

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389606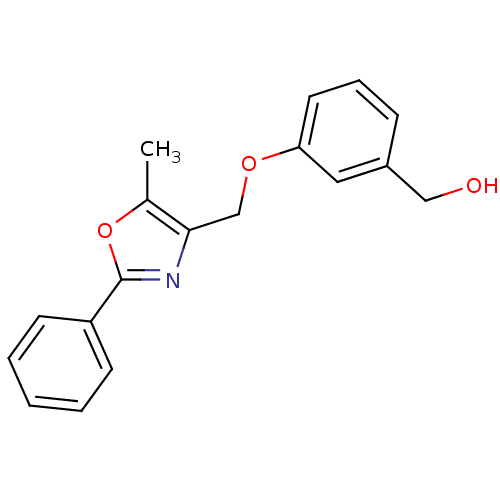 (CHEMBL2069625) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human unphosphorylated N-terminal-His6 tagged VEGFR2 catalytic domain using 5-FAM-EEPLYWSFPAKKK-CONH2 as substrate after 60 mins by mob... | ACS Med Chem Lett 3: 342-346 (2012) Article DOI: 10.1021/ml3000403 BindingDB Entry DOI: 10.7270/Q2FN1782 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50254020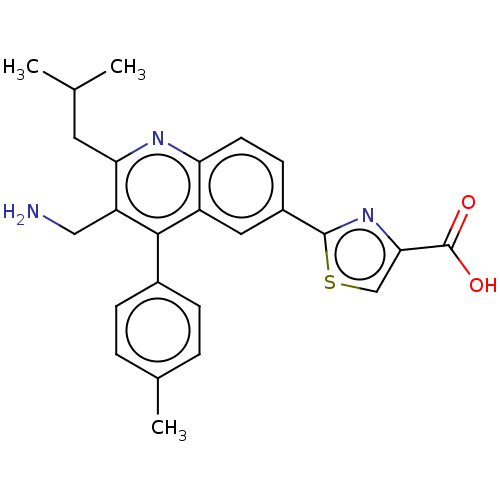 (CHEMBL4068446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human DPP4 purified from Caco2 cells pre-incubated for 15 mins before Gly-Pro-pNA substrate addition and measured after 60 mins | Bioorg Med Chem Lett 27: 3565-3571 (2017) Article DOI: 10.1016/j.bmcl.2017.05.048 BindingDB Entry DOI: 10.7270/Q2PK0JKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195224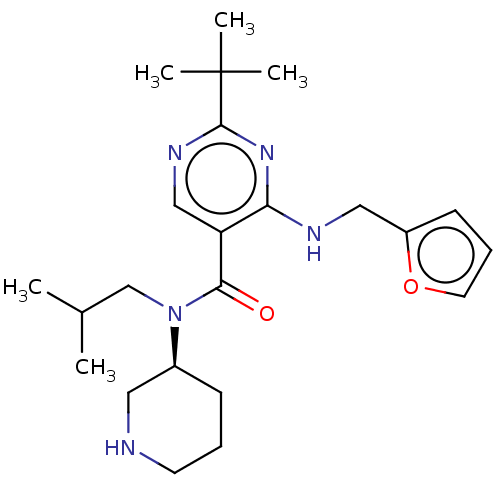 (CHEMBL3916240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin expressed in FreeStyle 293 expression system using recombinant human angiotensinogen as substrate preincubated ... | Bioorg Med Chem 24: 5771-5780 (2016) Article DOI: 10.1016/j.bmc.2016.09.030 BindingDB Entry DOI: 10.7270/Q22V2J24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444208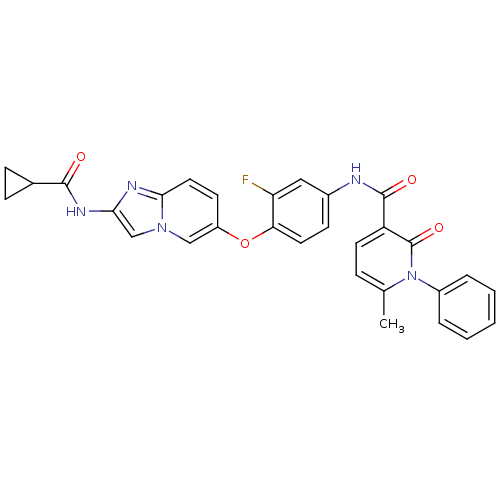 (CHEMBL3093579 | D3RKN_15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 60 mins followed by 1000 uM of ATP addition m... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444209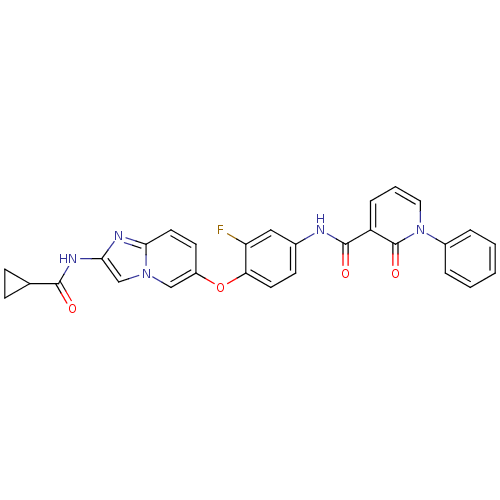 (CHEMBL3093581) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 60 mins followed by 1000 uM of ATP addition m... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444210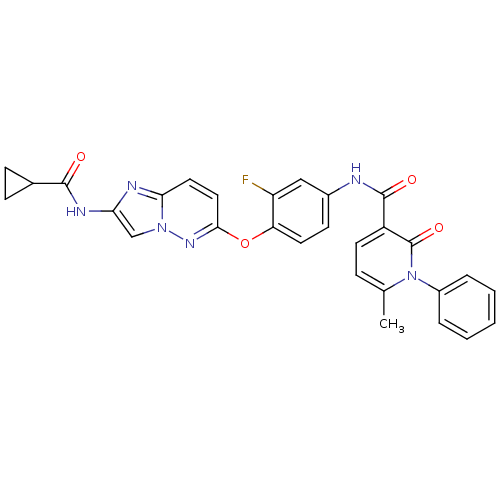 (CHEMBL3093582) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 60 mins followed by 1000 uM of ATP addition m... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50357496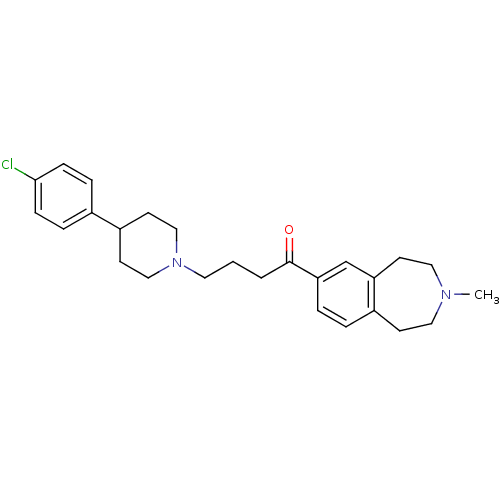 (CHEMBL1914623) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-MCH(4-19) from human MCHR1 expressed in CHO cells | Bioorg Med Chem 19: 6261-73 (2011) Article DOI: 10.1016/j.bmc.2011.09.007 BindingDB Entry DOI: 10.7270/Q26T0N2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267928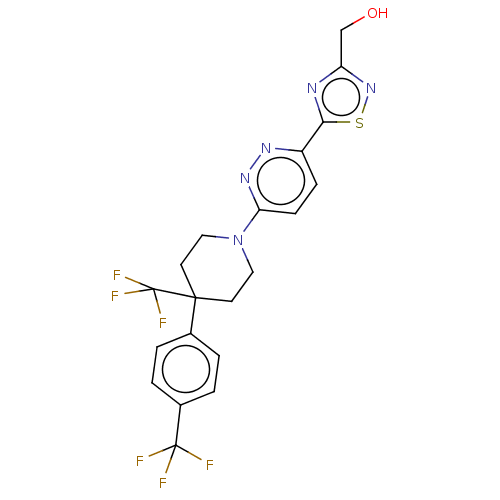 (CHEMBL4066506) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50357495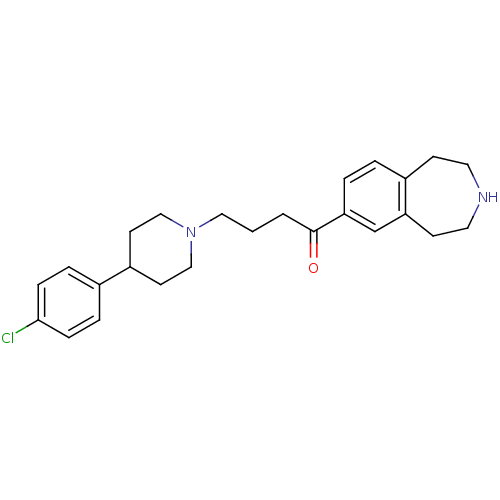 (CHEMBL1914630) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-MCH(4-19) from human MCHR1 expressed in CHO cells | Bioorg Med Chem 19: 6261-73 (2011) Article DOI: 10.1016/j.bmc.2011.09.007 BindingDB Entry DOI: 10.7270/Q26T0N2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195228 (CHEMBL3925242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin expressed in FreeStyle 293 expression system using recombinant human angiotensinogen as substrate preincubated ... | Bioorg Med Chem 24: 5771-5780 (2016) Article DOI: 10.1016/j.bmc.2016.09.030 BindingDB Entry DOI: 10.7270/Q22V2J24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50336806 (1-(3-(aminomethyl)-2-isobutyl-4-p-tolylquinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase-4 expressed in Caco-2 cells using Gly-Pro-pNA.Tos as substrate after 60 mins by microplate reader analysis | Bioorg Med Chem 19: 4482-98 (2011) Article DOI: 10.1016/j.bmc.2011.06.032 BindingDB Entry DOI: 10.7270/Q2P26ZGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50351312 (CHEMBL1818800) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50351322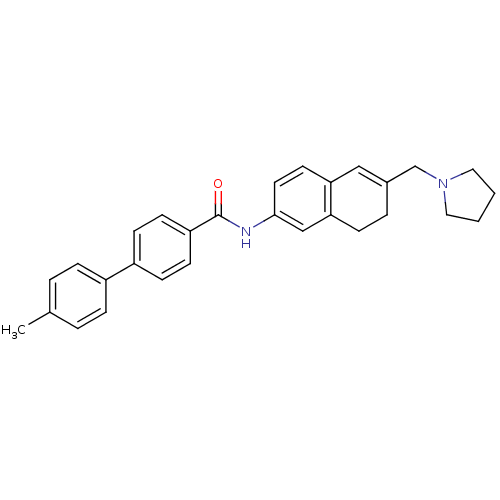 (CHEMBL1818810) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444212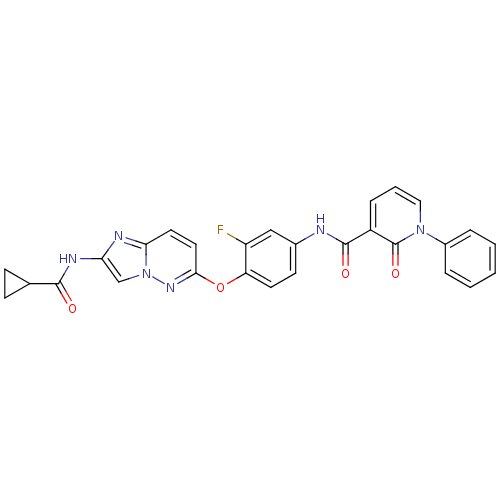 (CHEMBL3093583 | D3RKN_14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444209 (CHEMBL3093581) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50351308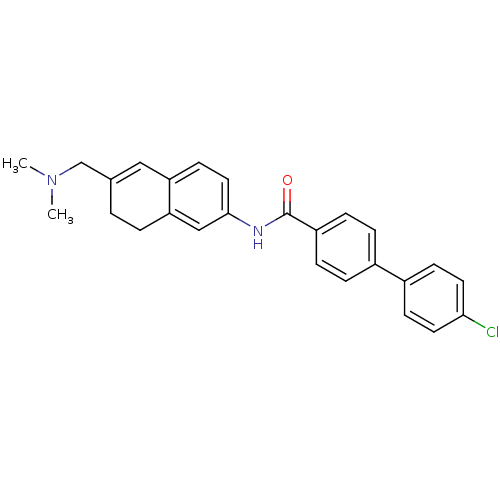 (CHEMBL1818795) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50349380 (CHEMBL1808468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase-4 expressed in Caco-2 cells using Gly-Pro-pNA.Tos as substrate after 60 