 Found 273 hits with Last Name = 'tanabe' and Initial = 'g'
Found 273 hits with Last Name = 'tanabe' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612995

(CHEMBL5276586)Show SMILES Cl.[H][C@@]1(OC(OC[C@H]1O)c1ccccc1[N+]([O-])=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612998
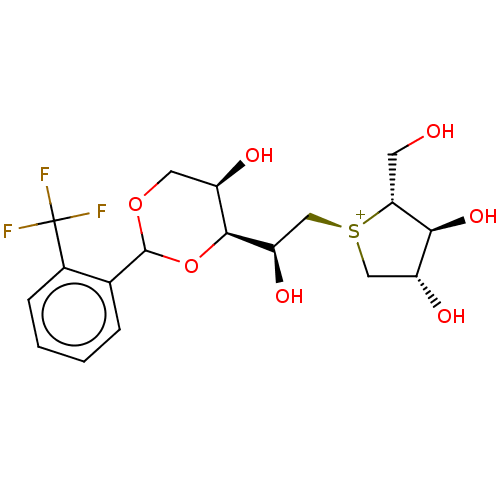
(CHEMBL5269400)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1C(F)(F)F)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50613001

(CHEMBL5268739)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1Cl)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50613004

(CHEMBL5282029)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1F)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612994

(CHEMBL5283777)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50612995

(CHEMBL5276586)Show SMILES Cl.[H][C@@]1(OC(OC[C@H]1O)c1ccccc1[N+]([O-])=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50612998

(CHEMBL5269400)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1C(F)(F)F)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50613001

(CHEMBL5268739)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1Cl)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50613004

(CHEMBL5282029)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1F)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50612994

(CHEMBL5283777)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147013
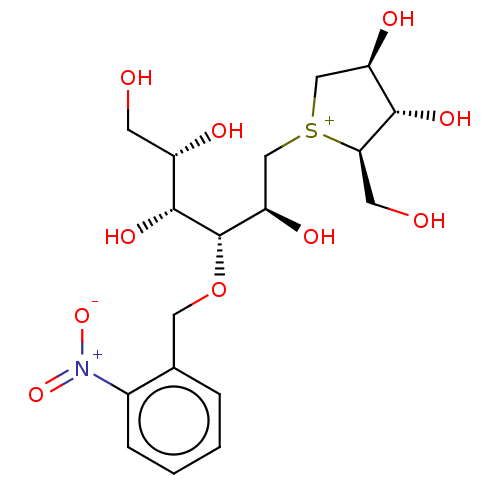
(CHEMBL3764559)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1[N+]([O-])=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C18H28NO10S.ClH/c20-5-12(22)17(26)18(29-7-10-3-1-2-4-11(10)19(27)28)14(24)9-30-8-13(23)16(25)15(30)6-21;/h1-4,12-18,20-26H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,30?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50330955
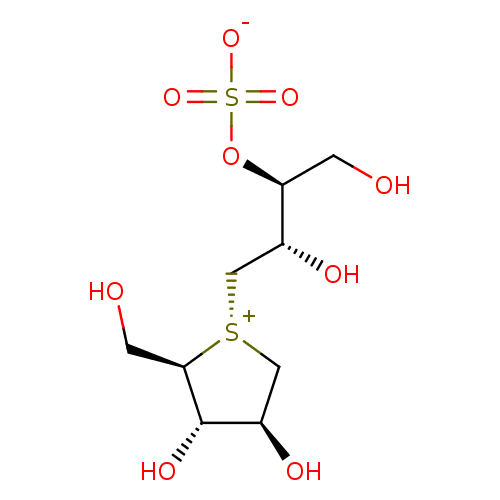
((1S,2S)-3-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymeth...)Show SMILES OC[C@H](OS([O-])(=O)=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C9H18O9S2/c10-1-7(18-20(15,16)17)5(12)3-19-4-6(13)9(14)8(19)2-11/h5-14H,1-4H2/t5-,6-,7+,8-,9+,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127751
BindingDB Entry DOI: 10.7270/Q22B92NF |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147016
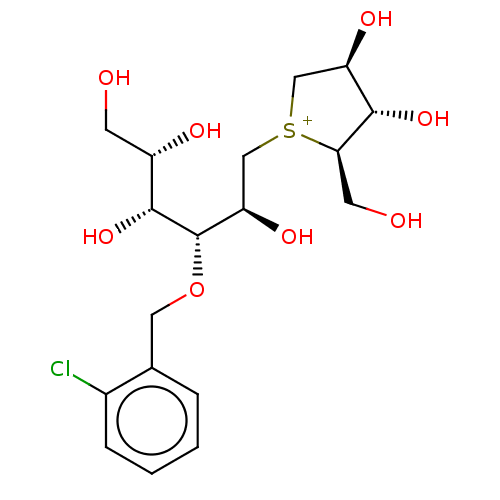
(CHEMBL3763300)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1Cl)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C18H28ClO8S.ClH/c19-11-4-2-1-3-10(11)7-27-18(17(26)12(22)5-20)14(24)9-28-8-13(23)16(25)15(28)6-21;/h1-4,12-18,20-26H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,28?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147107
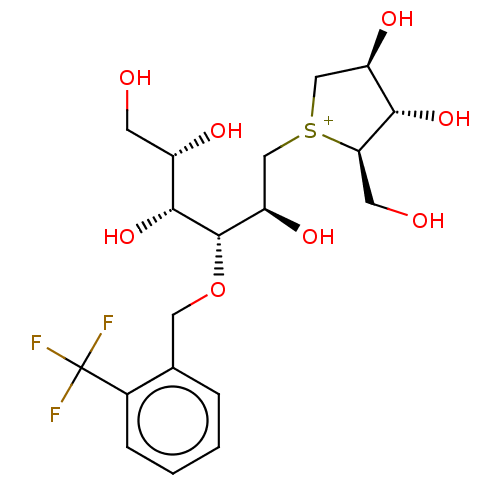
(CHEMBL3763319)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1C(F)(F)F)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C19H28F3O8S.ClH/c20-19(21,22)11-4-2-1-3-10(11)7-30-18(17(29)12(25)5-23)14(27)9-31-8-13(26)16(28)15(31)6-24;/h1-4,12-18,23-29H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,31?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147158
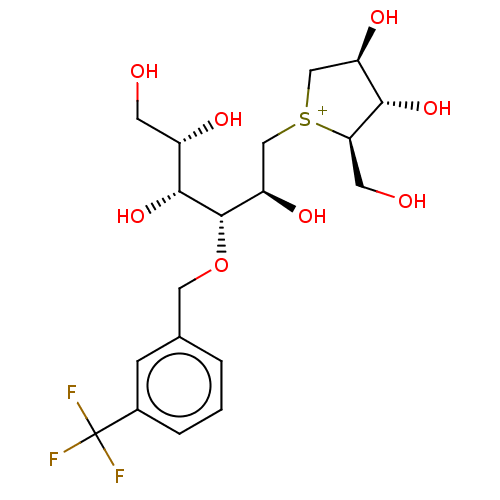
(CHEMBL3764745)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1cccc(c1)C(F)(F)F)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C19H28F3O8S.ClH/c20-19(21,22)11-3-1-2-10(4-11)7-30-18(17(29)12(25)5-23)14(27)9-31-8-13(26)16(28)15(31)6-24;/h1-4,12-18,23-29H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,31?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147159
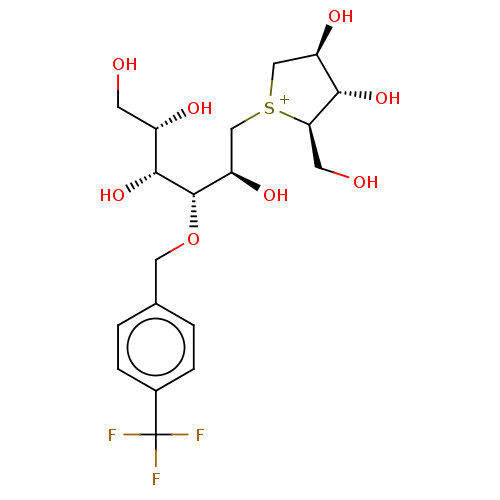
(CHEMBL3764813)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)C(F)(F)F)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C19H28F3O8S.ClH/c20-19(21,22)11-3-1-10(2-4-11)7-30-18(17(29)12(25)5-23)14(27)9-31-8-13(26)16(28)15(31)6-24;/h1-4,12-18,23-29H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,31?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by Lineweaver-Burk plot analysis |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50549692

(CHEMBL4749306)Show SMILES [Cl-].CCCCCCCCCCCCCC[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127751
BindingDB Entry DOI: 10.7270/Q22B92NF |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50549691
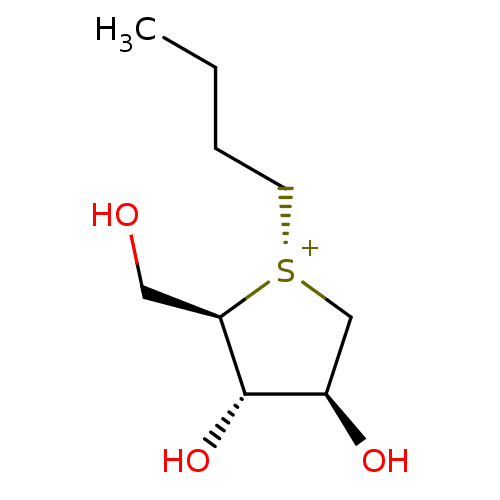
(CHEMBL4754034)Show SMILES [Cl-].CCCC[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127751
BindingDB Entry DOI: 10.7270/Q22B92NF |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50549693
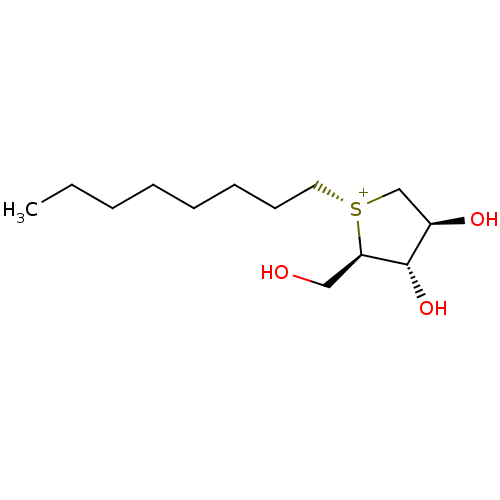
(CHEMBL4750690)Show SMILES [Cl-].CCCCCCCC[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127751
BindingDB Entry DOI: 10.7270/Q22B92NF |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180584
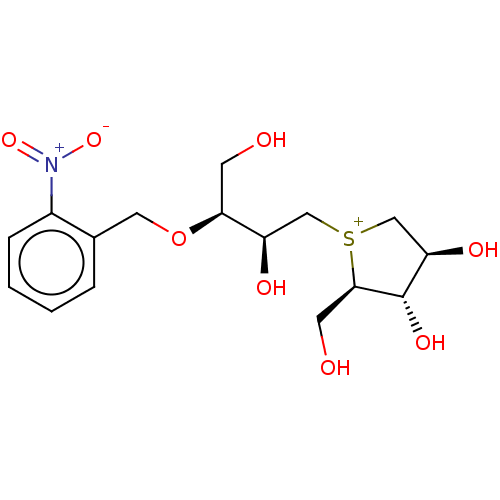
(CHEMBL3814496)Show SMILES [Cl-].OC[C@H](OCc1ccccc1[N+]([O-])=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C16H24NO8S.ClH/c18-5-14(25-7-10-3-1-2-4-11(10)17(23)24)12(20)8-26-9-13(21)16(22)15(26)6-19;/h1-4,12-16,18-22H,5-9H2;1H/q+1;/p-1/t12-,13-,14+,15-,16+,26?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180582
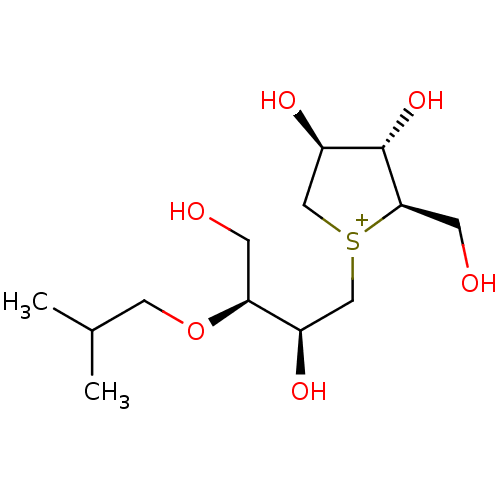
(CHEMBL3814988)Show SMILES [Cl-].CC(C)CO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C13H27O6S.ClH/c1-8(2)5-19-11(3-14)9(16)6-20-7-10(17)13(18)12(20)4-15;/h8-18H,3-7H2,1-2H3;1H/q+1;/p-1/t9-,10-,11+,12-,13+,20?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180583
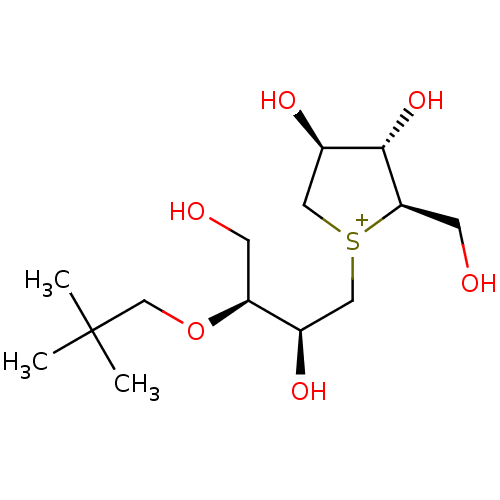
(CHEMBL3815109)Show SMILES [Cl-].CC(C)(C)CO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C14H29O6S.ClH/c1-14(2,3)8-20-11(4-15)9(17)6-21-7-10(18)13(19)12(21)5-16;/h9-13,15-19H,4-8H2,1-3H3;1H/q+1;/p-1/t9-,10-,11+,12-,13+,21?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612995

