 Found 7452 hits with Last Name = 'tam' and Initial = 's'
Found 7452 hits with Last Name = 'tam' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase
(Stylophora pistillata) | BDBM26995
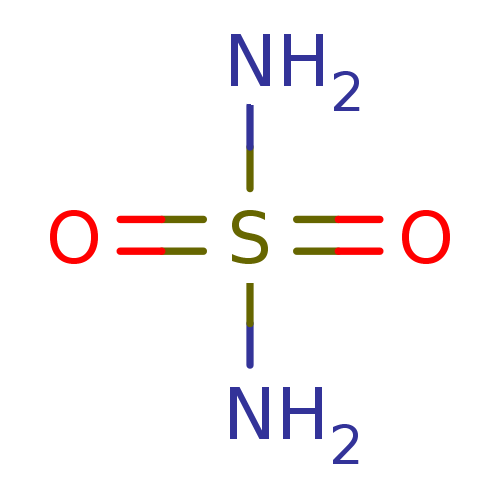
(CHEMBL355001 | H2NSO2NH2 | sulfamamide | sulfamide...)Show InChI InChI=1S/H4N2O2S/c1-5(2,3)4/h(H4,1,2,3,4) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of Stylophora pistillata carbonic anhydrase 2 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase
(Stylophora pistillata) | BDBM26996
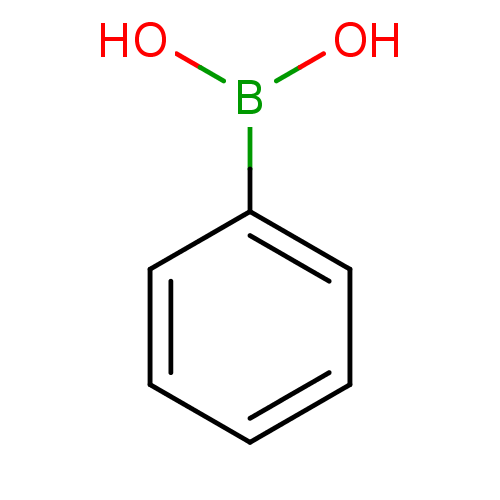
(CHEMBL21485 | PhB(OH)2 | Phenyl-boronic acid | Phe...)Show InChI InChI=1S/C6H7BO2/c8-7(9)6-4-2-1-3-5-6/h1-5,8-9H | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of Stylophora pistillata carbonic anhydrase 2 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase
(Stylophora pistillata) | BDBM26994
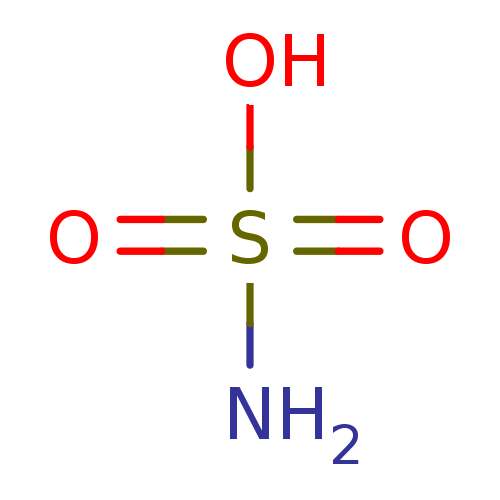
(CHEMBL68253 | H2NSO3H | sulfamic acid)Show InChI InChI=1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of Stylophora pistillata carbonic anhydrase 2 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase
(Stylophora pistillata) | BDBM26995

(CHEMBL355001 | H2NSO2NH2 | sulfamamide | sulfamide...)Show InChI InChI=1S/H4N2O2S/c1-5(2,3)4/h(H4,1,2,3,4) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of Stylophora pistillata carbonic anhydrase by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110121
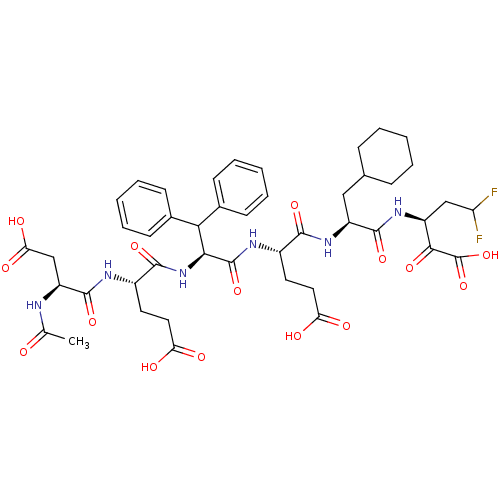
(3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)C(O)=O Show InChI InChI=1S/C45H56F2N6O15/c1-24(54)48-32(23-36(59)60)43(65)49-29(18-20-35(57)58)41(63)53-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)44(66)50-28(17-19-34(55)56)40(62)52-31(21-25-11-5-2-6-12-25)42(64)51-30(22-33(46)47)39(61)45(67)68/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,48,54)(H,49,65)(H,50,66)(H,51,64)(H,52,62)(H,53,63)(H,55,56)(H,57,58)(H,59,60)(H,67,68)/t28-,29-,30-,31-,32-,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031568
(CHEMBL2369874 | [D-Asp2]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C77H129N25O16/c1-7-45(6)62(73(117)97-54(25-17-35-90-77(86)87)74(118)102-36-18-26-59(102)72(116)96-51(22-12-14-32-79)67(111)99-55(37-43(2)3)69(113)93-50(63(81)107)21-11-13-31-78)101-68(112)53(24-16-34-89-76(84)85)94-66(110)52(23-15-33-88-75(82)83)95-70(114)56(38-44(4)5)100-71(115)57(40-46-19-9-8-10-20-46)92-60(104)42-91-65(109)58(41-61(105)106)98-64(108)49(80)39-47-27-29-48(103)30-28-47/h8-10,19-20,27-30,43-45,49-59,62,103H,7,11-18,21-26,31-42,78-80H2,1-6H3,(H2,81,107)(H,91,109)(H,92,104)(H,93,113)(H,94,110)(H,95,114)(H,96,116)(H,97,117)(H,98,108)(H,99,111)(H,100,115)(H,101,112)(H,105,106)(H4,82,83,88)(H4,84,85,89)(H4,86,87,90)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58+,59+,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against delta opioid receptor of rat forebrain using [3H]-DPDPE as the radioligand using competition bind... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031568

(CHEMBL2369874 | [D-Asp2]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C77H129N25O16/c1-7-45(6)62(73(117)97-54(25-17-35-90-77(86)87)74(118)102-36-18-26-59(102)72(116)96-51(22-12-14-32-79)67(111)99-55(37-43(2)3)69(113)93-50(63(81)107)21-11-13-31-78)101-68(112)53(24-16-34-89-76(84)85)94-66(110)52(23-15-33-88-75(82)83)95-70(114)56(38-44(4)5)100-71(115)57(40-46-19-9-8-10-20-46)92-60(104)42-91-65(109)58(41-61(105)106)98-64(108)49(80)39-47-27-29-48(103)30-28-47/h8-10,19-20,27-30,43-45,49-59,62,103H,7,11-18,21-26,31-42,78-80H2,1-6H3,(H2,81,107)(H,91,109)(H,92,104)(H,93,113)(H,94,110)(H,95,114)(H,96,116)(H,97,117)(H,98,108)(H,99,111)(H,100,115)(H,101,112)(H,105,106)(H4,82,83,88)(H4,84,85,89)(H4,86,87,90)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58+,59+,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor mu 1 of rat forebrain using [3H]-DAMGO as the radioligand using competition bindi... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26994

(CHEMBL68253 | H2NSO3H | sulfamic acid)Show InChI InChI=1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA1 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50499777
(CHEMBL3740883)Show SMILES O=Cc1cn(nc1-c1ccccc1)-c1nc(cs1)-c1cc2c(ccc3ccccc23)oc1=O Show InChI InChI=1S/C26H15N3O3S/c30-14-18-13-29(28-24(18)17-7-2-1-3-8-17)26-27-22(15-33-26)21-12-20-19-9-5-4-6-16(19)10-11-23(20)32-25(21)31/h1-15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal CYP2A6 |
Bioorg Med Chem Lett 25: 5797-803 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.042
BindingDB Entry DOI: 10.7270/Q2J67KXF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase
(Stylophora pistillata) | BDBM26997
(CHEMBL364571 | PhAsO3H2 | benzenarsonic acid | ben...)Show InChI InChI=1S/C6H7AsO3/c8-7(9,10)6-4-2-1-3-5-6/h1-5H,(H2,8,9,10) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of Stylophora pistillata carbonic anhydrase 2 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM26995

(CHEMBL355001 | H2NSO2NH2 | sulfamamide | sulfamide...)Show InChI InChI=1S/H4N2O2S/c1-5(2,3)4/h(H4,1,2,3,4) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA6 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM26994

(CHEMBL68253 | H2NSO3H | sulfamic acid)Show InChI InChI=1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA6 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031574
(CHEMBL2369884 | cyclo[D-Asp2,Dab5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C75H124N26O15/c1-5-43(4)60(71(115)97-53(23-15-34-89-75(84)85)72(116)101-36-16-24-57(101)70(114)96-49(20-10-12-31-77)65(109)99-54(37-42(2)3)68(112)92-48(61(79)105)19-9-11-30-76)100-67(111)51(22-14-33-88-74(82)83)94-64(108)50(21-13-32-87-73(80)81)93-66(110)52-29-35-86-58(103)40-56(98-62(106)47(78)38-45-25-27-46(102)28-26-45)63(107)90-41-59(104)91-55(69(113)95-52)39-44-17-7-6-8-18-44/h6-8,17-18,25-28,42-43,47-57,60,102H,5,9-16,19-24,29-41,76-78H2,1-4H3,(H2,79,105)(H,86,103)(H,90,107)(H,91,104)(H,92,112)(H,93,110)(H,94,108)(H,95,113)(H,96,114)(H,97,115)(H,98,106)(H,99,109)(H,100,111)(H4,80,81,87)(H4,82,83,88)(H4,84,85,89)/t43-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56+,57+,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor mu 1 of rat forebrain using [3H]-DAMGO as the radioligand using competition bindi... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50517085

(CHEMBL4545711)Show SMILES NS(=O)(=O)c1nnc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)s1 Show InChI InChI=1S/C10H7ClF3N5O3S2/c11-6-2-1-4(3-5(6)10(12,13)14)16-7(20)17-8-18-19-9(23-8)24(15,21)22/h1-3H,(H2,15,21,22)(H2,16,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50031574

(CHEMBL2369884 | cyclo[D-Asp2,Dab5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C75H124N26O15/c1-5-43(4)60(71(115)97-53(23-15-34-89-75(84)85)72(116)101-36-16-24-57(101)70(114)96-49(20-10-12-31-77)65(109)99-54(37-42(2)3)68(112)92-48(61(79)105)19-9-11-30-76)100-67(111)51(22-14-33-88-74(82)83)94-64(108)50(21-13-32-87-73(80)81)93-66(110)52-29-35-86-58(103)40-56(98-62(106)47(78)38-45-25-27-46(102)28-26-45)63(107)90-41-59(104)91-55(69(113)95-52)39-44-17-7-6-8-18-44/h6-8,17-18,25-28,42-43,47-57,60,102H,5,9-16,19-24,29-41,76-78H2,1-4H3,(H2,79,105)(H,86,103)(H,90,107)(H,91,104)(H,92,112)(H,93,110)(H,94,108)(H,95,113)(H,96,114)(H,97,115)(H,98,106)(H,99,109)(H,100,111)(H4,80,81,87)(H4,82,83,88)(H4,84,85,89)/t43-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56+,57+,60-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor kappa 1 of guinea pig cerebellum using [3H]-bremazocine as the radioligand using ... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031574

(CHEMBL2369884 | cyclo[D-Asp2,Dab5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C75H124N26O15/c1-5-43(4)60(71(115)97-53(23-15-34-89-75(84)85)72(116)101-36-16-24-57(101)70(114)96-49(20-10-12-31-77)65(109)99-54(37-42(2)3)68(112)92-48(61(79)105)19-9-11-30-76)100-67(111)51(22-14-33-88-74(82)83)94-64(108)50(21-13-32-87-73(80)81)93-66(110)52-29-35-86-58(103)40-56(98-62(106)47(78)38-45-25-27-46(102)28-26-45)63(107)90-41-59(104)91-55(69(113)95-52)39-44-17-7-6-8-18-44/h6-8,17-18,25-28,42-43,47-57,60,102H,5,9-16,19-24,29-41,76-78H2,1-4H3,(H2,79,105)(H,86,103)(H,90,107)(H,91,104)(H,92,112)(H,93,110)(H,94,108)(H,95,113)(H,96,114)(H,97,115)(H,98,106)(H,99,109)(H,100,111)(H4,80,81,87)(H4,82,83,88)(H4,84,85,89)/t43-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56+,57+,60-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against delta opioid receptor of rat forebrain using [3H]-DPDPE as the radioligand using competition bind... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50031573
(CHEMBL2369870 | [D-Asp2,Dab(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#7]=[#6](-[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C80H136N28O16/c1-9-47(4)64(75(123)102-57(27-19-38-93-79(89)90)76(124)108-40-20-28-61(108)74(122)101-53(24-14-16-35-82)69(117)104-58(41-46(2)3)72(120)97-52(65(84)113)23-13-15-34-81)105-71(119)55(26-18-37-92-78(87)88)99-68(116)54(25-17-36-91-77(85)86)98-70(118)56(33-39-94-80(106(5)6)107(7)8)100-73(121)59(43-48-21-11-10-12-22-48)96-62(110)45-95-67(115)60(44-63(111)112)103-66(114)51(83)42-49-29-31-50(109)32-30-49/h10-12,21-22,29-32,46-47,51-61,64,109H,9,13-20,23-28,33-45,81-83H2,1-8H3,(H2,84,113)(H,95,115)(H,96,110)(H,97,120)(H,98,118)(H,99,116)(H,100,121)(H,101,122)(H,102,123)(H,103,114)(H,104,117)(H,105,119)(H,111,112)(H4,85,86,91)(H4,87,88,92)(H4,89,90,93)/t47-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60+,61-,64-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor kappa 1 of guinea pig cerebellum using [3H]-bremazocine as the radioligand using ... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50031569
(CHEMBL414773 | Dyn A-(1-13)NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C75H127N25O14/c1-7-45(6)61(71(113)96-54(25-17-35-88-75(84)85)72(114)100-36-18-26-58(100)70(112)95-51(22-12-14-32-77)65(107)97-55(37-43(2)3)67(109)92-50(62(79)104)21-11-13-31-76)99-66(108)53(24-16-34-87-74(82)83)93-64(106)52(23-15-33-86-73(80)81)94-68(110)56(38-44(4)5)98-69(111)57(40-46-19-9-8-10-20-46)91-60(103)42-89-59(102)41-90-63(105)49(78)39-47-27-29-48(101)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,101H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H2,79,104)(H,89,102)(H,90,105)(H,91,103)(H,92,109)(H,93,106)(H,94,110)(H,95,112)(H,96,113)(H,97,107)(H,98,111)(H,99,108)(H4,80,81,86)(H4,82,83,87)(H4,84,85,88)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58+,61-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor kappa 1 of guinea pig cerebellum using [3H]-bremazocine as the radioligand using ... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031570
(CHEMBL2369885 | [D-Asp2,Dap(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]\[#7]=[#6](\[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C79H134N28O16/c1-9-46(4)63(74(122)100-55(27-19-37-92-78(88)89)75(123)107-38-20-28-60(107)73(121)99-52(24-14-16-34-81)68(116)102-56(39-45(2)3)70(118)96-51(64(83)112)23-13-15-33-80)104-69(117)54(26-18-36-91-77(86)87)97-67(115)53(25-17-35-90-76(84)85)98-72(120)59(43-94-79(105(5)6)106(7)8)103-71(119)57(41-47-21-11-10-12-22-47)95-61(109)44-93-66(114)58(42-62(110)111)101-65(113)50(82)40-48-29-31-49(108)32-30-48/h10-12,21-22,29-32,45-46,50-60,63,108H,9,13-20,23-28,33-44,80-82H2,1-8H3,(H2,83,112)(H,93,114)(H,95,109)(H,96,118)(H,97,115)(H,98,120)(H,99,121)(H,100,122)(H,101,113)(H,102,116)(H,103,119)(H,104,117)(H,110,111)(H4,84,85,90)(H4,86,87,91)(H4,88,89,92)/t46-,50-,51-,52-,53-,54-,55-,56-,57-,58+,59-,60-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor mu 1 of rat forebrain using [3H]-DAMGO as the radioligand using competition bindi... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031570

(CHEMBL2369885 | [D-Asp2,Dap(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]\[#7]=[#6](\[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C79H134N28O16/c1-9-46(4)63(74(122)100-55(27-19-37-92-78(88)89)75(123)107-38-20-28-60(107)73(121)99-52(24-14-16-34-81)68(116)102-56(39-45(2)3)70(118)96-51(64(83)112)23-13-15-33-80)104-69(117)54(26-18-36-91-77(86)87)97-67(115)53(25-17-35-90-76(84)85)98-72(120)59(43-94-79(105(5)6)106(7)8)103-71(119)57(41-47-21-11-10-12-22-47)95-61(109)44-93-66(114)58(42-62(110)111)101-65(113)50(82)40-48-29-31-49(108)32-30-48/h10-12,21-22,29-32,45-46,50-60,63,108H,9,13-20,23-28,33-44,80-82H2,1-8H3,(H2,83,112)(H,93,114)(H,95,109)(H,96,118)(H,97,115)(H,98,120)(H,99,121)(H,100,122)(H,101,113)(H,102,116)(H,103,119)(H,104,117)(H,110,111)(H4,84,85,90)(H4,86,87,91)(H4,88,89,92)/t46-,50-,51-,52-,53-,54-,55-,56-,57-,58+,59-,60-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against delta opioid receptor of rat forebrain using [3H]-DPDPE as the radioligand using competition bind... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031569

(CHEMBL414773 | Dyn A-(1-13)NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C75H127N25O14/c1-7-45(6)61(71(113)96-54(25-17-35-88-75(84)85)72(114)100-36-18-26-58(100)70(112)95-51(22-12-14-32-77)65(107)97-55(37-43(2)3)67(109)92-50(62(79)104)21-11-13-31-76)99-66(108)53(24-16-34-87-74(82)83)93-64(106)52(23-15-33-86-73(80)81)94-68(110)56(38-44(4)5)98-69(111)57(40-46-19-9-8-10-20-46)91-60(103)42-89-59(102)41-90-63(105)49(78)39-47-27-29-48(101)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,101H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H2,79,104)(H,89,102)(H,90,105)(H,91,103)(H,92,109)(H,93,106)(H,94,110)(H,95,112)(H,96,113)(H,97,107)(H,98,111)(H,99,108)(H4,80,81,86)(H4,82,83,87)(H4,84,85,88)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58+,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor mu 1 of rat forebrain using [3H]-DAMGO as the radioligand using competition bindi... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603932

((+)-trans-Ethyl 2-ethyl-2-{[6-({-2-[(fluoromethoxy...)Show SMILES CCOC(=O)C(CC)(CC)NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2COCF)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50031570

(CHEMBL2369885 | [D-Asp2,Dap(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]\[#7]=[#6](\[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C79H134N28O16/c1-9-46(4)63(74(122)100-55(27-19-37-92-78(88)89)75(123)107-38-20-28-60(107)73(121)99-52(24-14-16-34-81)68(116)102-56(39-45(2)3)70(118)96-51(64(83)112)23-13-15-33-80)104-69(117)54(26-18-36-91-77(86)87)97-67(115)53(25-17-35-90-76(84)85)98-72(120)59(43-94-79(105(5)6)106(7)8)103-71(119)57(41-47-21-11-10-12-22-47)95-61(109)44-93-66(114)58(42-62(110)111)101-65(113)50(82)40-48-29-31-49(108)32-30-48/h10-12,21-22,29-32,45-46,50-60,63,108H,9,13-20,23-28,33-44,80-82H2,1-8H3,(H2,83,112)(H,93,114)(H,95,109)(H,96,118)(H,97,115)(H,98,120)(H,99,121)(H,100,122)(H,101,113)(H,102,116)(H,103,119)(H,104,117)(H,110,111)(H4,84,85,90)(H4,86,87,91)(H4,88,89,92)/t46-,50-,51-,52-,53-,54-,55-,56-,57-,58+,59-,60-,63-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor kappa 1 of guinea pig cerebellum using [3H]-bremazocine as the radioligand using ... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50031571
(CHEMBL2369883 | cyclo[D-Asp2,Dap5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#7]-[#6](=O)-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C74H122N26O15/c1-5-42(4)59(70(114)95-51(23-15-33-87-74(83)84)71(115)100-34-16-24-56(100)69(113)94-48(20-10-12-30-76)64(108)97-52(35-41(2)3)66(110)91-47(60(78)104)19-9-11-29-75)99-65(109)50(22-14-32-86-73(81)82)92-63(107)49(21-13-31-85-72(79)80)93-68(112)55-39-88-57(102)38-54(96-61(105)46(77)36-44-25-27-45(101)28-26-44)62(106)89-40-58(103)90-53(67(111)98-55)37-43-17-7-6-8-18-43/h6-8,17-18,25-28,41-42,46-56,59,101H,5,9-16,19-24,29-40,75-77H2,1-4H3,(H2,78,104)(H,88,102)(H,89,106)(H,90,103)(H,91,110)(H,92,107)(H,93,112)(H,94,113)(H,95,114)(H,96,105)(H,97,108)(H,98,111)(H,99,109)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t42-,46-,47-,48-,49-,50-,51-,52-,53-,54+,55-,56+,59-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor kappa 1 of guinea pig cerebellum using [3H]-bremazocine as the radioligand using ... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50517085

(CHEMBL4545711)Show SMILES NS(=O)(=O)c1nnc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)s1 Show InChI InChI=1S/C10H7ClF3N5O3S2/c11-6-2-1-4(3-5(6)10(12,13)14)16-7(20)17-8-18-19-9(23-8)24(15,21)22/h1-3H,(H2,15,21,22)(H2,16,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50037135
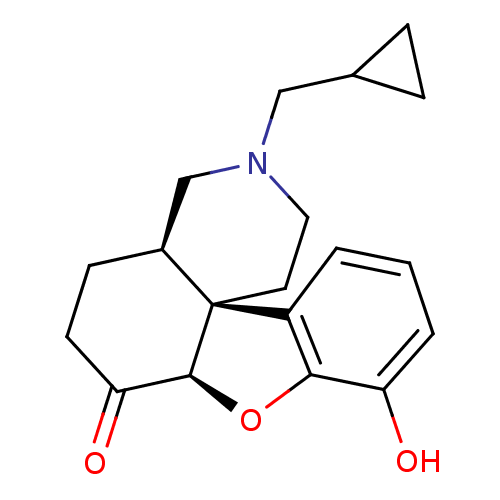
((4aR,7aR,12bS)-3-Cyclopropylmethyl-9-hydroxy-2,3,4...)Show SMILES Oc1cccc2c1O[C@H]1C(=O)CC[C@H]3CN(CC4CC4)CC[C@]213 Show InChI InChI=1S/C19H23NO3/c21-15-3-1-2-14-17(15)23-18-16(22)7-6-13-11-20(10-12-4-5-12)9-8-19(13,14)18/h1-3,12-13,18,21H,4-11H2/t13-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
In vivo binding affinity against mu opioid receptor was measured by using labeled ligand [3H]-Naloxone (0.5 nM) |
J Med Chem 37: 3121-7 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F6R |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031572
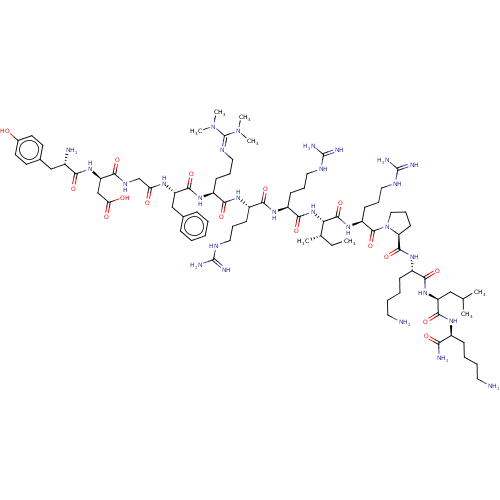
(CHEMBL2369873 | [D-Asp2,Orn(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C81H138N28O16/c1-9-48(4)65(76(124)103-58(29-20-39-94-80(90)91)77(125)109-41-21-30-62(109)75(123)102-54(25-14-16-36-83)71(119)105-59(42-47(2)3)73(121)98-53(66(85)114)24-13-15-35-82)106-72(120)57(27-18-38-93-79(88)89)100-69(117)55(26-17-37-92-78(86)87)99-70(118)56(28-19-40-95-81(107(5)6)108(7)8)101-74(122)60(44-49-22-11-10-12-23-49)97-63(111)46-96-68(116)61(45-64(112)113)104-67(115)52(84)43-50-31-33-51(110)34-32-50/h10-12,22-23,31-34,47-48,52-62,65,110H,9,13-21,24-30,35-46,82-84H2,1-8H3,(H2,85,114)(H,96,116)(H,97,111)(H,98,121)(H,99,118)(H,100,117)(H,101,122)(H,102,123)(H,103,124)(H,104,115)(H,105,119)(H,106,120)(H,112,113)(H4,86,87,92)(H4,88,89,93)(H4,90,91,94)/t48-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61+,62-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against delta opioid receptor of rat forebrain using [3H]-DPDPE as the radioligand using competition bind... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031572

(CHEMBL2369873 | [D-Asp2,Orn(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C81H138N28O16/c1-9-48(4)65(76(124)103-58(29-20-39-94-80(90)91)77(125)109-41-21-30-62(109)75(123)102-54(25-14-16-36-83)71(119)105-59(42-47(2)3)73(121)98-53(66(85)114)24-13-15-35-82)106-72(120)57(27-18-38-93-79(88)89)100-69(117)55(26-17-37-92-78(86)87)99-70(118)56(28-19-40-95-81(107(5)6)108(7)8)101-74(122)60(44-49-22-11-10-12-23-49)97-63(111)46-96-68(116)61(45-64(112)113)104-67(115)52(84)43-50-31-33-51(110)34-32-50/h10-12,22-23,31-34,47-48,52-62,65,110H,9,13-21,24-30,35-46,82-84H2,1-8H3,(H2,85,114)(H,96,116)(H,97,111)(H,98,121)(H,99,118)(H,100,117)(H,101,122)(H,102,123)(H,103,124)(H,104,115)(H,105,119)(H,106,120)(H,112,113)(H4,86,87,92)(H4,88,89,93)(H4,90,91,94)/t48-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61+,62-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor mu 1 of rat forebrain using [3H]-DAMGO as the radioligand using competition bindi... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603936

((+)-trans-2-Fluoroethyl 2-ethyl-2-{[6-{[-2-(hydrox...)Show SMILES CCC(CC)(NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2CO)n1)C(=O)OCCF |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM26995

(CHEMBL355001 | H2NSO2NH2 | sulfamamide | sulfamide...)Show InChI InChI=1S/H4N2O2S/c1-5(2,3)4/h(H4,1,2,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA1 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM224024

(BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Binding affinity to Opioid receptor kappa 1 by competitive inhibition of radioligand [3H]-diprenorphine using cloned receptors transiently expressed ... |
J Med Chem 40: 1211-8 (1997)
Article DOI: 10.1021/jm960753p
BindingDB Entry DOI: 10.7270/Q2BG2N3H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031573

(CHEMBL2369870 | [D-Asp2,Dab(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#7]=[#6](-[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C80H136N28O16/c1-9-47(4)64(75(123)102-57(27-19-38-93-79(89)90)76(124)108-40-20-28-61(108)74(122)101-53(24-14-16-35-82)69(117)104-58(41-46(2)3)72(120)97-52(65(84)113)23-13-15-34-81)105-71(119)55(26-18-37-92-78(87)88)99-68(116)54(25-17-36-91-77(85)86)98-70(118)56(33-39-94-80(106(5)6)107(7)8)100-73(121)59(43-48-21-11-10-12-22-48)96-62(110)45-95-67(115)60(44-63(111)112)103-66(114)51(83)42-49-29-31-50(109)32-30-49/h10-12,21-22,29-32,46-47,51-61,64,109H,9,13-20,23-28,33-45,81-83H2,1-8H3,(H2,84,113)(H,95,115)(H,96,110)(H,97,120)(H,98,118)(H,99,116)(H,100,121)(H,101,122)(H,102,123)(H,103,114)(H,104,117)(H,105,119)(H,111,112)(H4,85,86,91)(H4,87,88,92)(H4,89,90,93)/t47-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60+,61-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor mu 1 of rat forebrain using [3H]-DAMGO as the radioligand using competition bindi... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031573

(CHEMBL2369870 | [D-Asp2,Dab(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#7]=[#6](-[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C80H136N28O16/c1-9-47(4)64(75(123)102-57(27-19-38-93-79(89)90)76(124)108-40-20-28-61(108)74(122)101-53(24-14-16-35-82)69(117)104-58(41-46(2)3)72(120)97-52(65(84)113)23-13-15-34-81)105-71(119)55(26-18-37-92-78(87)88)99-68(116)54(25-17-36-91-77(85)86)98-70(118)56(33-39-94-80(106(5)6)107(7)8)100-73(121)59(43-48-21-11-10-12-22-48)96-62(110)45-95-67(115)60(44-63(111)112)103-66(114)51(83)42-49-29-31-50(109)32-30-49/h10-12,21-22,29-32,46-47,51-61,64,109H,9,13-20,23-28,33-45,81-83H2,1-8H3,(H2,84,113)(H,95,115)(H,96,110)(H,97,120)(H,98,118)(H,99,116)(H,100,121)(H,101,122)(H,102,123)(H,103,114)(H,104,117)(H,105,119)(H,111,112)(H4,85,86,91)(H4,87,88,92)(H4,89,90,93)/t47-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60+,61-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against delta opioid receptor of rat forebrain using [3H]-DPDPE as the radioligand using competition bind... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50031572

(CHEMBL2369873 | [D-Asp2,Orn(Tmg)5]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7](-[#6])-[#6])-[#7](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C81H138N28O16/c1-9-48(4)65(76(124)103-58(29-20-39-94-80(90)91)77(125)109-41-21-30-62(109)75(123)102-54(25-14-16-36-83)71(119)105-59(42-47(2)3)73(121)98-53(66(85)114)24-13-15-35-82)106-72(120)57(27-18-38-93-79(88)89)100-69(117)55(26-17-37-92-78(86)87)99-70(118)56(28-19-40-95-81(107(5)6)108(7)8)101-74(122)60(44-49-22-11-10-12-23-49)97-63(111)46-96-68(116)61(45-64(112)113)104-67(115)52(84)43-50-31-33-51(110)34-32-50/h10-12,22-23,31-34,47-48,52-62,65,110H,9,13-21,24-30,35-46,82-84H2,1-8H3,(H2,85,114)(H,96,116)(H,97,111)(H,98,121)(H,99,118)(H,100,117)(H,101,122)(H,102,123)(H,103,124)(H,104,115)(H,105,119)(H,106,120)(H,112,113)(H4,86,87,92)(H4,88,89,93)(H4,90,91,94)/t48-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61+,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor kappa 1 of guinea pig cerebellum using [3H]-bremazocine as the radioligand using ... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 of guinea pig brain membranes using 0.5 nM of [3H]naloxone as radioligand |
J Med Chem 29: 1222-5 (1987)
BindingDB Entry DOI: 10.7270/Q2M04606 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50517085

(CHEMBL4545711)Show SMILES NS(=O)(=O)c1nnc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)s1 Show InChI InChI=1S/C10H7ClF3N5O3S2/c11-6-2-1-4(3-5(6)10(12,13)14)16-7(20)17-8-18-19-9(23-8)24(15,21)22/h1-3H,(H2,15,21,22)(H2,16,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111600
BindingDB Entry DOI: 10.7270/Q2542RZZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM26994

(CHEMBL68253 | H2NSO3H | sulfamic acid)Show InChI InChI=1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Scientifique de Monaco
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 by stopped-flow CO2 hydration assay |
Bioorg Med Chem Lett 21: 710-4 (2011)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.11.124
BindingDB Entry DOI: 10.7270/Q26D5T7K |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603935
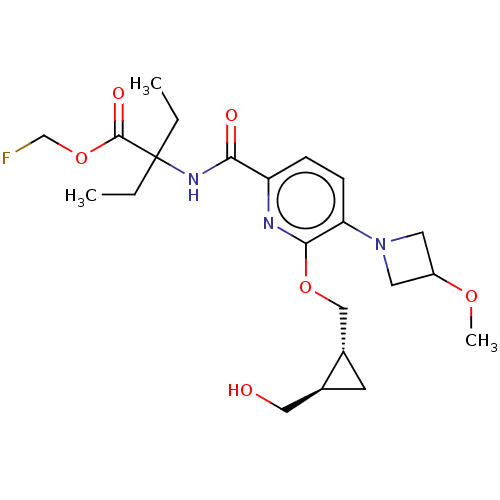
((−)-trans-Fluoromethyl 2-ethyl-2-{[6-{[-2-(h...)Show SMILES CCC(CC)(NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@@H]2C[C@H]2CO)n1)C(=O)OCF | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50031568

(CHEMBL2369874 | [D-Asp2]Dyn A-(1-13)NH2)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C77H129N25O16/c1-7-45(6)62(73(117)97-54(25-17-35-90-77(86)87)74(118)102-36-18-26-59(102)72(116)96-51(22-12-14-32-79)67(111)99-55(37-43(2)3)69(113)93-50(63(81)107)21-11-13-31-78)101-68(112)53(24-16-34-89-76(84)85)94-66(110)52(23-15-33-88-75(82)83)95-70(114)56(38-44(4)5)100-71(115)57(40-46-19-9-8-10-20-46)92-60(104)42-91-65(109)58(41-61(105)106)98-64(108)49(80)39-47-27-29-48(103)30-28-47/h8-10,19-20,27-30,43-45,49-59,62,103H,7,11-18,21-26,31-42,78-80H2,1-6H3,(H2,81,107)(H,91,109)(H,92,104)(H,93,113)(H,94,110)(H,95,114)(H,96,116)(H,97,117)(H,98,108)(H,99,111)(H,100,115)(H,101,112)(H,105,106)(H4,82,83,88)(H4,84,85,89)(H4,86,87,90)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58+,59+,62-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Opioid receptor kappa 1 of guinea pig cerebellum using [3H]-bremazocine as the radioligand using ... |
J Med Chem 38: 2410-7 (1995)
BindingDB Entry DOI: 10.7270/Q2CV4GS8 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50343682

(((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-ylox...)Show SMILES [#6]-[#6]-[#6](-[#6]-[#6])-[#8]-[#6@@H]-1-[#6]-[#6](=[#6]-[#6@H](\[#7]=[#6](/[#7])-[#7])-[#6@H]-1-[#7]-[#6](-[#6])=O)-[#6](-[#8])=O |r,c:8| Show InChI InChI=1S/C15H26N4O4/c1-4-10(5-2)23-12-7-9(14(21)22)6-11(19-15(16)17)13(12)18-8(3)20/h6,10-13H,4-5,7H2,1-3H3,(H,18,20)(H,21,22)(H4,16,17,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of influenza A nuraminidase N1 |
J Med Chem 53: 7377-91 (2010)
Article DOI: 10.1021/jm100822f
BindingDB Entry DOI: 10.7270/Q2S75K5B |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50190142
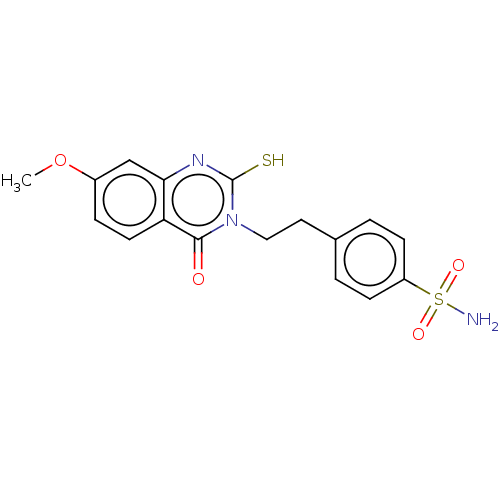
(CHEMBL3828241)Show SMILES COc1ccc2c(c1)nc(S)n(CCc1ccc(cc1)S(N)(=O)=O)c2=O Show InChI InChI=1S/C17H17N3O4S2/c1-24-12-4-7-14-15(10-12)19-17(25)20(16(14)21)9-8-11-2-5-13(6-3-11)26(18,22)23/h2-7,10H,8-9H2,1H3,(H,19,25)(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 24: 4100-4107 (2016)
Article DOI: 10.1016/j.bmc.2016.06.052
BindingDB Entry DOI: 10.7270/Q2FJ2JPH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50019647
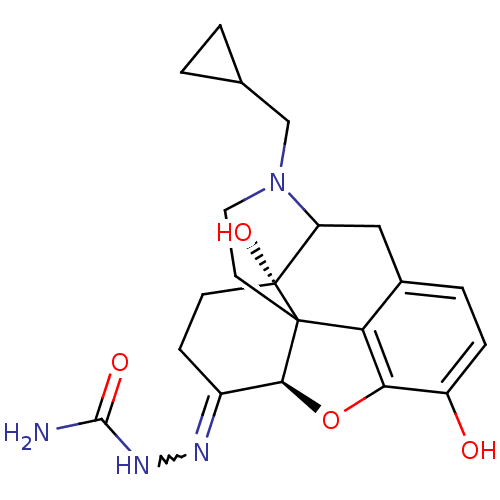
(CHEMBL19982 | Naltrexone semicarbazone)Show SMILES NC(=O)NN=C1CC[C@@]2(O)C3Cc4ccc(O)c5O[C@@H]1C2(CCN3CC1CC1)c45 |w:4.3,TLB:24:23:8:28.12.11,17:28:8:23.21.22,9:8:28.12.11:23.21.22| Show InChI InChI=1S/C21H26N4O4/c22-19(27)24-23-13-5-6-21(28)15-9-12-3-4-14(26)17-16(12)20(21,18(13)29-17)7-8-25(15)10-11-1-2-11/h3-4,11,15,18,26,28H,1-2,5-10H2,(H3,22,24,27)/t15?,18-,20?,21+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 of guinea pig brain membranes using 0.5 nM of [3H]naloxone as radioligand |
J Med Chem 29: 1222-5 (1987)
BindingDB Entry DOI: 10.7270/Q2M04606 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM603926
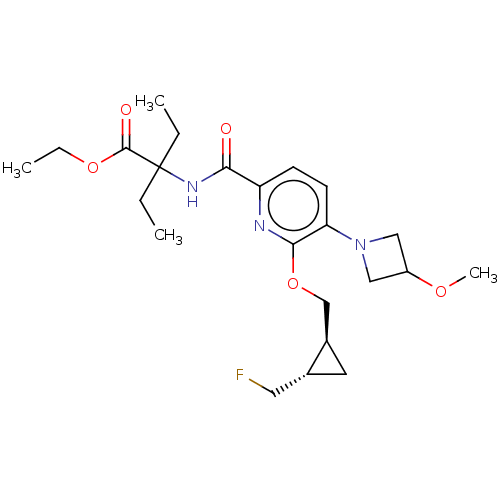
(Ethyl 2-ethyl-2-{[6-{[(1S,2S)-2-(fluoromethyl)cycl...)Show SMILES CCOC(=O)C(CC)(CC)NC(=O)c1ccc(N2CC(C2)OC)c(OC[C@H]2C[C@@H]2CF)n1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93CHD |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110117

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C=O Show InChI InChI=1S/C44H56F2N6O13/c1-25(54)47-33(23-37(59)60)43(64)49-31(18-20-36(57)58)41(62)52-39(38(27-13-7-3-8-14-27)28-15-9-4-10-16-28)44(65)50-30(17-19-35(55)56)40(61)51-32(21-26-11-5-2-6-12-26)42(63)48-29(24-53)22-34(45)46/h3-4,7-10,13-16,24,26,29-34,38-39H,2,5-6,11-12,17-23H2,1H3,(H,47,54)(H,48,63)(H,49,64)(H,50,65)(H,51,61)(H,52,62)(H,55,56)(H,57,58)(H,59,60)/t29-,30-,31-,32-,33-,39-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50190147
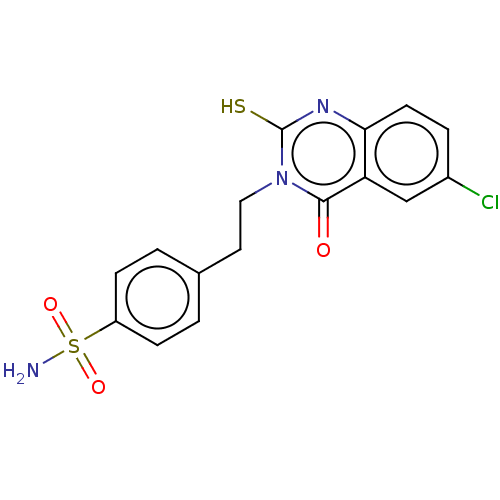
(CHEMBL3828207)Show SMILES NS(=O)(=O)c1ccc(CCn2c(S)nc3ccc(Cl)cc3c2=O)cc1 Show InChI InChI=1S/C16H14ClN3O3S2/c17-11-3-6-14-13(9-11)15(21)20(16(24)19-14)8-7-10-1-4-12(5-2-10)25(18,22)23/h1-6,9H,7-8H2,(H,19,24)(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 24: 4100-4107 (2016)
Article DOI: 10.1016/j.bmc.2016.06.052
BindingDB Entry DOI: 10.7270/Q2FJ2JPH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50190143
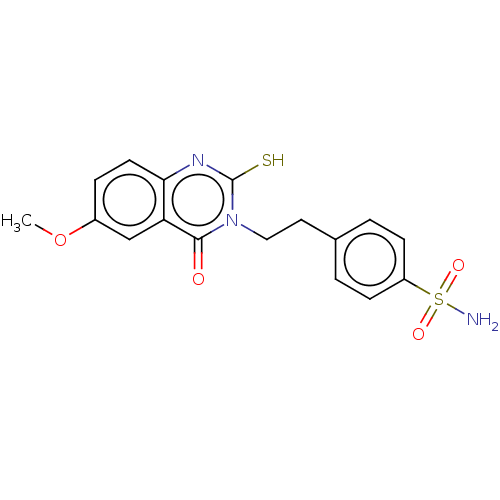
(CHEMBL3827651)Show SMILES COc1ccc2nc(S)n(CCc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C17H17N3O4S2/c1-24-12-4-7-15-14(10-12)16(21)20(17(25)19-15)9-8-11-2-5-13(6-3-11)26(18,22)23/h2-7,10H,8-9H2,1H3,(H,19,25)(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 24: 4100-4107 (2016)
Article DOI: 10.1016/j.bmc.2016.06.052
BindingDB Entry DOI: 10.7270/Q2FJ2JPH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50037134

((+)-trans-3-Cyclopropylmethyl-2,3,4,4aalpha,5,6,7,...)Show SMILES Oc1cccc2c1O[C@H]1CCC[C@H]3CN(CC4CC4)CC[C@]213 Show InChI InChI=1S/C19H25NO2/c21-16-5-2-4-15-18(16)22-17-6-1-3-14-12-20(11-13-7-8-13)10-9-19(14,15)17/h2,4-5,13-14,17,21H,1,3,6-12H2/t14-,17-,19-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
In vivo binding affinity against mu opioid receptor was measured by using labeled ligand [3H]-Naloxone (0.5 nM) |
J Med Chem 37: 3121-7 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F6R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich (Swiss Federal Institute of Technology)
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant histamine H3 receptor |
Bioorg Med Chem 20: 2889-96 (2012)
Article DOI: 10.1016/j.bmc.2012.03.024
BindingDB Entry DOI: 10.7270/Q2JQ1258 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50190134

(CHEMBL1433252)Show SMILES NS(=O)(=O)c1ccc(CCn2c(=S)[nH]c3ccccc3c2=O)cc1 Show InChI InChI=1S/C16H15N3O3S2/c17-24(21,22)12-7-5-11(6-8-12)9-10-19-15(20)13-3-1-2-4-14(13)18-16(19)23/h1-8H,9-10H2,(H,18,23)(H2,17,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 24: 4100-4107 (2016)
Article DOI: 10.1016/j.bmc.2016.06.052
BindingDB Entry DOI: 10.7270/Q2FJ2JPH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