mins by microplate reader analysis | Bioorg Med Chem 19: 4482-98 (2011) Article DOI: 10.1016/j.bmc.2011.06.032 BindingDB Entry DOI: 10.7270/Q2P26ZGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444210 (CHEMBL3093582) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50351324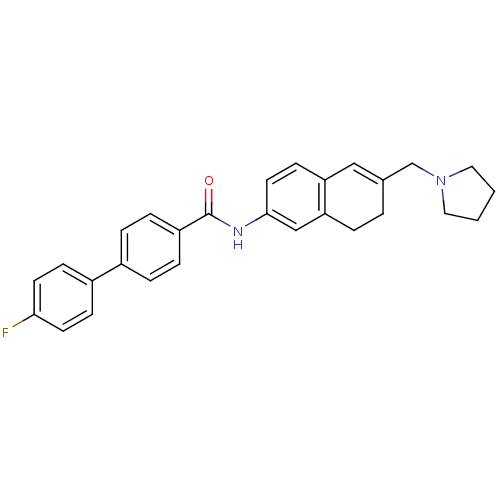 (CHEMBL1818901) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444208 (CHEMBL3093579 | D3RKN_15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50351313 (CHEMBL1818801) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195233 (CHEMBL3897200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin expressed in FreeStyle 293 expression system using recombinant human angiotensinogen as substrate preincubated ... | Bioorg Med Chem 24: 5771-5780 (2016) Article DOI: 10.1016/j.bmc.2016.09.030 BindingDB Entry DOI: 10.7270/Q22V2J24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253979 (CHEMBL4068477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human DPP4 purified from Caco2 cells pre-incubated for 15 mins before Gly-Pro-pNA substrate addition and measured after 60 mins | Bioorg Med Chem Lett 27: 3565-3571 (2017) Article DOI: 10.1016/j.bmcl.2017.05.048 BindingDB Entry DOI: 10.7270/Q2PK0JKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50351323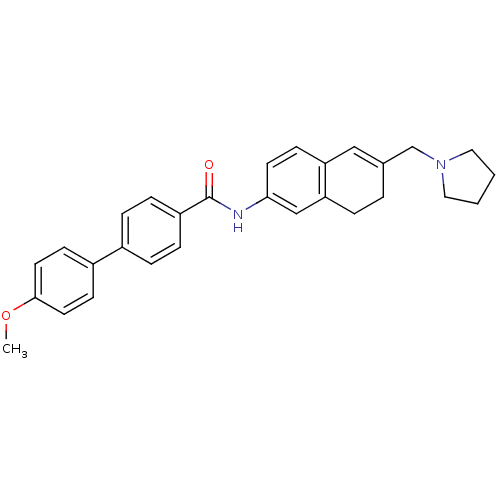 (CHEMBL1818811) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50349384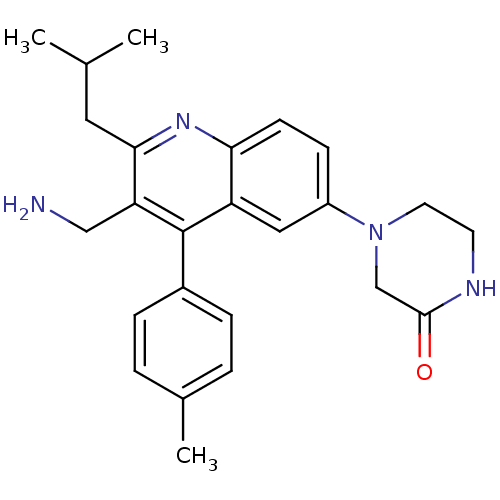 (CHEMBL1808473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase-4 expressed in Caco-2 cells using Gly-Pro-pNA.Tos as substrate after 60 mins by microplate reader analysis | Bioorg Med Chem 19: 4482-98 (2011) Article DOI: 10.1016/j.bmc.2011.06.032 BindingDB Entry DOI: 10.7270/Q2P26ZGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50351312 (CHEMBL1818800) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from rat MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444213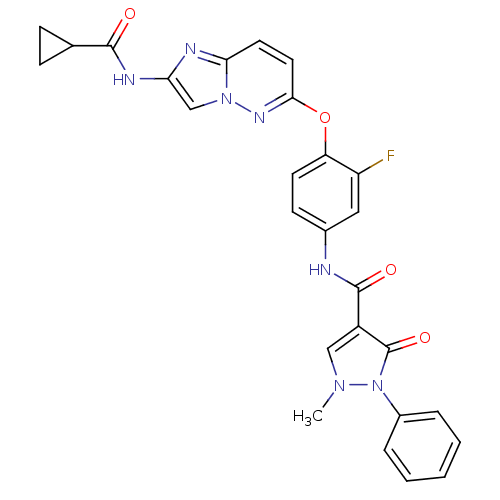 (CHEMBL3093585) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267930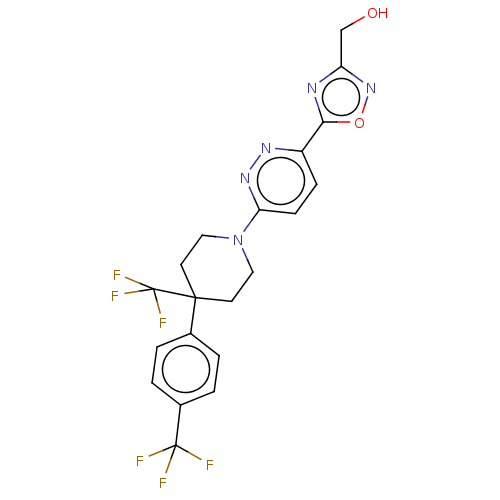 (CHEMBL4104190) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50351322 (CHEMBL1818810) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from rat MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267945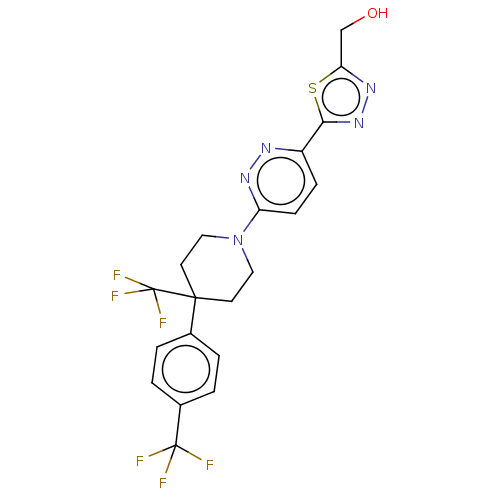 (CHEMBL4094042) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50351313 (CHEMBL1818801) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from rat MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50254019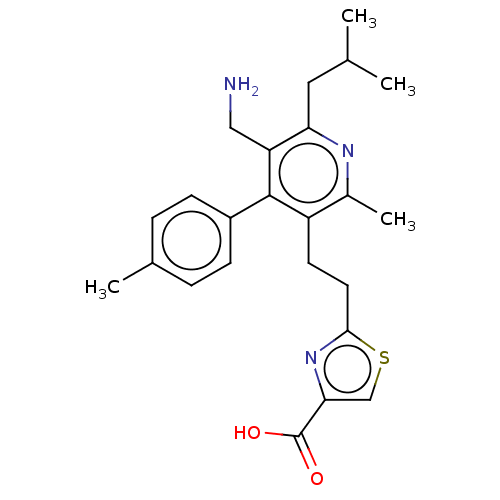 (CHEMBL4098218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human DPP4 purified from Caco2 cells pre-incubated for 15 mins before Gly-Pro-pNA substrate addition and measured after 60 mins | Bioorg Med Chem Lett 27: 3565-3571 (2017) Article DOI: 10.1016/j.bmcl.2017.05.048 BindingDB Entry DOI: 10.7270/Q2PK0JKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50349385 (CHEMBL1808474) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase-4 expressed in Caco-2 cells using Gly-Pro-pNA.Tos as substrate after 60 mins by microplate reader analysis | Bioorg Med Chem 19: 4482-98 (2011) Article DOI: 10.1016/j.bmc.2011.06.032 BindingDB Entry DOI: 10.7270/Q2P26ZGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50351308 (CHEMBL1818795) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from rat MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50357502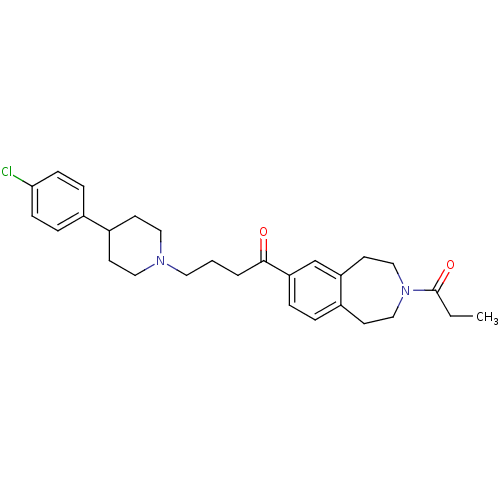 (CHEMBL1914631) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at human MCHR1 expressed in CHO cells assessed as inhibition of MCH-stimulated arachidonic acid release | Bioorg Med Chem 19: 6261-73 (2011) Article DOI: 10.1016/j.bmc.2011.09.007 BindingDB Entry DOI: 10.7270/Q26T0N2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50351314 (CHEMBL1818802) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50452856 (CHEMBL4217811) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of BODIPY-labeled ligand from human His-tagged RORgammat after 20 mins by TR-FRET assay | Bioorg Med Chem 26: 470-482 (2018) Article DOI: 10.1016/j.bmc.2017.12.008 BindingDB Entry DOI: 10.7270/Q2GQ71BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50452853 (CHEMBL4087736) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of BODIPY-labeled ligand from human His-tagged RORgammat after 20 mins by TR-FRET assay | Bioorg Med Chem 26: 470-482 (2018) Article DOI: 10.1016/j.bmc.2017.12.008 BindingDB Entry DOI: 10.7270/Q2GQ71BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50452850 (CHEMBL4203255) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of BODIPY-labeled ligand from human His-tagged RORgammat after 20 mins by TR-FRET assay | Bioorg Med Chem 26: 470-482 (2018) Article DOI: 10.1016/j.bmc.2017.12.008 BindingDB Entry DOI: 10.7270/Q2GQ71BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444214 (CHEMBL3093587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 5 mins followed by 2 uM of ATP addition measu... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50195227 (CHEMBL3986004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin expressed in FreeStyle 293 expression system using recombinant human angiotensinogen as substrate preincubated ... | Bioorg Med Chem 24: 5771-5780 (2016) Article DOI: 10.1016/j.bmc.2016.09.030 BindingDB Entry DOI: 10.7270/Q22V2J24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50452854 (CHEMBL4205112) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of BODIPY-labeled ligand from human His-tagged RORgammat after 20 mins by TR-FRET assay | Bioorg Med Chem 26: 470-482 (2018) Article DOI: 10.1016/j.bmc.2017.12.008 BindingDB Entry DOI: 10.7270/Q2GQ71BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50351323 (CHEMBL1818811) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from rat MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50452843 (CHEMBL4217478) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of BODIPY-labeled ligand from human His-tagged RORgammat after 20 mins by TR-FRET assay | Bioorg Med Chem 26: 470-482 (2018) Article DOI: 10.1016/j.bmc.2017.12.008 BindingDB Entry DOI: 10.7270/Q2GQ71BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50357504 (CHEMBL1914633) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-MCH(4-19) from human MCHR1 expressed in CHO cells | Bioorg Med Chem 19: 6261-73 (2011) Article DOI: 10.1016/j.bmc.2011.09.007 BindingDB Entry DOI: 10.7270/Q26T0N2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444212 (CHEMBL3093583 | D3RKN_14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using biotinylated poly-GluTyr (4:1) as substrate preincubated for 60 mins followed by 1000 uM of ATP addition m... | Bioorg Med Chem 21: 7686-98 (2013) Article DOI: 10.1016/j.bmc.2013.10.028 BindingDB Entry DOI: 10.7270/Q28W3FRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50351324 (CHEMBL1818901) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]-MCH from rat MCHR1 expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 19: 5539-52 (2011) Article DOI: 10.1016/j.bmc.2011.07.038 BindingDB Entry DOI: 10.7270/Q26H4HT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50452840 (CHEMBL4209940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of BODIPY-labeled ligand from human His-tagged RORgammat after 20 mins by TR-FRET assay | Bioorg Med Chem 26: 470-482 (2018) Article DOI: 10.1016/j.bmc.2017.12.008 BindingDB Entry DOI: 10.7270/Q2GQ71BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50349376 (CHEMBL1808464) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase-4 expressed in Caco-2 cells using Gly-Pro-pNA.Tos as substrate after 60 mins by microplate reader analysis | Bioorg Med Chem 19: 4482-98 (2011) Article DOI: 10.1016/j.bmc.2011.06.032 BindingDB Entry DOI: 10.7270/Q2P26ZGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50452846 (CHEMBL4205579) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of BODIPY-labeled ligand from human His-tagged RORgammat after 20 mins by TR-FRET assay | Bioorg Med Chem 26: 470-482 (2018) Article DOI: 10.1016/j.bmc.2017.12.008 BindingDB Entry DOI: 10.7270/Q2GQ71BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 414 total ) | Next | Last >> |