@@H](C(O)=O)c1cc(CN)ccc1NC(=O)C[C@@H]1CCc2cc(Cl)cc3[nH]c(C(O)=O)c1c23 Show InChI InChI=1S/C24H24ClN3O5/c1-11(23(30)31)16-6-12(10-26)2-5-17(16)27-19(29)8-14-4-3-13-7-15(25)9-18-20(13)21(14)22(28-18)24(32)33/h2,5-7,9,11,14,28H,3-4,8,10,26H2,1H3,(H,27,29)(H,30,31)(H,32,33)/t11-,14+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity for glycine site of N-methyl-D-aspartate glutamate receptor determined by displacement of [3H]- glycine in rat cortical membranes |
J Med Chem 46: 691-701 (2003)
Article DOI: 10.1021/jm020239l
BindingDB Entry DOI: 10.7270/Q2G44PP3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50032630
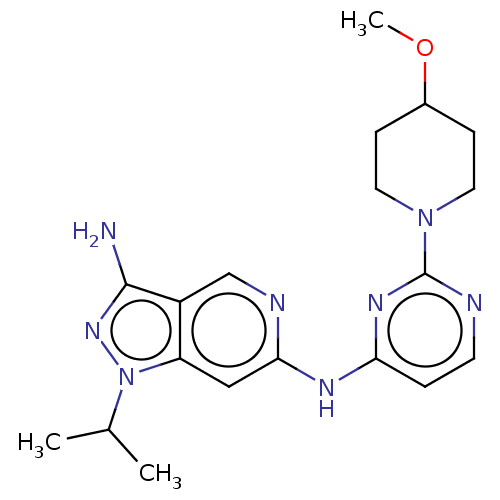
(CHEMBL3354191)Show SMILES COC1CCN(CC1)c1nccc(Nc2cc3n(nc(N)c3cn2)C(C)C)n1 Show InChI InChI=1S/C19H26N8O/c1-12(2)27-15-10-17(22-11-14(15)18(20)25-27)23-16-4-7-21-19(24-16)26-8-5-13(28-3)6-9-26/h4,7,10-13H,5-6,8-9H2,1-3H3,(H2,20,25)(H,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442144
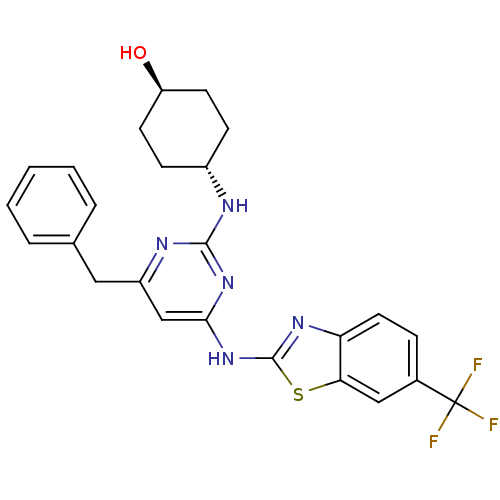
(CHEMBL2441272)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccc(cc3s2)C(F)(F)F)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;33.51,-3.74,;34.44,-4.96,;34.1,-2.32,;35.04,-3.73,;27.67,-9.6,)| Show InChI InChI=1S/C25H24F3N5OS/c26-25(27,28)16-6-11-20-21(13-16)35-24(31-20)33-22-14-18(12-15-4-2-1-3-5-15)30-23(32-22)29-17-7-9-19(34)10-8-17/h1-6,11,13-14,17,19,34H,7-10,12H2,(H2,29,30,31,32,33)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50564776

(CHEMBL4781863)Show SMILES COc1ccc(cc1)-c1ccccc1N1CCN(CCCCOc2cnccn2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50032612
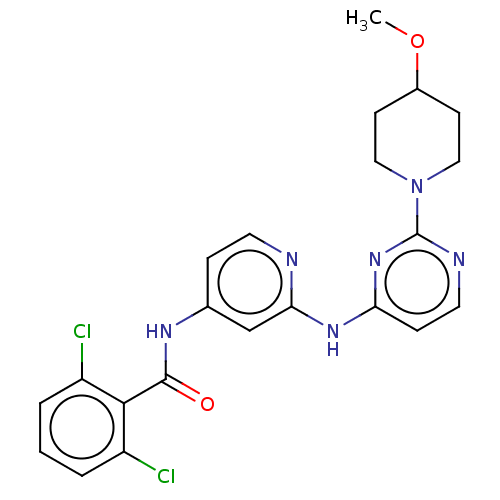
(CHEMBL3352835)Show SMILES COC1CCN(CC1)c1nccc(Nc2cc(NC(=O)c3c(Cl)cccc3Cl)ccn2)n1 Show InChI InChI=1S/C22H22Cl2N6O2/c1-32-15-7-11-30(12-8-15)22-26-10-6-18(29-22)28-19-13-14(5-9-25-19)27-21(31)20-16(23)3-2-4-17(20)24/h2-6,9-10,13,15H,7-8,11-12H2,1H3,(H2,25,26,27,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM101536
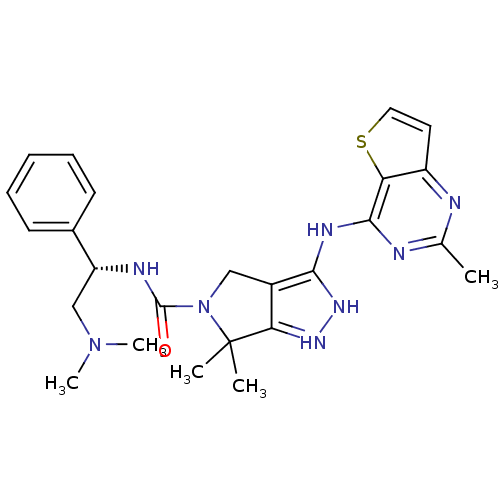
(CHEMBL3128043 | US8530494, 203 | US8530652, 114 | ...)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST-tagged recombinant human PAK4 kinase domain expressed in Escherichia coli using KKRNRRLSVA as substrate preincubat... |
J Med Chem 57: 1033-45 (2014)
Article DOI: 10.1021/jm401768t
BindingDB Entry DOI: 10.7270/Q2F47QNW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50564783
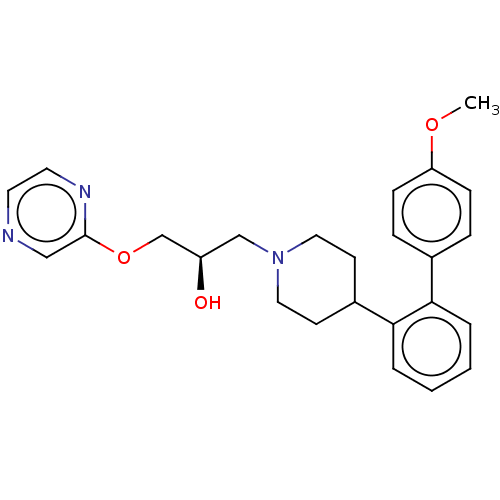
(CHEMBL4789865)Show SMILES COc1ccc(cc1)-c1ccccc1C1CCN(C[C@@H](O)COc2cnccn2)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50564782

(CHEMBL4799274)Show SMILES COc1ccc(cc1)-c1ccccc1N1CCN(CCCCOc2ccc3OC(C)C(=O)Nc3c2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM101536

(CHEMBL3128043 | US8530494, 203 | US8530652, 114 | ...)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(Nc3nc(C)nc4ccsc34)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C25H30N8OS/c1-15-26-18-11-12-35-20(18)23(27-15)29-22-17-13-33(25(2,3)21(17)30-31-22)24(34)28-19(14-32(4)5)16-9-7-6-8-10-16/h6-12,19H,13-14H2,1-5H3,(H,28,34)(H2,26,27,29,30,31)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal His-6-tagged recombinant human PAK1 using peptide substrate |
J Med Chem 57: 1033-45 (2014)
Article DOI: 10.1021/jm401768t
BindingDB Entry DOI: 10.7270/Q2F47QNW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50564772
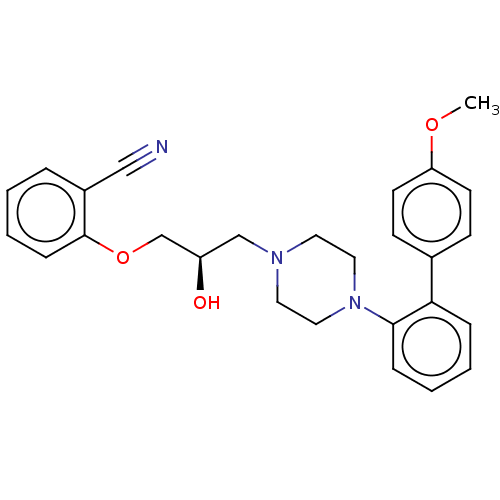
(CHEMBL4794688)Show SMILES COc1ccc(cc1)-c1ccccc1N1CCN(C[C@@H](O)COc2ccccc2C#N)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442161
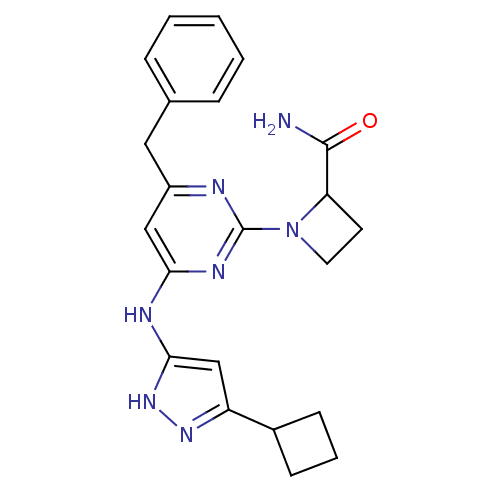
(CHEMBL2441278)Show SMILES NC(=O)C1CCN1c1nc(Cc2ccccc2)cc(Nc2cc(n[nH]2)C2CCC2)n1 Show InChI InChI=1S/C22H25N7O/c23-21(30)18-9-10-29(18)22-24-16(11-14-5-2-1-3-6-14)12-19(26-22)25-20-13-17(27-28-20)15-7-4-8-15/h1-3,5-6,12-13,15,18H,4,7-11H2,(H2,23,30)(H2,24,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ITK (unknown origin) |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50564769
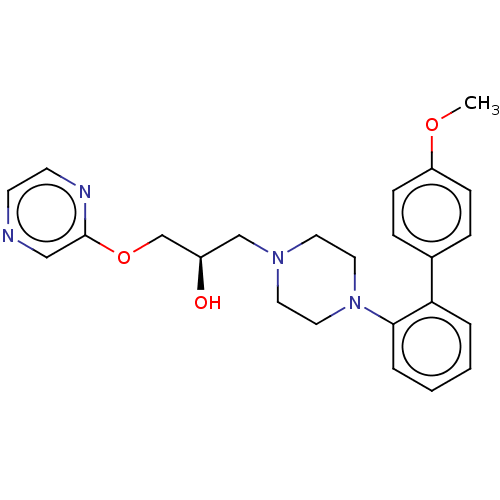
(CHEMBL4780452)Show SMILES COc1ccc(cc1)-c1ccccc1N1CCN(C[C@@H](O)COc2cnccn2)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442159
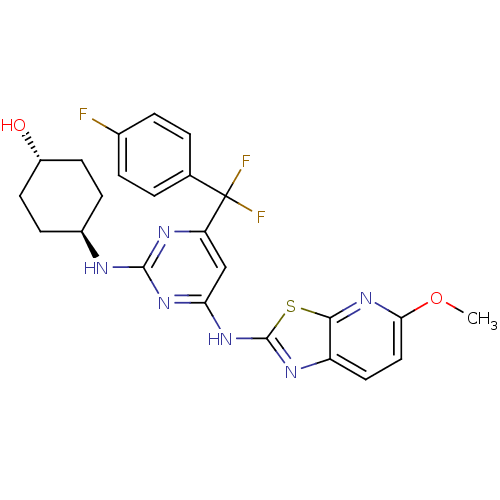
(CHEMBL2441285)Show SMILES COc1ccc2nc(Nc3cc(nc(N[C@H]4CC[C@H](O)CC4)n3)C(F)(F)c3ccc(F)cc3)sc2n1 |r,wU:18.18,wD:15.14,(18.65,-21.99,;17.9,-20.64,;16.36,-20.61,;15.62,-19.27,;14.09,-19.24,;13.3,-20.55,;11.79,-20.85,;11.6,-22.37,;10.25,-23.11,;10.23,-24.65,;8.88,-25.4,;8.85,-26.94,;10.17,-27.73,;11.52,-26.99,;12.84,-27.78,;12.81,-29.32,;11.46,-30.06,;11.43,-31.6,;12.75,-32.4,;12.73,-33.94,;14.1,-31.65,;14.13,-30.11,;11.55,-25.45,;7.51,-27.68,;6.32,-28.66,;8.28,-29.01,;6.14,-26.97,;4.84,-27.8,;3.47,-27.09,;3.4,-25.56,;2.03,-24.85,;4.7,-24.72,;6.06,-25.43,;13,-23.03,;14.04,-21.9,;15.57,-21.93,)| Show InChI InChI=1S/C24H23F3N6O2S/c1-35-20-11-10-17-21(33-20)36-23(29-17)32-19-12-18(24(26,27)13-2-4-14(25)5-3-13)30-22(31-19)28-15-6-8-16(34)9-7-15/h2-5,10-12,15-16,34H,6-9H2,1H3,(H2,28,29,30,31,32)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50032625
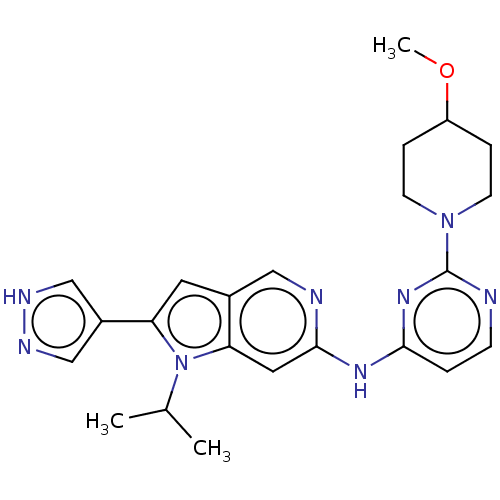
(CHEMBL3354187)Show SMILES COC1CCN(CC1)c1nccc(Nc2cc3n(C(C)C)c(cc3cn2)-c2cn[nH]c2)n1 Show InChI InChI=1S/C23H28N8O/c1-15(2)31-19(17-13-26-27-14-17)10-16-12-25-22(11-20(16)31)28-21-4-7-24-23(29-21)30-8-5-18(32-3)6-9-30/h4,7,10-15,18H,5-6,8-9H2,1-3H3,(H,26,27)(H,24,25,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50032612

(CHEMBL3352835)Show SMILES COC1CCN(CC1)c1nccc(Nc2cc(NC(=O)c3c(Cl)cccc3Cl)ccn2)n1 Show InChI InChI=1S/C22H22Cl2N6O2/c1-32-15-7-11-30(12-8-15)22-26-10-6-18(29-22)28-19-13-14(5-9-25-19)27-21(31)20-16(23)3-2-4-17(20)24/h2-6,9-10,13,15H,7-8,11-12H2,1H3,(H2,25,26,27,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Tyk2 (unknown origin) |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50032627
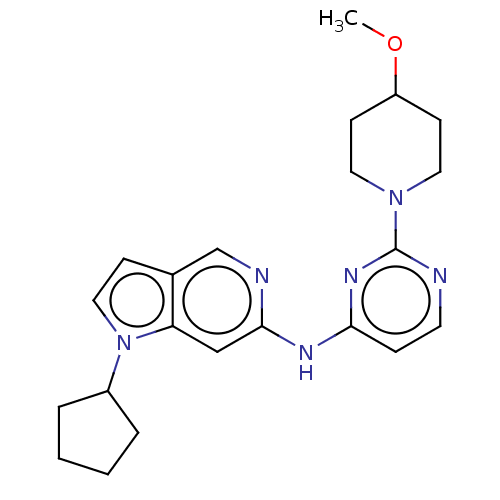
(CHEMBL3354189)Show SMILES COC1CCN(CC1)c1nccc(Nc2cc3n(ccc3cn2)C2CCCC2)n1 Show InChI InChI=1S/C22H28N6O/c1-29-18-8-11-27(12-9-18)22-23-10-6-20(26-22)25-21-14-19-16(15-24-21)7-13-28(19)17-4-2-3-5-17/h6-7,10,13-15,17-18H,2-5,8-9,11-12H2,1H3,(H,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50032631
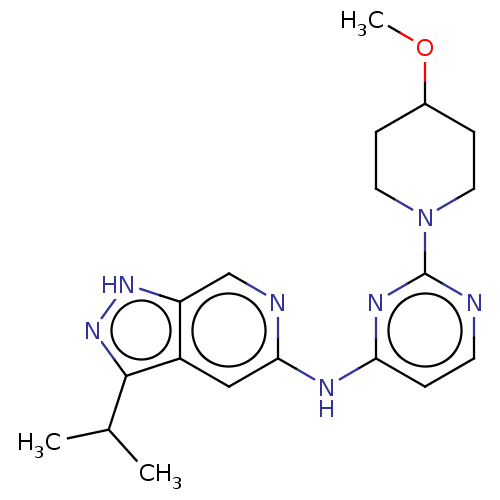
(CHEMBL3354192)Show SMILES COC1CCN(CC1)c1nccc(Nc2cc3c(n[nH]c3cn2)C(C)C)n1 Show InChI InChI=1S/C19H25N7O/c1-12(2)18-14-10-17(21-11-15(14)24-25-18)22-16-4-7-20-19(23-16)26-8-5-13(27-3)6-9-26/h4,7,10-13H,5-6,8-9H2,1-3H3,(H,24,25)(H,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50564779
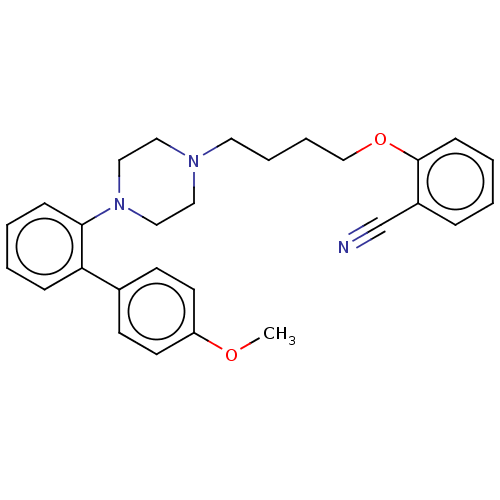
(CHEMBL4783043)Show SMILES COc1ccc(cc1)-c1ccccc1N1CCN(CCCCOc2ccccc2C#N)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50032627

(CHEMBL3354189)Show SMILES COC1CCN(CC1)c1nccc(Nc2cc3n(ccc3cn2)C2CCCC2)n1 Show InChI InChI=1S/C22H28N6O/c1-29-18-8-11-27(12-9-18)22-23-10-6-20(26-22)25-21-14-19-16(15-24-21)7-13-28(19)17-4-2-3-5-17/h6-7,10,13-15,17-18H,2-5,8-9,11-12H2,1H3,(H,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50564775

(CHEMBL4795056)Show SMILES COc1ccc(cc1)-c1ccccc1N1CCN(C[C@@H](O)COc2ccc3OC(C)C(=O)Nc3c2)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442151
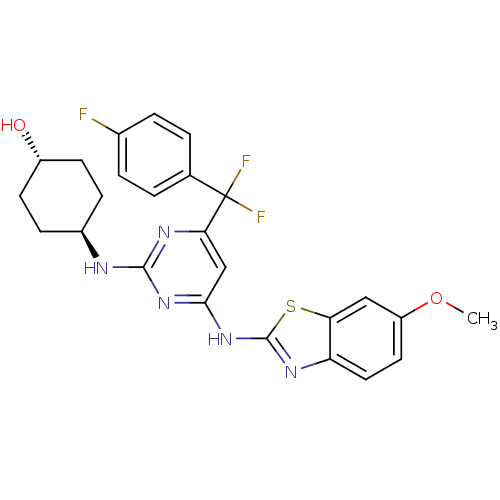
(CHEMBL2441286)Show SMILES COc1ccc2nc(Nc3cc(nc(N[C@H]4CC[C@H](O)CC4)n3)C(F)(F)c3ccc(F)cc3)sc2c1 |r,wU:18.18,wD:15.14,(40.74,-21.58,;39.99,-20.23,;38.45,-20.2,;37.7,-18.85,;36.17,-18.83,;35.39,-20.14,;33.88,-20.44,;33.69,-21.95,;32.34,-22.7,;32.31,-24.24,;30.96,-24.98,;30.93,-26.52,;32.26,-27.32,;33.61,-26.57,;34.93,-27.37,;34.9,-28.91,;33.55,-29.65,;33.52,-31.19,;34.84,-31.98,;34.81,-33.52,;36.19,-31.24,;36.21,-29.7,;33.64,-25.03,;29.59,-27.26,;28.4,-28.25,;30.69,-28.36,;28.22,-26.55,;26.93,-27.39,;25.56,-26.68,;25.49,-25.14,;24.12,-24.43,;26.79,-24.31,;28.15,-25.01,;35.08,-22.61,;36.13,-21.48,;37.66,-21.51,)| Show InChI InChI=1S/C25H24F3N5O2S/c1-35-18-10-11-19-20(12-18)36-24(30-19)33-22-13-21(25(27,28)14-2-4-15(26)5-3-14)31-23(32-22)29-16-6-8-17(34)9-7-16/h2-5,10-13,16-17,34H,6-9H2,1H3,(H2,29,30,31,32,33)/t16-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins |
ACS Med Chem Lett 4: 948-52 (2013)
Article DOI: 10.1021/ml400206q
BindingDB Entry DOI: 10.7270/Q28P61Z7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50032623

(CHEMBL3354183)Show SMILES C[C@@H](CO)Nc1nc(C)cc(Nc2cc(NC(=O)c3c(Cl)cccc3Cl)ccn2)n1 |r| Show InChI InChI=1S/C20H20Cl2N6O2/c1-11-8-17(28-20(24-11)25-12(2)10-29)27-16-9-13(6-7-23-16)26-19(30)18-14(21)4-3-5-15(18)22/h3-9,12,29H,10H2,1-2H3,(H3,23,24,25,26,27,28,30)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50564780
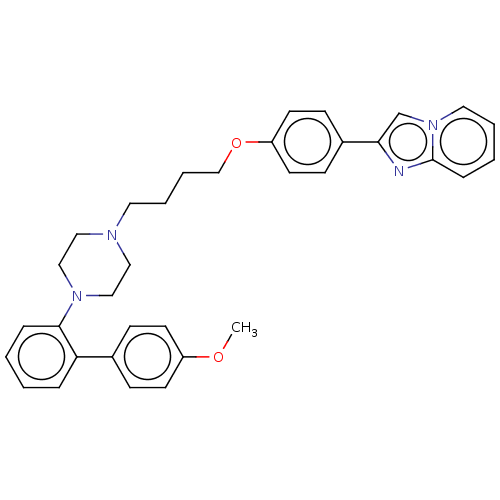
(CHEMBL4785158)Show SMILES COc1ccc(cc1)-c1ccccc1N1CCN(CCCCOc2ccc(cc2)-c2cn3ccccc3n2)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-5-CT from human 5-HT7R expressed in human HEK293 cells assessed as inhibitory constant incubated for 60 mins by radioligand bind... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112395
BindingDB Entry DOI: 10.7270/Q2MW2MW1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50055580
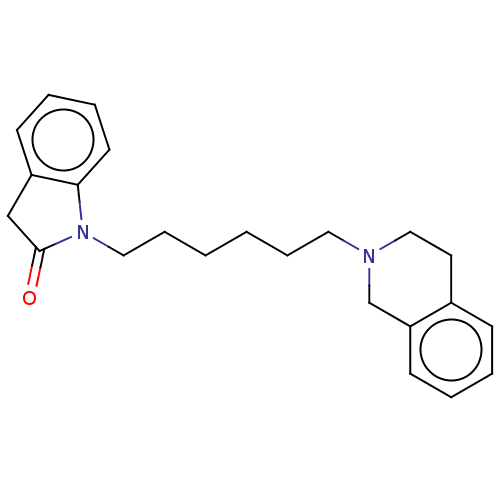
(CHEMBL3321778)Show InChI InChI=1S/C23H28N2O/c26-23-17-20-10-5-6-12-22(20)25(23)15-8-2-1-7-14-24-16-13-19-9-3-4-11-21(19)18-24/h3-6,9-12H,1-2,7-8,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT7 expressed in HEK-293 cells after 120 mins by scintillation spectrometry |
J Med Chem 57: 6879-84 (2014)
Article DOI: 10.1021/jm500880c
BindingDB Entry DOI: 10.7270/Q2J104TB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data