 Found 68 hits with Last Name = 'teall' and Initial = 'mr'
Found 68 hits with Last Name = 'teall' and Initial = 'mr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290032
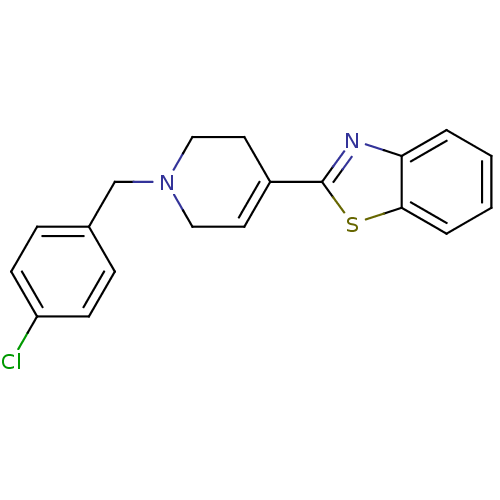
(2-[1-(4-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccccc3s2)cc1 |c:9| Show InChI InChI=1S/C19H17ClN2S/c20-16-7-5-14(6-8-16)13-22-11-9-15(10-12-22)19-21-17-3-1-2-4-18(17)23-19/h1-9H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290030
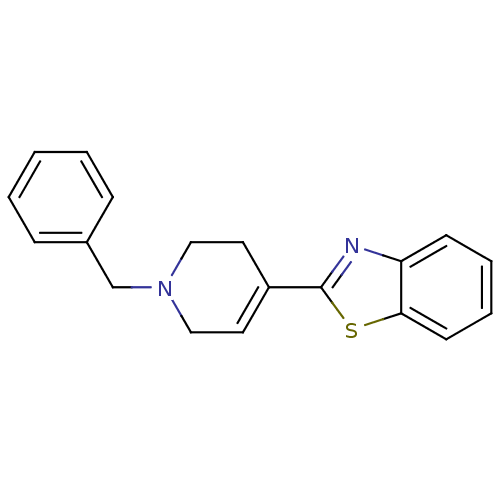
(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-benzo...)Show InChI InChI=1S/C19H18N2S/c1-2-6-15(7-3-1)14-21-12-10-16(11-13-21)19-20-17-8-4-5-9-18(17)22-19/h1-10H,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290034
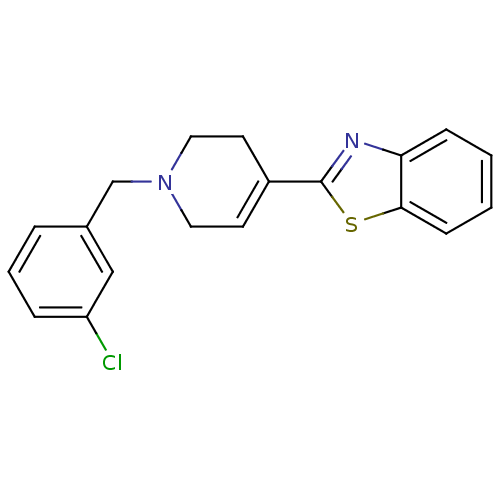
(2-[1-(3-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1cccc(CN2CCC(=CC2)c2nc3ccccc3s2)c1 |c:10| Show InChI InChI=1S/C19H17ClN2S/c20-16-5-3-4-14(12-16)13-22-10-8-15(9-11-22)19-21-17-6-1-2-7-18(17)23-19/h1-8,12H,9-11,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290025
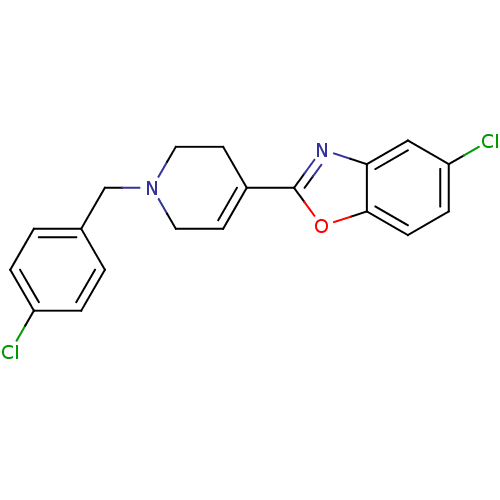
(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3cc(Cl)ccc3o2)cc1 |c:9| Show InChI InChI=1S/C19H16Cl2N2O/c20-15-3-1-13(2-4-15)12-23-9-7-14(8-10-23)19-22-17-11-16(21)5-6-18(17)24-19/h1-7,11H,8-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290029
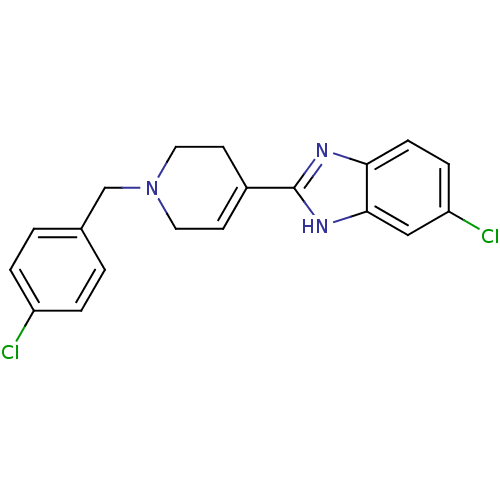
(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccc(Cl)cc3[nH]2)cc1 |c:9| Show InChI InChI=1S/C19H17Cl2N3/c20-15-3-1-13(2-4-15)12-24-9-7-14(8-10-24)19-22-17-6-5-16(21)11-18(17)23-19/h1-7,11H,8-10,12H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290036
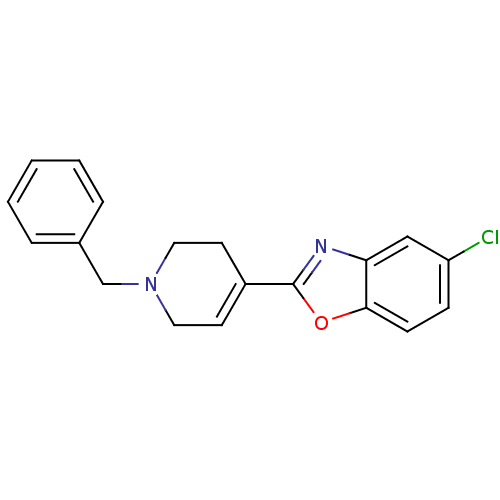
(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2oc(nc2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H17ClN2O/c20-16-6-7-18-17(12-16)21-19(23-18)15-8-10-22(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290031
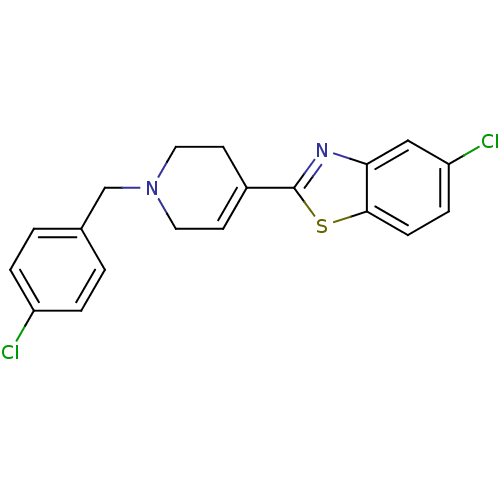
(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3cc(Cl)ccc3s2)cc1 |c:9| Show InChI InChI=1S/C19H16Cl2N2S/c20-15-3-1-13(2-4-15)12-23-9-7-14(8-10-23)19-22-17-11-16(21)5-6-18(17)24-19/h1-7,11H,8-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290028
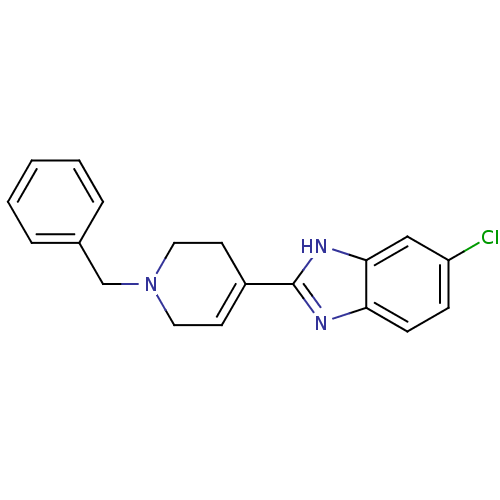
(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2nc([nH]c2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H18ClN3/c20-16-6-7-17-18(12-16)22-19(21-17)15-8-10-23(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290035
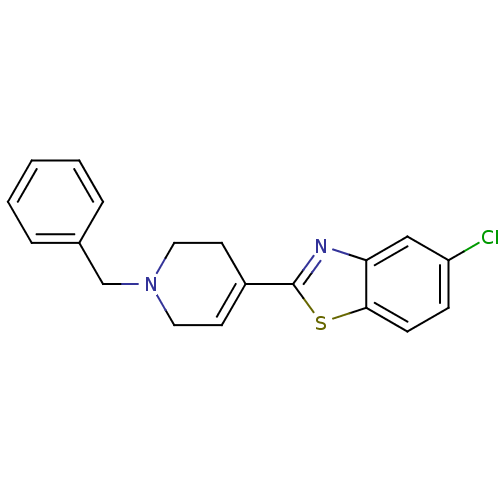
(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2sc(nc2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H17ClN2S/c20-16-6-7-18-17(12-16)21-19(23-18)15-8-10-22(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290037
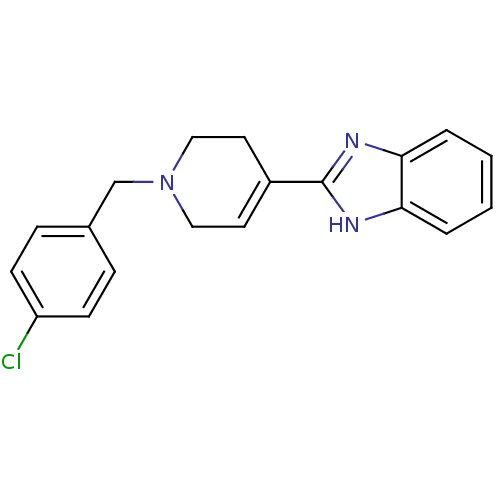
(2-[1-(4-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccccc3[nH]2)cc1 |c:9| Show InChI InChI=1S/C19H18ClN3/c20-16-7-5-14(6-8-16)13-23-11-9-15(10-12-23)19-21-17-3-1-2-4-18(17)22-19/h1-9H,10-13H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290033
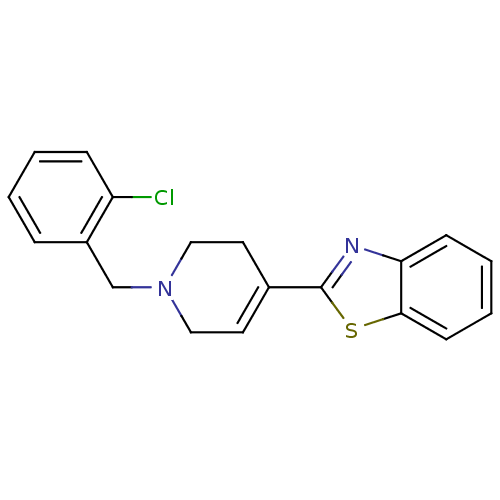
(2-[1-(2-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show InChI InChI=1S/C19H17ClN2S/c20-16-6-2-1-5-15(16)13-22-11-9-14(10-12-22)19-21-17-7-3-4-8-18(17)23-19/h1-9H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290026
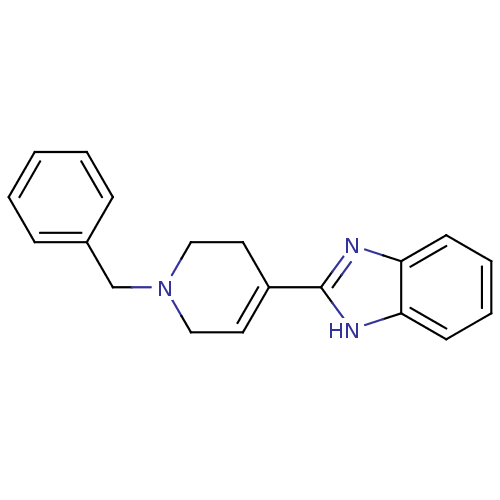
(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-1H-be...)Show SMILES C(N1CCC(=CC1)c1nc2ccccc2[nH]1)c1ccccc1 |c:4| Show InChI InChI=1S/C19H19N3/c1-2-6-15(7-3-1)14-22-12-10-16(11-13-22)19-20-17-8-4-5-9-18(17)21-19/h1-10H,11-14H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290027
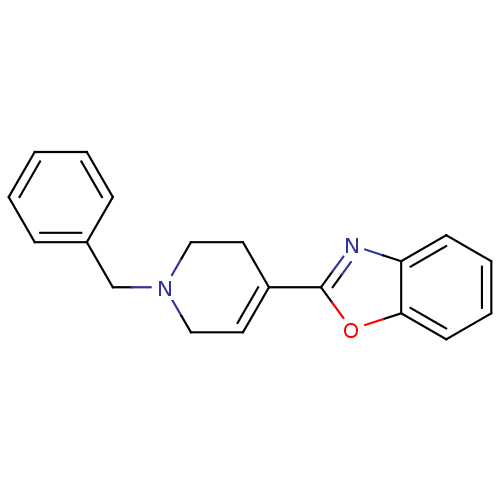
(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-benzo...)Show InChI InChI=1S/C19H18N2O/c1-2-6-15(7-3-1)14-21-12-10-16(11-13-21)19-20-17-8-4-5-9-18(17)22-19/h1-10H,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290027

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-benzo...)Show InChI InChI=1S/C19H18N2O/c1-2-6-15(7-3-1)14-21-12-10-16(11-13-21)19-20-17-8-4-5-9-18(17)22-19/h1-10H,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290029

(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccc(Cl)cc3[nH]2)cc1 |c:9| Show InChI InChI=1S/C19H17Cl2N3/c20-15-3-1-13(2-4-15)12-24-9-7-14(8-10-24)19-22-17-6-5-16(21)11-18(17)23-19/h1-7,11H,8-10,12H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290034

(2-[1-(3-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1cccc(CN2CCC(=CC2)c2nc3ccccc3s2)c1 |c:10| Show InChI InChI=1S/C19H17ClN2S/c20-16-5-3-4-14(12-16)13-22-10-8-15(9-11-22)19-21-17-6-1-2-7-18(17)23-19/h1-8,12H,9-11,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290030

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-benzo...)Show InChI InChI=1S/C19H18N2S/c1-2-6-15(7-3-1)14-21-12-10-16(11-13-21)19-20-17-8-4-5-9-18(17)22-19/h1-10H,11-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290028

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2nc([nH]c2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H18ClN3/c20-16-6-7-17-18(12-16)22-19(21-17)15-8-10-23(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290030

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-benzo...)Show InChI InChI=1S/C19H18N2S/c1-2-6-15(7-3-1)14-21-12-10-16(11-13-21)19-20-17-8-4-5-9-18(17)22-19/h1-10H,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290034

(2-[1-(3-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1cccc(CN2CCC(=CC2)c2nc3ccccc3s2)c1 |c:10| Show InChI InChI=1S/C19H17ClN2S/c20-16-5-3-4-14(12-16)13-22-10-8-15(9-11-22)19-21-17-6-1-2-7-18(17)23-19/h1-8,12H,9-11,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290031

(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3cc(Cl)ccc3s2)cc1 |c:9| Show InChI InChI=1S/C19H16Cl2N2S/c20-15-3-1-13(2-4-15)12-23-9-7-14(8-10-23)19-22-17-11-16(21)5-6-18(17)24-19/h1-7,11H,8-10,12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290037

(2-[1-(4-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccccc3[nH]2)cc1 |c:9| Show InChI InChI=1S/C19H18ClN3/c20-16-7-5-14(6-8-16)13-23-11-9-15(10-12-23)19-21-17-3-1-2-4-18(17)22-19/h1-9H,10-13H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290029

(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccc(Cl)cc3[nH]2)cc1 |c:9| Show InChI InChI=1S/C19H17Cl2N3/c20-15-3-1-13(2-4-15)12-24-9-7-14(8-10-24)19-22-17-6-5-16(21)11-18(17)23-19/h1-7,11H,8-10,12H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290026

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-1H-be...)Show SMILES C(N1CCC(=CC1)c1nc2ccccc2[nH]1)c1ccccc1 |c:4| Show InChI InChI=1S/C19H19N3/c1-2-6-15(7-3-1)14-22-12-10-16(11-13-22)19-20-17-8-4-5-9-18(17)21-19/h1-10H,11-14H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290033

(2-[1-(2-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show InChI InChI=1S/C19H17ClN2S/c20-16-6-2-1-5-15(16)13-22-11-9-14(10-12-22)19-21-17-7-3-4-8-18(17)23-19/h1-9H,10-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290035

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2sc(nc2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H17ClN2S/c20-16-6-7-18-17(12-16)21-19(23-18)15-8-10-22(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290036

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2oc(nc2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H17ClN2O/c20-16-6-7-18-17(12-16)21-19(23-18)15-8-10-22(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290032

(2-[1-(4-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccccc3s2)cc1 |c:9| Show InChI InChI=1S/C19H17ClN2S/c20-16-7-5-14(6-8-16)13-22-11-9-15(10-12-22)19-21-17-3-1-2-4-18(17)23-19/h1-9H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290037

(2-[1-(4-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccccc3[nH]2)cc1 |c:9| Show InChI InChI=1S/C19H18ClN3/c20-16-7-5-14(6-8-16)13-23-11-9-15(10-12-23)19-21-17-3-1-2-4-18(17)22-19/h1-9H,10-13H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290025

(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3cc(Cl)ccc3o2)cc1 |c:9| Show InChI InChI=1S/C19H16Cl2N2O/c20-15-3-1-13(2-4-15)12-23-9-7-14(8-10-23)19-22-17-11-16(21)5-6-18(17)24-19/h1-7,11H,8-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290035

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2sc(nc2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H17ClN2S/c20-16-6-7-18-17(12-16)21-19(23-18)15-8-10-22(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290036

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2oc(nc2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H17ClN2O/c20-16-6-7-18-17(12-16)21-19(23-18)15-8-10-22(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290031

(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3cc(Cl)ccc3s2)cc1 |c:9| Show InChI InChI=1S/C19H16Cl2N2S/c20-15-3-1-13(2-4-15)12-23-9-7-14(8-10-23)19-22-17-11-16(21)5-6-18(17)24-19/h1-7,11H,8-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50290033

(2-[1-(2-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show InChI InChI=1S/C19H17ClN2S/c20-16-6-2-1-5-15(16)13-22-11-9-14(10-12-22)19-21-17-7-3-4-8-18(17)23-19/h1-9H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine D2 receptor in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290028

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-5-chl...)Show SMILES Clc1ccc2nc([nH]c2c1)C1=CCN(Cc2ccccc2)CC1 |t:12| Show InChI InChI=1S/C19H18ClN3/c20-16-6-7-17-18(12-16)22-19(21-17)15-8-10-23(11-9-15)13-14-4-2-1-3-5-14/h1-8,12H,9-11,13H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290032

(2-[1-(4-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccccc3s2)cc1 |c:9| Show InChI InChI=1S/C19H17ClN2S/c20-16-7-5-14(6-8-16)13-22-11-9-15(10-12-22)19-21-17-3-1-2-4-18(17)23-19/h1-9H,10-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290026

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-1H-be...)Show SMILES C(N1CCC(=CC1)c1nc2ccccc2[nH]1)c1ccccc1 |c:4| Show InChI InChI=1S/C19H19N3/c1-2-6-15(7-3-1)14-22-12-10-16(11-13-22)19-20-17-8-4-5-9-18(17)21-19/h1-10H,11-14H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290025

(5-Chloro-2-[1-(4-chloro-benzyl)-1,2,3,6-tetrahydro...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3cc(Cl)ccc3o2)cc1 |c:9| Show InChI InChI=1S/C19H16Cl2N2O/c20-15-3-1-13(2-4-15)12-23-9-7-14(8-10-23)19-22-17-11-16(21)5-6-18(17)24-19/h1-7,11H,8-10,12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| >4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290027

(2-(1-Benzyl-1,2,3,6-tetrahydro-pyridin-4-yl)-benzo...)Show InChI InChI=1S/C19H18N2O/c1-2-6-15(7-3-1)14-21-12-10-16(11-13-21)19-20-17-8-4-5-9-18(17)22-19/h1-10H,11-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD3 expressed in CHO cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28962

(cyclohexyl sulfone-based compound, 5 | {4-[(4-chlo...)Show SMILES OC[C@H]1CC[C@@](CC1)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:2.1,wD:5.8,(6.8,4.91,;5.47,5.68,;4.13,4.91,;4.13,3.37,;2.8,2.6,;1.47,3.37,;1.47,4.91,;2.8,5.68,;-.12,3.9,;-.12,5.44,;-1.46,6.21,;-1.46,7.75,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C19H19ClF2O3S/c20-14-1-4-16(5-2-14)26(24,25)19(9-7-13(12-23)8-10-19)17-11-15(21)3-6-18(17)22/h1-6,11,13,23H,7-10,12H2/t13-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28964
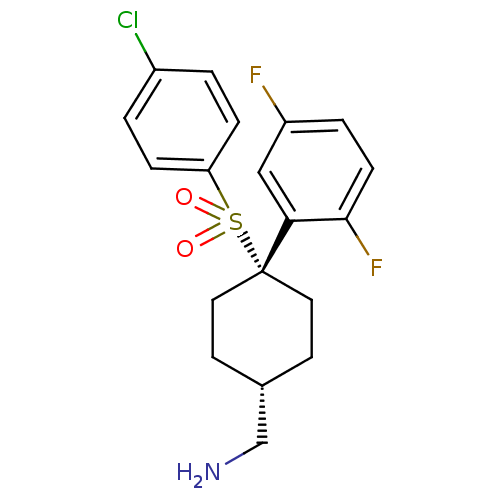
(cyclohexyl sulfone-based compound, 7 | {4-[(4-chlo...)Show SMILES NC[C@H]1CC[C@@](CC1)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:2.1,wD:5.8,(6.8,4.91,;5.47,5.68,;4.13,4.91,;4.13,3.37,;2.8,2.6,;1.47,3.37,;1.47,4.91,;2.8,5.68,;-.12,3.9,;-.12,5.44,;-1.46,6.21,;-1.46,7.75,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C19H20ClF2NO2S/c20-14-1-4-16(5-2-14)26(24,25)19(9-7-13(12-23)8-10-19)17-11-15(21)3-6-18(17)22/h1-6,11,13H,7-10,12,23H2/t13-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 148 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28966
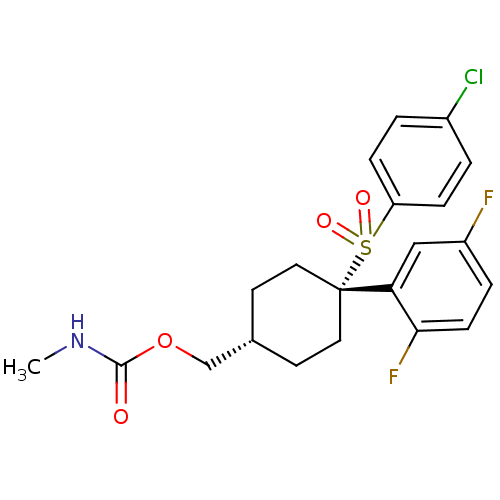
(cyclohexyl sulfone-based compound, 9 | {4-[(4-chlo...)Show SMILES CNC(=O)OC[C@H]1CC[C@@](CC1)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:6.5,wD:9.12,(10.8,5.68,;9.47,4.91,;8.13,5.68,;8.13,7.22,;6.8,4.91,;5.47,5.68,;4.13,4.91,;4.13,3.37,;2.8,2.6,;1.47,3.37,;1.47,4.91,;2.8,5.68,;-.12,3.9,;-.12,5.44,;-1.46,6.21,;-1.46,7.75,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C21H22ClF2NO4S/c1-25-20(26)29-13-14-8-10-21(11-9-14,18-12-16(23)4-7-19(18)24)30(27,28)17-5-2-15(22)3-6-17/h2-7,12,14H,8-11,13H2,1H3,(H,25,26)/t14-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28968
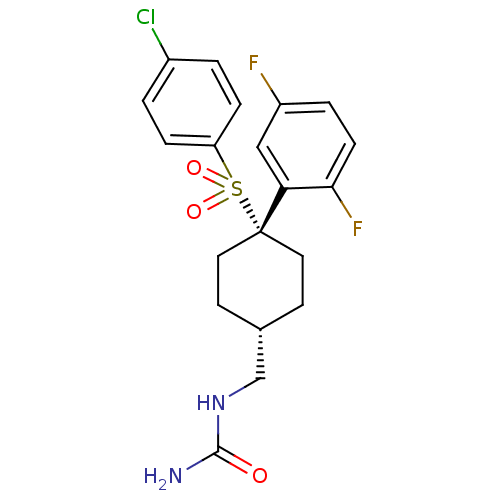
(({4-[(4-chlorobenzene)sulfonyl]-4-(2,5-difluorophe...)Show SMILES NC(=O)NC[C@H]1CC[C@@](CC1)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:5.4,wD:8.11,(9.47,4.91,;8.13,5.68,;8.13,7.22,;6.8,4.91,;5.47,5.68,;4.13,4.91,;4.13,3.37,;2.8,2.6,;1.47,3.37,;1.47,4.91,;2.8,5.68,;-.12,3.9,;-.12,5.44,;-1.46,6.21,;-1.46,7.75,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C20H21ClF2N2O3S/c21-14-1-4-16(5-2-14)29(27,28)20(17-11-15(22)3-6-18(17)23)9-7-13(8-10-20)12-25-19(24)26/h1-6,11,13H,7-10,12H2,(H3,24,25,26)/t13-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28970
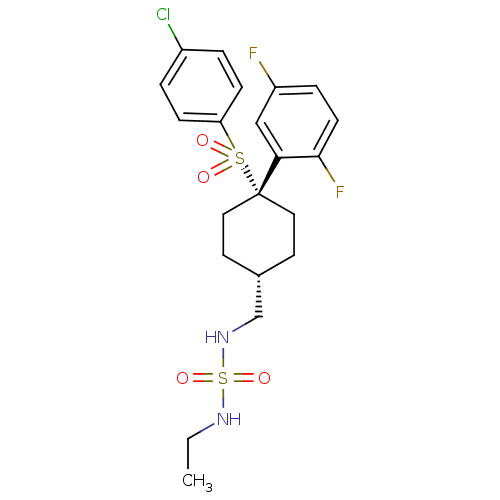
([({4-[(4-chlorobenzene)sulfonyl]-4-(2,5-difluoroph...)Show SMILES CCNS(=O)(=O)NC[C@H]1CC[C@@](CC1)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:8.7,wD:11.14,(12.14,4.91,;10.8,5.68,;9.47,4.91,;8.13,5.68,;7.05,6.77,;8.9,7.02,;6.8,4.91,;5.47,5.68,;4.13,4.91,;4.13,3.37,;2.8,2.6,;1.47,3.37,;1.47,4.91,;2.8,5.68,;-.12,3.9,;-.12,5.44,;-1.46,6.21,;-1.46,7.75,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C21H25ClF2N2O4S2/c1-2-25-32(29,30)26-14-15-9-11-21(12-10-15,19-13-17(23)5-8-20(19)24)31(27,28)18-6-3-16(22)4-7-18/h3-8,13,15,25-26H,2,9-12,14H2,1H3/t15-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28972
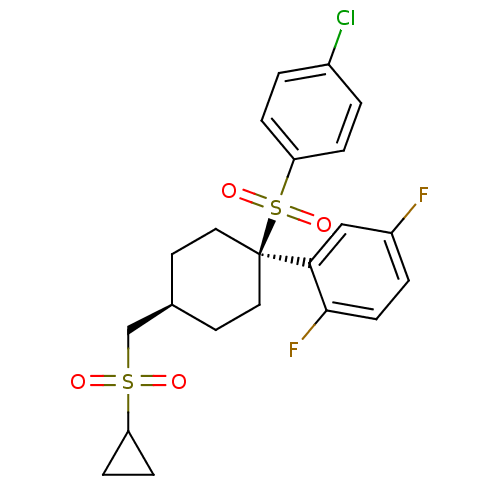
(2-{1-[(4-chlorobenzene)sulfonyl]-4-[(cyclopropanes...)Show SMILES Fc1ccc(F)c(c1)[C@]1(CC[C@H](CS(=O)(=O)C2CC2)CC1)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:11.12,wD:8.8,(-1.46,7.75,;-1.46,6.21,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;-.12,3.9,;-.12,5.44,;1.47,3.37,;2.8,2.6,;4.13,3.37,;4.13,4.91,;5.47,5.68,;6.8,4.91,;6.03,3.58,;7.89,3.82,;8.13,5.68,;8.9,7.02,;9.67,5.68,;2.8,5.68,;1.47,4.91,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C22H23ClF2O4S2/c23-16-1-4-19(5-2-16)31(28,29)22(20-13-17(24)3-8-21(20)25)11-9-15(10-12-22)14-30(26,27)18-6-7-18/h1-5,8,13,15,18H,6-7,9-12,14H2/t15-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28974
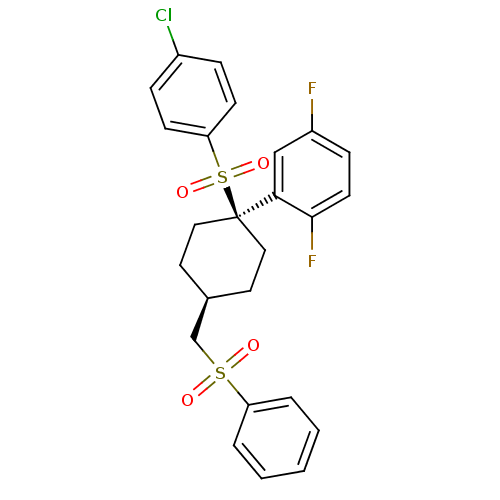
(2-{4-[(benzenesulfonyl)methyl]-1-[(4-chlorobenzene...)Show SMILES Fc1ccc(F)c(c1)[C@]1(CC[C@H](CS(=O)(=O)c2ccccc2)CC1)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:11.12,wD:8.8,(-1.46,7.75,;-1.46,6.21,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;-.12,3.9,;-.12,5.44,;1.47,3.37,;2.8,2.6,;4.13,3.37,;4.13,4.91,;5.47,5.68,;6.8,4.91,;6.03,3.58,;7.89,3.82,;8.13,5.68,;8.13,7.22,;9.47,7.99,;10.8,7.22,;10.8,5.68,;9.47,4.91,;2.8,5.68,;1.47,4.91,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C25H23ClF2O4S2/c26-19-6-9-22(10-7-19)34(31,32)25(23-16-20(27)8-11-24(23)28)14-12-18(13-15-25)17-33(29,30)21-4-2-1-3-5-21/h1-11,16,18H,12-15,17H2/t18-,25+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28976
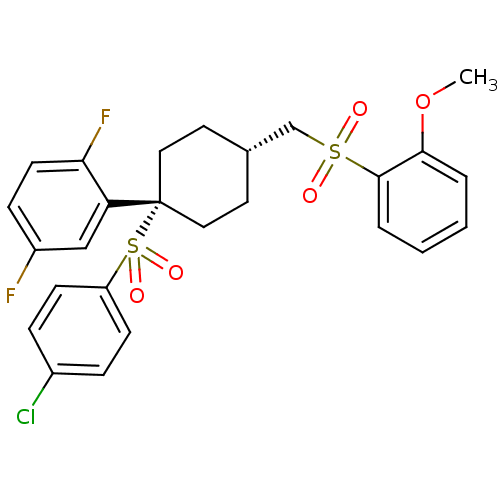
(2-{1-[(4-chlorobenzene)sulfonyl]-4-{[(2-methoxyben...)Show SMILES COc1ccccc1S(=O)(=O)C[C@H]1CC[C@@](CC1)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:12.12,wD:15.19,(10.8,2.6,;9.47,3.37,;9.47,4.91,;10.8,5.68,;10.8,7.22,;9.47,7.99,;8.13,7.22,;8.13,5.68,;6.8,4.91,;6.03,3.58,;7.89,3.82,;5.47,5.68,;4.13,4.91,;4.13,3.37,;2.8,2.6,;1.47,3.37,;1.47,4.91,;2.8,5.68,;-.12,3.9,;-.12,5.44,;-1.46,6.21,;-1.46,7.75,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C26H25ClF2O5S2/c1-34-24-4-2-3-5-25(24)35(30,31)17-18-12-14-26(15-13-18,22-16-20(28)8-11-23(22)29)36(32,33)21-9-6-19(27)7-10-21/h2-11,16,18H,12-15,17H2,1H3/t18-,26+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28978
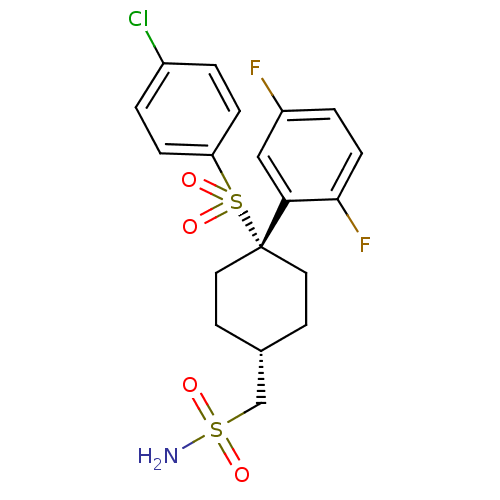
(cyclohexyl sulfone-based compound, 22 | {4-[(4-chl...)Show SMILES NS(=O)(=O)C[C@H]1CC[C@@](CC1)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:5.4,wD:8.11,(8.13,5.68,;6.8,4.91,;6.03,3.58,;7.89,3.82,;5.47,5.68,;4.13,4.91,;4.13,3.37,;2.8,2.6,;1.47,3.37,;1.47,4.91,;2.8,5.68,;-.12,3.9,;-.12,5.44,;-1.46,6.21,;-1.46,7.75,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C19H20ClF2NO4S2/c20-14-1-4-16(5-2-14)29(26,27)19(17-11-15(21)3-6-18(17)22)9-7-13(8-10-19)12-28(23,24)25/h1-6,11,13H,7-10,12H2,(H2,23,24,25)/t13-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28980
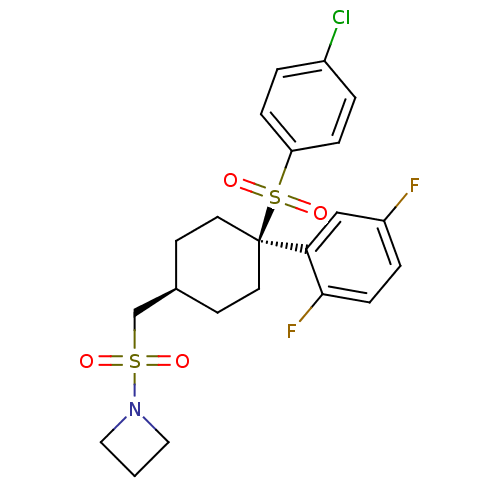
(1-[({4-[(4-chlorobenzene)sulfonyl]-4-(2,5-difluoro...)Show SMILES Fc1ccc(F)c(c1)[C@]1(CC[C@H](CS(=O)(=O)N2CCC2)CC1)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:11.12,wD:8.8,(-1.46,7.75,;-1.46,6.21,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;-.12,3.9,;-.12,5.44,;1.47,3.37,;2.8,2.6,;4.13,3.37,;4.13,4.91,;5.47,5.68,;6.8,4.91,;6.03,3.58,;7.89,3.82,;8.13,5.68,;8.13,7.22,;9.67,7.22,;9.67,5.68,;2.8,5.68,;1.47,4.91,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C22H24ClF2NO4S2/c23-17-2-5-19(6-3-17)32(29,30)22(20-14-18(24)4-7-21(20)25)10-8-16(9-11-22)15-31(27,28)26-12-1-13-26/h2-7,14,16H,1,8-13,15H2/t16-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit PEN-2/Nicastrin [34-709]/Presenilin-1
(Homo sapiens (Human)) | BDBM28982
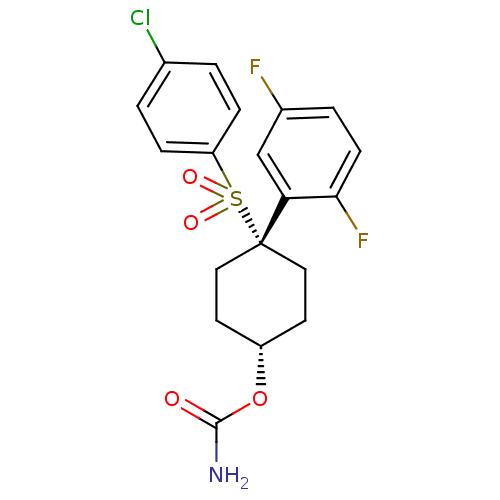
(cyclohexyl sulfone-based compound, 28 | {4-[(4-chl...)Show SMILES NC(=O)O[C@H]1CC[C@@](CC1)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:4.3,wD:7.10,(6.8,7.99,;5.47,7.22,;4.13,7.99,;5.47,5.68,;4.13,4.91,;4.13,3.37,;2.8,2.6,;1.47,3.37,;1.47,4.91,;2.8,5.68,;-.12,3.9,;-.12,5.44,;-1.46,6.21,;-1.46,7.75,;-2.79,5.44,;-2.79,3.9,;-1.46,3.13,;-1.46,1.59,;1.47,1.83,;3.01,1.83,;-.07,1.83,;1.47,.29,;.13,-.48,;.13,-2.02,;1.47,-2.79,;1.47,-4.33,;2.8,-2.02,;2.8,-.48,)| Show InChI InChI=1S/C19H18ClF2NO4S/c20-12-1-4-15(5-2-12)28(25,26)19(16-11-13(21)3-6-17(16)22)9-7-14(8-10-19)27-18(23)24/h1-6,11,14H,7-10H2,(H2,23,24)/t14-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
A whole-cell gamma-secretase inhibition assay using SHSY5Y neuroblastoma cells in which human gamma-secretase catalyzes the breakdown of the overexpr... |
Bioorg Med Chem Lett 16: 280-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.009
BindingDB Entry DOI: 10.7270/Q22Z13VQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data