(CHEMBL5276586)Show SMILES Cl.[H][C@@]1(OC(OC[C@H]1O)c1ccccc1[N+]([O-])=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50342937

((1S,2R,3S,4S)-1-((2S,3S)-3-ethoxy-2,4-dihydroxybut...)Show SMILES CCO[C@@H](CO)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23O6S/c1-2-17-9(3-12)7(14)5-18-6-8(15)11(16)10(18)4-13/h7-16H,2-6H2,1H3/q+1/t7-,8-,9+,10-,11+,18-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase after 30 mins by glucose-oxidase method |
Bioorg Med Chem Lett 21: 3159-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.109
BindingDB Entry DOI: 10.7270/Q2DF6RJC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180520
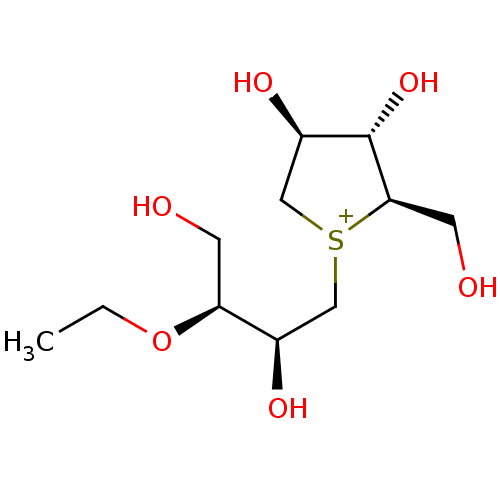
(CHEMBL3814838)Show SMILES [Cl-].CCO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23O6S.ClH/c1-2-17-9(3-12)7(14)5-18-6-8(15)11(16)10(18)4-13;/h7-16H,2-6H2,1H3;1H/q+1;/p-1/t7-,8-,9+,10-,11+,18?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50342939

((1S,2R,3S,4S)-1-((2S,3S)-3-(benzyloxy)-2,4-dihydro...)Show SMILES OC[C@H](OCc1ccccc1)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C16H25O6S/c17-6-14(22-8-11-4-2-1-3-5-11)12(19)9-23-10-13(20)16(21)15(23)7-18/h1-5,12-21H,6-10H2/q+1/t12-,13-,14+,15-,16+,23-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal isomaltase after 30 mins by glucose-oxidase method |
Bioorg Med Chem Lett 21: 3159-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.109
BindingDB Entry DOI: 10.7270/Q2DF6RJC |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50180584

(CHEMBL3814496)Show SMILES [Cl-].OC[C@H](OCc1ccccc1[N+]([O-])=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C16H24NO8S.ClH/c18-5-14(25-7-10-3-1-2-4-11(10)17(23)24)12(20)8-26-9-13(21)16(22)15(26)6-19;/h1-4,12-16,18-22H,5-9H2;1H/q+1;/p-1/t12-,13-,14+,15-,16+,26?;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of human small intestine microsomal maltase using maltose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50263044
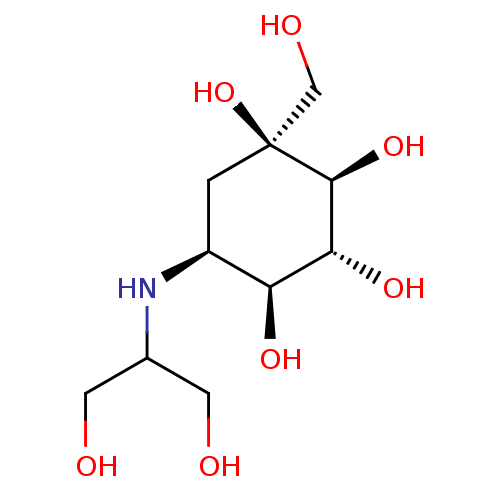
(CHEMBL476960 | Voglibose)Show SMILES OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase |
Bioorg Med Chem 19: 2015-22 (2011)
Article DOI: 10.1016/j.bmc.2011.01.052
BindingDB Entry DOI: 10.7270/Q2J1044C |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50263044

(CHEMBL476960 | Voglibose)Show SMILES OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase after 30 mins by glucose-oxidase method |
Bioorg Med Chem Lett 21: 3159-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.109
BindingDB Entry DOI: 10.7270/Q2DF6RJC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50263044

(CHEMBL476960 | Voglibose)Show SMILES OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50263044

(CHEMBL476960 | Voglibose)Show SMILES OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal maltase after 30 mins |
Bioorg Med Chem 19: 2252-62 (2011)
Article DOI: 10.1016/j.bmc.2011.02.028
BindingDB Entry DOI: 10.7270/Q2GH9J8R |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147107

(CHEMBL3763319)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1C(F)(F)F)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C19H28F3O8S.ClH/c20-19(21,22)11-4-2-1-3-10(11)7-30-18(17(29)12(25)5-23)14(27)9-31-8-13(26)16(28)15(31)6-24;/h1-4,12-18,23-29H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,31?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50612995

(CHEMBL5276586)Show SMILES Cl.[H][C@@]1(OC(OC[C@H]1O)c1ccccc1[N+]([O-])=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147014
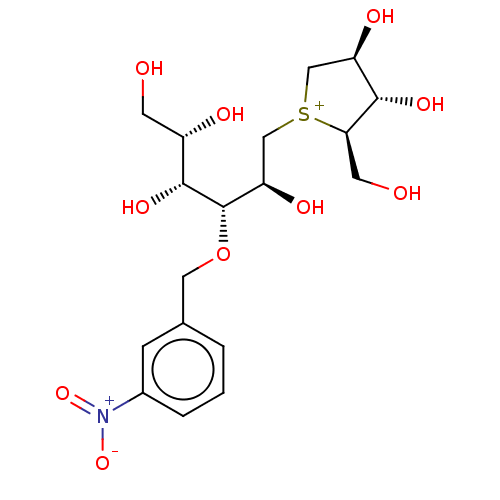
(CHEMBL3764223)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](OCc1cccc(c1)[N+]([O-])=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C18H28NO10S.ClH/c20-5-12(22)17(26)18(29-7-10-2-1-3-11(4-10)19(27)28)14(24)9-30-8-13(23)16(25)15(30)6-21;/h1-4,12-18,20-26H,5-9H2;1H/q+1;/p-1/t12-,13+,14+,15+,16-,17+,18-,30?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180584

(CHEMBL3814496)Show SMILES [Cl-].OC[C@H](OCc1ccccc1[N+]([O-])=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C16H24NO8S.ClH/c18-5-14(25-7-10-3-1-2-4-11(10)17(23)24)12(20)8-26-9-13(21)16(22)15(26)6-19;/h1-4,12-16,18-22H,5-9H2;1H/q+1;/p-1/t12-,13-,14+,15-,16+,26?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612998

(CHEMBL5269400)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1C(F)(F)F)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50612998

(CHEMBL5269400)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1C(F)(F)F)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180580
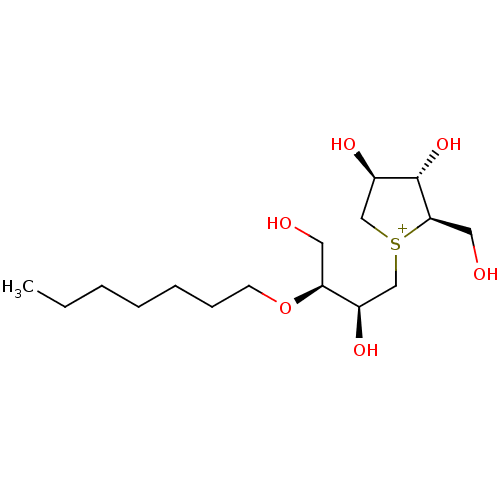
(CHEMBL3814911)Show SMILES [Cl-].CCCCCCCO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C16H33O6S.ClH/c1-2-3-4-5-6-7-22-14(8-17)12(19)10-23-11-13(20)16(21)15(23)9-18;/h12-21H,2-11H2,1H3;1H/q+1;/p-1/t12-,13-,14+,15-,16+,23?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180580

(CHEMBL3814911)Show SMILES [Cl-].CCCCCCCO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C16H33O6S.ClH/c1-2-3-4-5-6-7-22-14(8-17)12(19)10-23-11-13(20)16(21)15(23)9-18;/h12-21H,2-11H2,1H3;1H/q+1;/p-1/t12-,13-,14+,15-,16+,23?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50342937

((1S,2R,3S,4S)-1-((2S,3S)-3-ethoxy-2,4-dihydroxybut...)Show SMILES CCO[C@@H](CO)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23O6S/c1-2-17-9(3-12)7(14)5-18-6-8(15)11(16)10(18)4-13/h7-16H,2-6H2,1H3/q+1/t7-,8-,9+,10-,11+,18-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal isomaltase after 30 mins by glucose-oxidase method |
Bioorg Med Chem Lett 21: 3159-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.109
BindingDB Entry DOI: 10.7270/Q2DF6RJC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180520

(CHEMBL3814838)Show SMILES [Cl-].CCO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23O6S.ClH/c1-2-17-9(3-12)7(14)5-18-6-8(15)11(16)10(18)4-13;/h7-16H,2-6H2,1H3;1H/q+1;/p-1/t7-,8-,9+,10-,11+,18?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147012
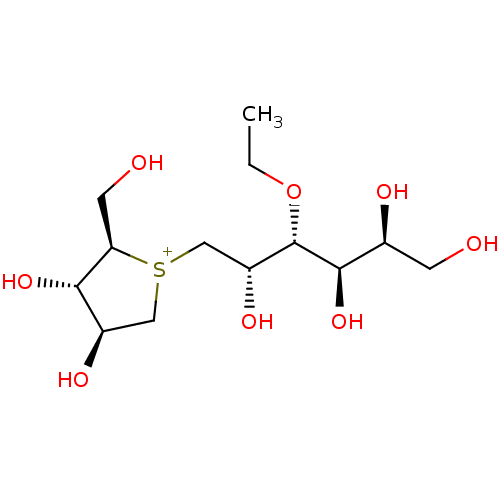
(CHEMBL3763591)Show SMILES [Cl-].CCO[C@@H]([C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO)[C@H](O)[C@@H](O)CO |r| Show InChI InChI=1S/C13H27O8S.ClH/c1-2-21-13(12(20)7(16)3-14)9(18)6-22-5-8(17)11(19)10(22)4-15;/h7-20H,2-6H2,1H3;1H/q+1;/p-1/t7-,8+,9+,10+,11-,12+,13-,22?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180583

(CHEMBL3815109)Show SMILES [Cl-].CC(C)(C)CO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C14H29O6S.ClH/c1-14(2,3)8-20-11(4-15)9(17)6-21-7-10(18)13(19)12(21)5-16;/h9-13,15-19H,4-8H2,1-3H3;1H/q+1;/p-1/t9-,10-,11+,12-,13+,21?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50327501
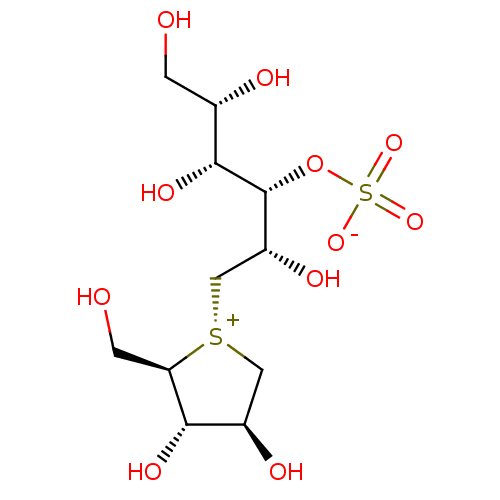
(CHEMBL1258528 | ponkoranol)Show SMILES OC[C@H](O)[C@@H](O)[C@H](OS([O-])(=O)=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H22O11S2/c12-1-5(14)10(18)11(22-24(19,20)21)7(16)4-23-3-6(15)9(17)8(23)2-13/h5-18H,1-4H2/t5-,6+,7+,8+,9-,10+,11+,23-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase |
Bioorg Med Chem 19: 2015-22 (2011)
Article DOI: 10.1016/j.bmc.2011.01.052
BindingDB Entry DOI: 10.7270/Q2J1044C |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50338672
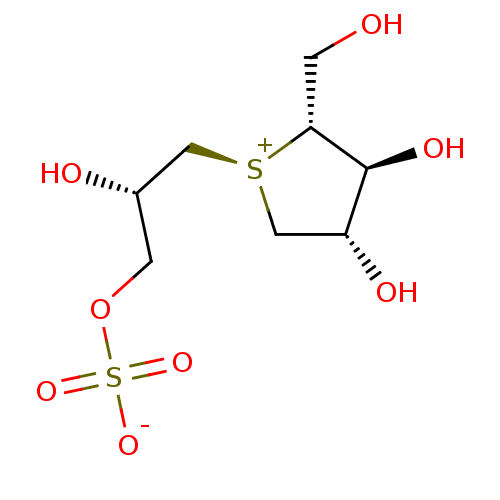
(CHEMBL1209027 | Salaprinol)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)C[S@+]1C[C@@H](O)COS([O-])(=O)=O |r| Show InChI InChI=1S/C8H16O8S2/c9-1-7-8(12)6(11)4-17(7)3-5(10)2-16-18(13,14)15/h5-12H,1-4H2/t5-,6+,7+,8-,17+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase after 30 mins |
Bioorg Med Chem 19: 2252-62 (2011)
Article DOI: 10.1016/j.bmc.2011.02.028
BindingDB Entry DOI: 10.7270/Q2GH9J8R |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180586

(CHEMBL1182462)Show SMILES OC[C@H](O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C9H19O6S/c10-1-5(12)6(13)3-16-4-7(14)9(15)8(16)2-11/h5-15H,1-4H2/q+1/t5-,6+,7+,8+,9-,16?/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal isomaltase using isomaltose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50338676

(1,4-Dideoxy-1,4-{(R)-[(2S,3R,4R,5S)-2,3,4,5,6-pent...)Show SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23O8S/c12-1-5(14)10(18)11(19)7(16)4-20-3-6(15)9(17)8(20)2-13/h5-19H,1-4H2/q+1/t5-,6+,7+,8+,9-,10+,11-,20-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase |
Bioorg Med Chem 19: 2015-22 (2011)
Article DOI: 10.1016/j.bmc.2011.01.052
BindingDB Entry DOI: 10.7270/Q2J1044C |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50338669
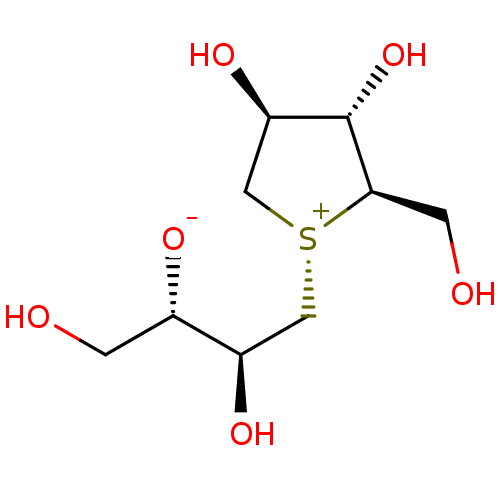
(CHEMBL1684162 | Neosalacinol)Show SMILES OC[C@H]([O-])[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C9H18O6S/c10-1-5(12)6(13)3-16-4-7(14)9(15)8(16)2-11/h5-11,13-15H,1-4H2/t5-,6+,7+,8+,9-,16+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal isomaltase |
Bioorg Med Chem 19: 2015-22 (2011)
Article DOI: 10.1016/j.bmc.2011.01.052
BindingDB Entry DOI: 10.7270/Q2J1044C |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612999
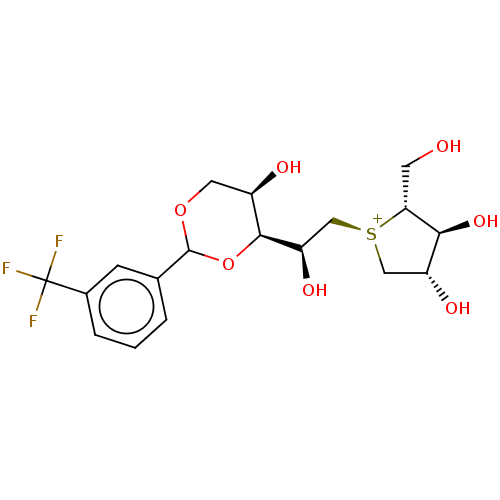
(CHEMBL5277322)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1cccc(c1)C(F)(F)F)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50147008
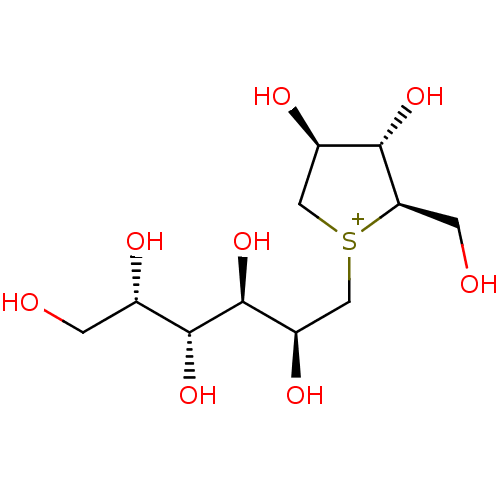
(CHEMBL3765363)Show SMILES [Cl-].OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23O8S.ClH/c12-1-5(14)10(18)11(19)7(16)4-20-3-6(15)9(17)8(20)2-13;/h5-19H,1-4H2;1H/q+1;/p-1/t5-,6+,7+,8+,9-,10+,11-,20?;/m0./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Eur J Med Chem 110: 224-36 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.029
BindingDB Entry DOI: 10.7270/Q2NS0WSH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data