

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sialidase (Clostridium perfringens) | BDBM50442400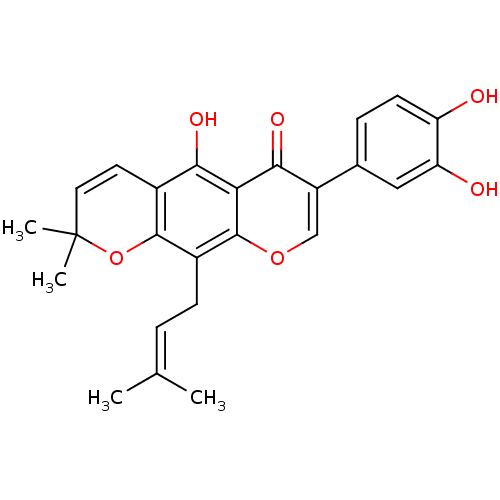 (AURICULASIN) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237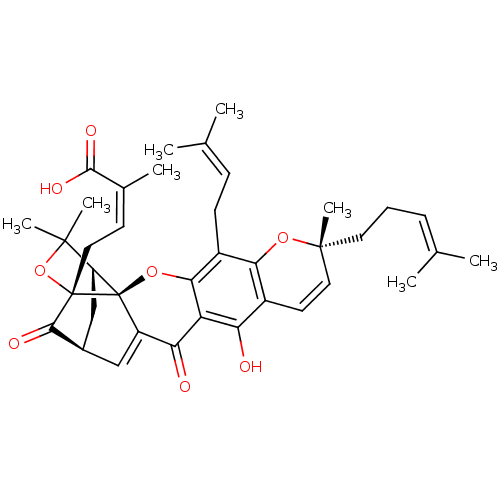 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256793 (CHEMBL4095800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256807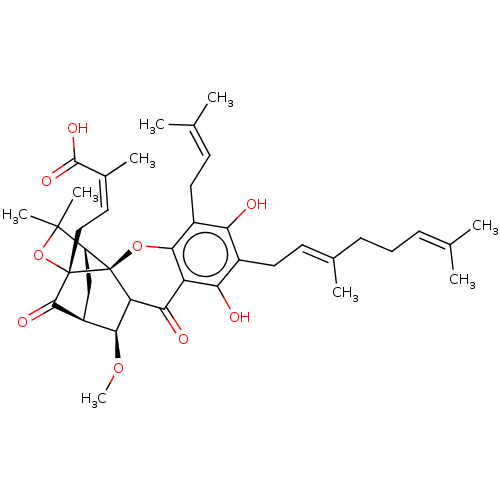 (CHEMBL4069859) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495308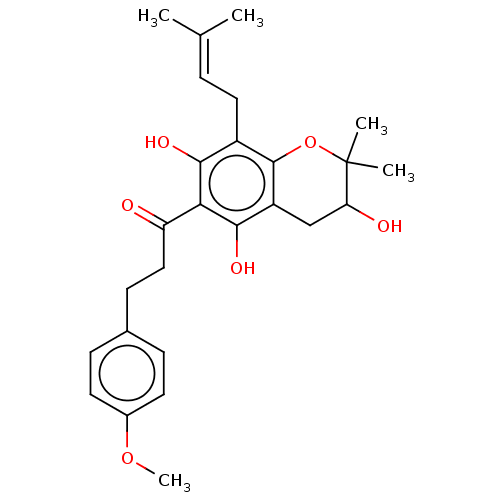 (CHEMBL3103546) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495305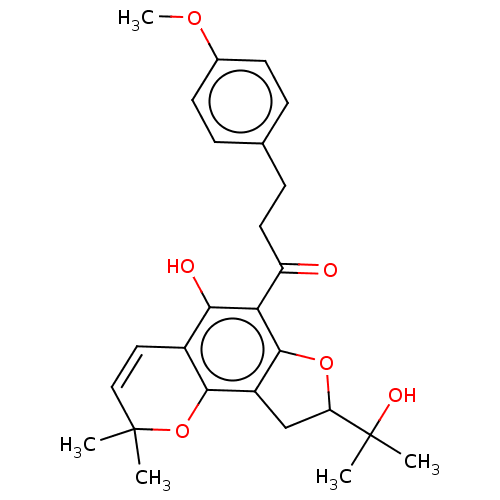 (CHEMBL3103544) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495309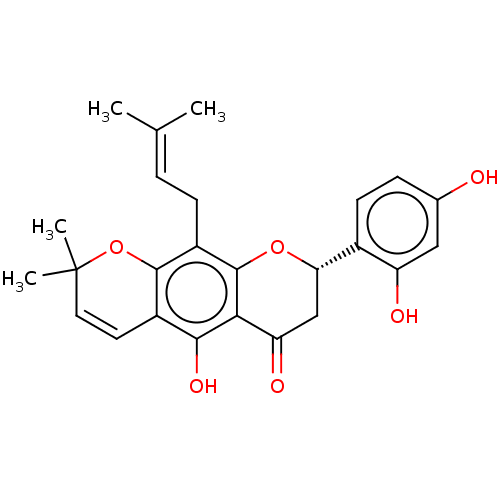 (Flemichin D) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442401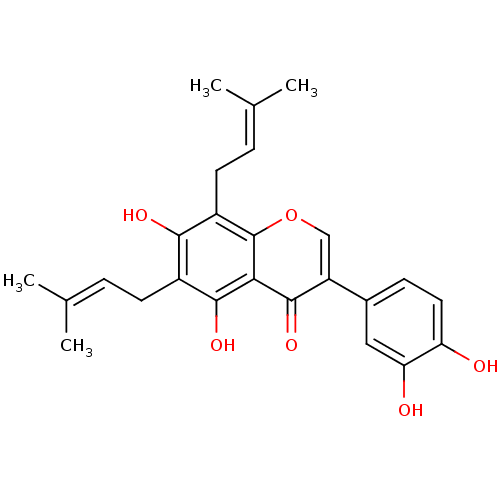 (CHEMBL2442947) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256792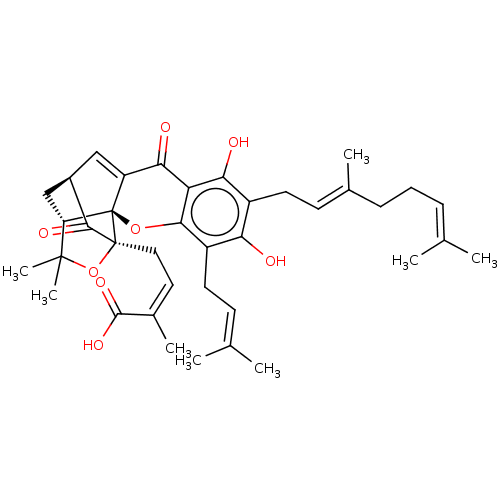 (Gambogenic Acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442399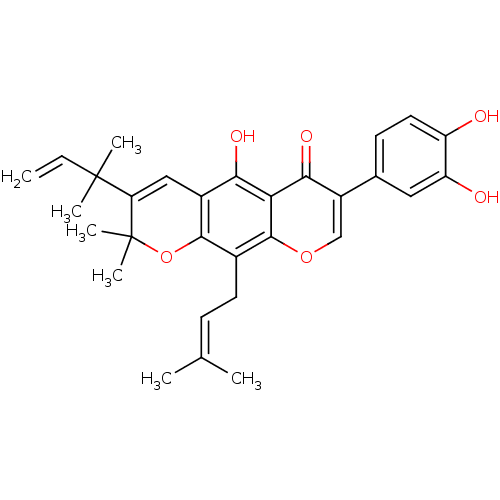 (CHEMBL2442948) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256790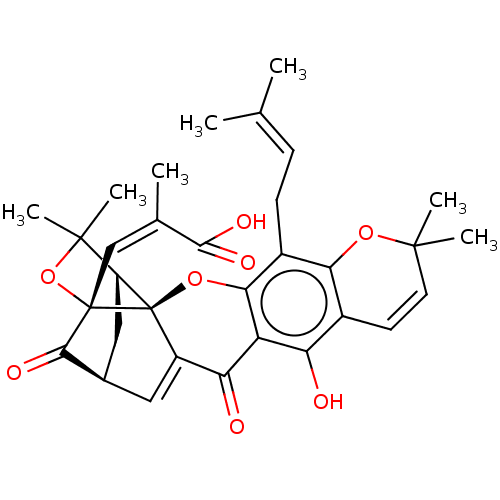 (CHEMBL4068566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256791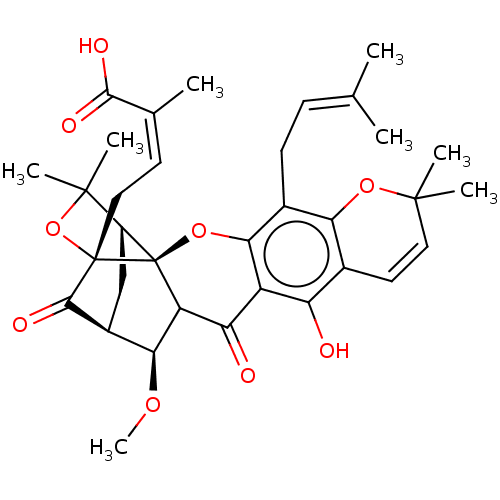 (CHEMBL4090412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495306 (khonklonginol H) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442401 (CHEMBL2442947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495305 (CHEMBL3103544) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442397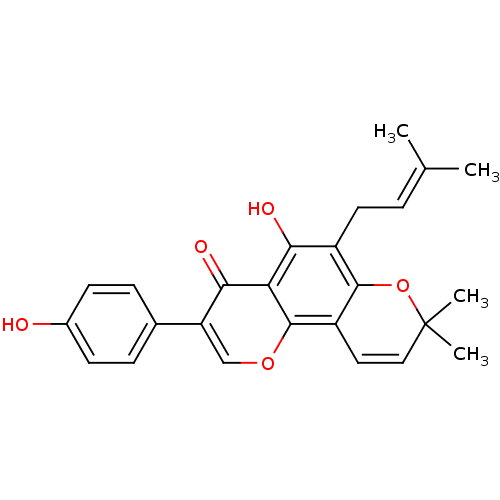 (OSAJIN) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136569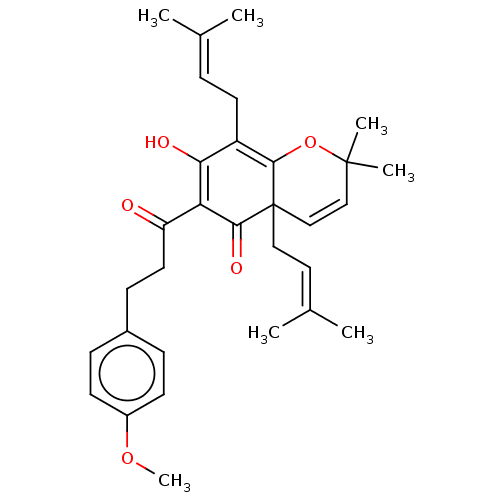 (CHEMBL3754629) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442402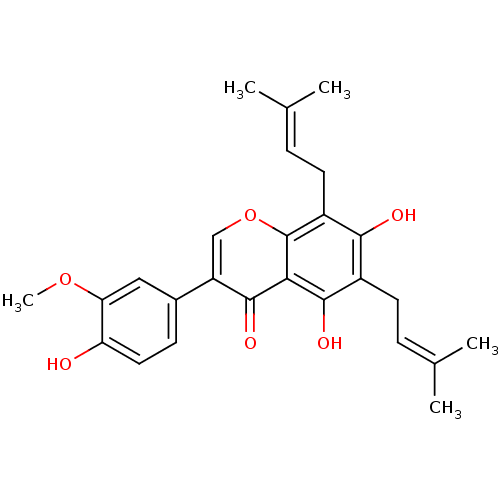 (FLEMINGSIN) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495309 (Flemichin D) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442403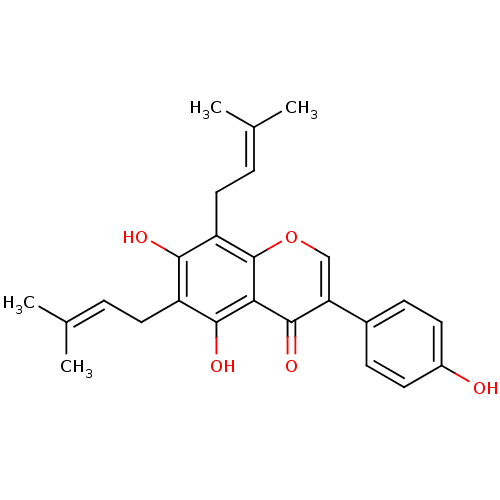 (CHEMBL494252) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256794 (CHEMBL4059800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442400 (AURICULASIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495310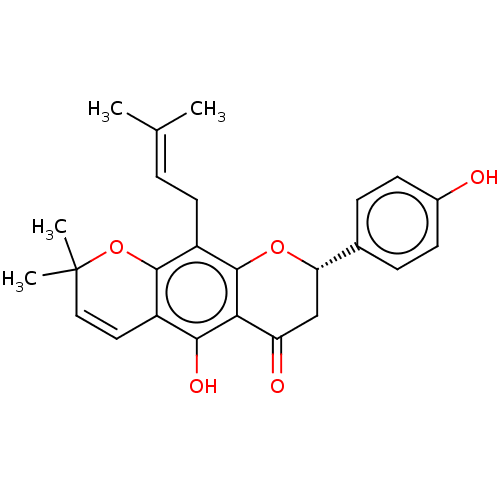 (Lupinifolin) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442398 (CHEMBL2442949) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442396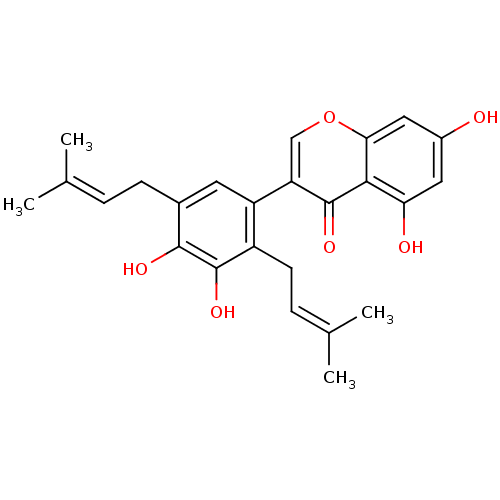 (CHEMBL2442950) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495306 (khonklonginol H) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495308 (CHEMBL3103546) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495307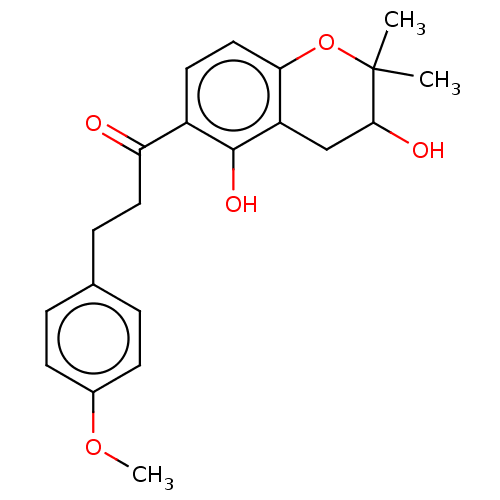 (CHEMBL3103545) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measu... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442403 (CHEMBL494252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136568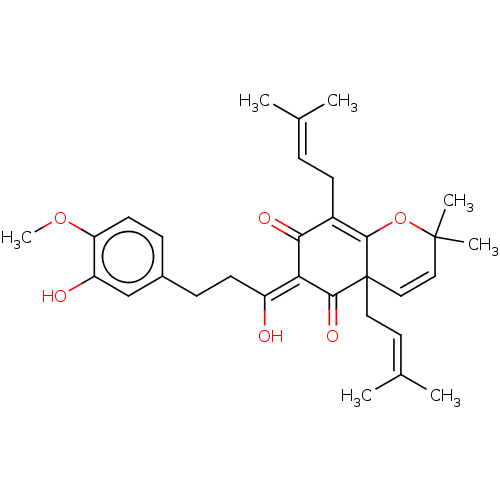 (CHEMBL3754570) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495307 (CHEMBL3103545) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50136567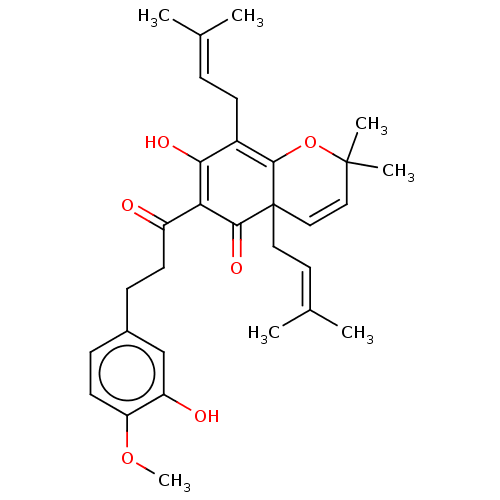 (CHEMBL3753821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442402 (FLEMINGSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256806 (CHEMBL4068458) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 60 mins followed by ... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442397 (OSAJIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plot | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495310 (Lupinifolin) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins with substrate followed by enzyme addition measured ... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442395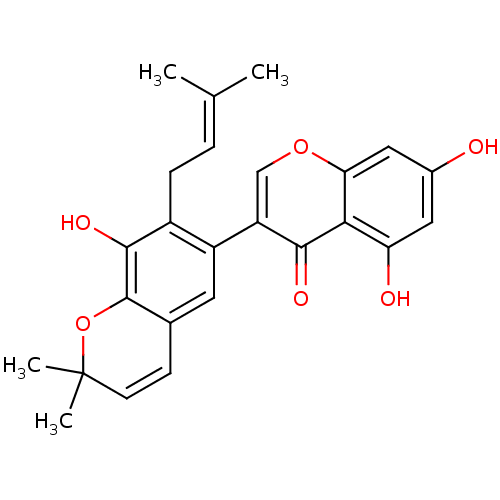 (CHEMBL2442951) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442400 (AURICULASIN) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as substrate by fl... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50366237 (GAMBOGIC ACID) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate incubated for 10 mins measured for 30... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256793 (CHEMBL4095800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate incubated for 10 mins measured for 30... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256807 (CHEMBL4069859) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate incubated for 10 mins measured for 30... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495308 (CHEMBL3103546) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measured after 15... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442401 (CHEMBL2442947) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as substrate by fl... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495305 (CHEMBL3103544) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measured after 15... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442399 (CHEMBL2442948) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as substrate by fl... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50495309 (Flemichin D) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-tyrosine as substrate preincubated for 10 mins with substrate followed by enzyme addition measured after 15... | Bioorg Med Chem 22: 1115-20 (2014) Article DOI: 10.1016/j.bmc.2013.12.047 BindingDB Entry DOI: 10.7270/Q2222XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50442401 (CHEMBL2442947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Qiqihar University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysis | Bioorg Med Chem Lett 26: 318-21 (2016) Article DOI: 10.1016/j.bmcl.2015.12.021 BindingDB Entry DOI: 10.7270/Q2C82C4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50442397 (OSAJIN) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate as substrate by fl... | Bioorg Med Chem 21: 6398-404 (2013) Article DOI: 10.1016/j.bmc.2013.08.049 BindingDB Entry DOI: 10.7270/Q2Z89DVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50256792 (Gambogenic Acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate incubated for 10 mins measured for 30... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |