 Found 131 hits with Last Name = 'thai' and Initial = 'a'
Found 131 hits with Last Name = 'thai' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lethal factor
(Bacillus anthracis) | BDBM50379543
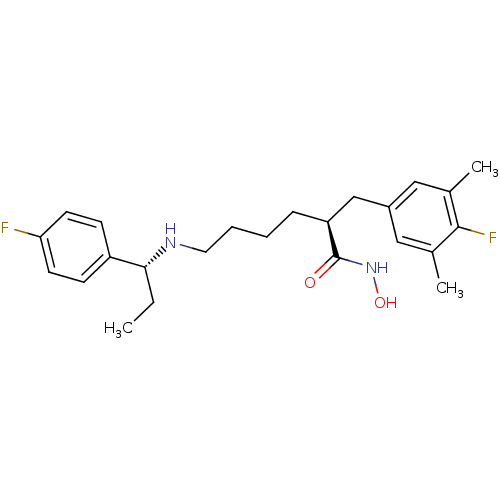
(CHEMBL2012752)Show SMILES CC[C@@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O2/c1-4-22(19-8-10-21(25)11-9-19)27-12-6-5-7-20(24(29)28-30)15-18-13-16(2)23(26)17(3)14-18/h8-11,13-14,20,22,27,30H,4-7,12,15H2,1-3H3,(H,28,29)/t20-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379542
(CHEMBL2012753)Show SMILES CC[C@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O2/c1-4-22(19-8-10-21(25)11-9-19)27-12-6-5-7-20(24(29)28-30)15-18-13-16(2)23(26)17(3)14-18/h8-11,13-14,20,22,27,30H,4-7,12,15H2,1-3H3,(H,28,29)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50340754
((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...)Show SMILES Cc1cc(C[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C22H28F2N2O2/c1-15-11-18(12-16(2)21(15)24)13-19(22(27)26-28)5-3-4-10-25-14-17-6-8-20(23)9-7-17/h6-9,11-12,19,25,28H,3-5,10,13-14H2,1-2H3,(H,26,27)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379536
(CHEMBL2012836)Show SMILES CCC(NCCCC[C@@H](Nc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H31F2N3O2/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(29)28-30)27-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26-27,30H,4-7,12H2,1-3H3,(H,28,29)/t20?,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379541
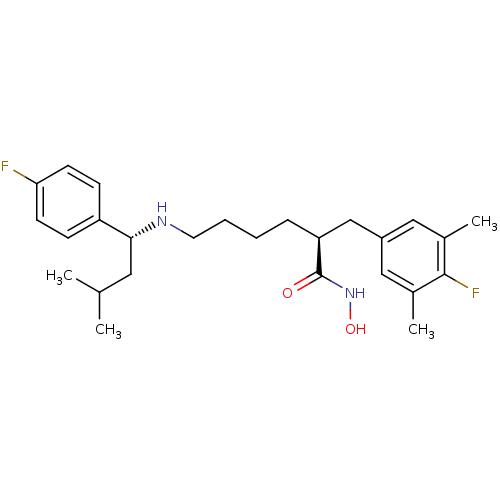
(CHEMBL2012832)Show SMILES CC(C)C[C@@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H36F2N2O2/c1-17(2)13-24(21-8-10-23(27)11-9-21)29-12-6-5-7-22(26(31)30-32)16-20-14-18(3)25(28)19(4)15-20/h8-11,14-15,17,22,24,29,32H,5-7,12-13,16H2,1-4H3,(H,30,31)/t22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50329265
((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...)Show SMILES Cc1cc(N[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H27F2N3O2/c1-14-11-18(12-15(2)20(14)23)25-19(21(27)26-28)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24-25,28H,3-5,10,13H2,1-2H3,(H,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379533
(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379540
(CHEMBL2012750)Show SMILES C[C@@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O2/c1-15-12-18(13-16(2)22(15)25)14-20(23(28)27-29)6-4-5-11-26-17(3)19-7-9-21(24)10-8-19/h7-10,12-13,17,20,26,29H,4-6,11,14H2,1-3H3,(H,27,28)/t17-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379535
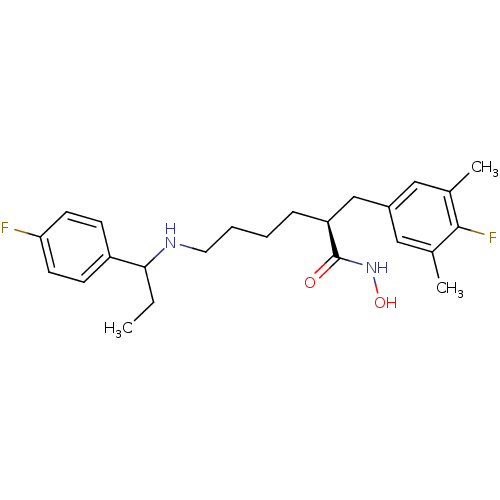
(CHEMBL2010824)Show SMILES CCC(NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O2/c1-4-22(19-8-10-21(25)11-9-19)27-12-6-5-7-20(24(29)28-30)15-18-13-16(2)23(26)17(3)14-18/h8-11,13-14,20,22,27,30H,4-7,12,15H2,1-3H3,(H,28,29)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379544
(CHEMBL2012751)Show SMILES C[C@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O2/c1-15-12-18(13-16(2)22(15)25)14-20(23(28)27-29)6-4-5-11-26-17(3)19-7-9-21(24)10-8-19/h7-10,12-13,17,20,26,29H,4-6,11,14H2,1-3H3,(H,27,28)/t17-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50340758
((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lethal factor
(Bacillus anthracis) | BDBM50379539
(CHEMBL2012834)Show SMILES Cc1cc(C[C@H](CCCCN[C@H](Cc2ccccc2)c2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C29H34F2N2O2/c1-20-16-23(17-21(2)28(20)31)18-25(29(34)33-35)10-6-7-15-32-27(19-22-8-4-3-5-9-22)24-11-13-26(30)14-12-24/h3-5,8-9,11-14,16-17,25,27,32,35H,6-7,10,15,18-19H2,1-2H3,(H,33,34)/t25-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379534
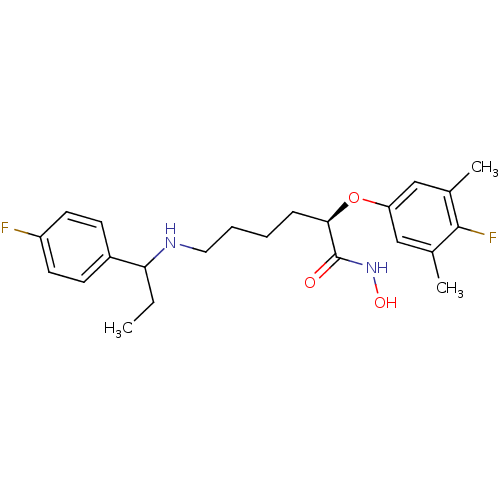
(CHEMBL2012837)Show SMILES CCC(NCCCC[C@@H](Oc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O3/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(28)27-29)30-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26,29H,4-7,12H2,1-3H3,(H,27,28)/t20?,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50340768
((R)-2-(4-fluoro-3,5-dimethylphenoxy)-6-(4-fluorobe...)Show SMILES Cc1cc(O[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H26F2N2O3/c1-14-11-18(12-15(2)20(14)23)28-19(21(26)25-27)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24,27H,3-5,10,13H2,1-2H3,(H,25,26)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379538
(CHEMBL2012833)Show SMILES CC(C)C[C@H](NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H36F2N2O2/c1-17(2)13-24(21-8-10-23(27)11-9-21)29-12-6-5-7-22(26(31)30-32)16-20-14-18(3)25(28)19(4)15-20/h8-11,14-15,17,22,24,29,32H,5-7,12-13,16H2,1-4H3,(H,30,31)/t22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50379537
(CHEMBL2012835)Show SMILES Cc1cc(C[C@H](CCCCN[C@@H](Cc2ccccc2)c2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C29H34F2N2O2/c1-20-16-23(17-21(2)28(20)31)18-25(29(34)33-35)10-6-7-15-32-27(19-22-8-4-3-5-9-22)24-11-13-26(30)14-12-24/h3-5,8-9,11-14,16-17,25,27,32,35H,6-7,10,15,18-19H2,1-2H3,(H,33,34)/t25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452691

(CHEMBL4218073)Show SMILES Nc1ncnc2n(ccc12)C1CCN(CC1)C(=O)c1ccc(cc1)S(F)(=O)=O Show InChI InChI=1S/C18H18FN5O3S/c19-28(26,27)14-3-1-12(2-4-14)18(25)23-8-5-13(6-9-23)24-10-7-15-16(20)21-11-22-17(15)24/h1-4,7,10-11,13H,5-6,8-9H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452676

(CHEMBL4206295)Show SMILES Cc1nc(N)c2ccn(C3CCN(CC3)C(=O)c3ccc(cc3)S(F)(=O)=O)c2n1 Show InChI InChI=1S/C19H20FN5O3S/c1-12-22-17(21)16-8-11-25(18(16)23-12)14-6-9-24(10-7-14)19(26)13-2-4-15(5-3-13)29(20,27)28/h2-5,8,11,14H,6-7,9-10H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452677
(CHEMBL4218072)Show SMILES Cc1nc(N)c2cnn(C3CCN(CC3)C(=O)c3ccc(cc3)S(F)(=O)=O)c2n1 Show InChI InChI=1S/C18H19FN6O3S/c1-11-22-16(20)15-10-21-25(17(15)23-11)13-6-8-24(9-7-13)18(26)12-2-4-14(5-3-12)29(19,27)28/h2-5,10,13H,6-9H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50340758

((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452684
(CHEMBL4217380)Show SMILES Nc1ncnc2n(ncc12)C1CCN(CC1)C(=O)c1ccc(cc1)S(F)(=O)=O Show InChI InChI=1S/C17H17FN6O3S/c18-28(26,27)13-3-1-11(2-4-13)17(25)23-7-5-12(6-8-23)24-16-14(9-22-24)15(19)20-10-21-16/h1-4,9-10,12H,5-8H2,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452675
(CHEMBL4211713)Show SMILES Cc1nc(N)c2cnn(C3CCN(Cc4ccc(cc4)S(F)(=O)=O)CC3)c2n1 Show InChI InChI=1S/C18H21FN6O2S/c1-12-22-17(20)16-10-21-25(18(16)23-12)14-6-8-24(9-7-14)11-13-2-4-15(5-3-13)28(19,26)27/h2-5,10,14H,6-9,11H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452681

(CHEMBL4209693)Show SMILES Nc1ncnc2n(cnc12)C1CCN(CC1)C(=O)c1ccc(cc1)S(F)(=O)=O Show InChI InChI=1S/C17H17FN6O3S/c18-28(26,27)13-3-1-11(2-4-13)17(25)23-7-5-12(6-8-23)24-10-22-14-15(19)20-9-21-16(14)24/h1-4,9-10,12H,5-8H2,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452678
(CHEMBL4211326)Show SMILES Nc1cc(OC2CN(C2)C(=O)c2ccc(cc2)S(F)(=O)=O)ccn1 Show InChI InChI=1S/C15H14FN3O4S/c16-24(21,22)13-3-1-10(2-4-13)15(20)19-8-12(9-19)23-11-5-6-18-14(17)7-11/h1-7,12H,8-9H2,(H2,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452682
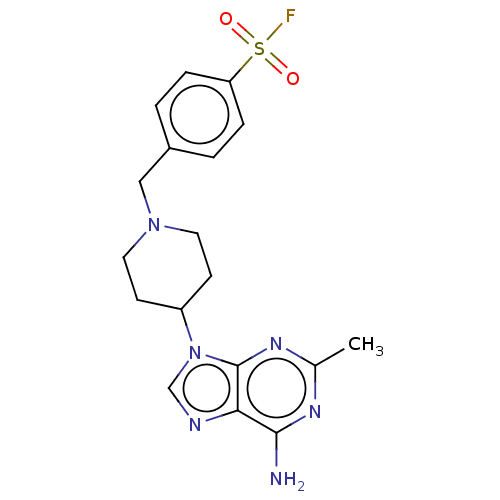
(CHEMBL4210973)Show SMILES Cc1nc(N)c2ncn(C3CCN(Cc4ccc(cc4)S(F)(=O)=O)CC3)c2n1 Show InChI InChI=1S/C18H21FN6O2S/c1-12-22-17(20)16-18(23-12)25(11-21-16)14-6-8-24(9-7-14)10-13-2-4-15(5-3-13)28(19,26)27/h2-5,11,14H,6-10H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452683

(CHEMBL4215512)Show SMILES Cc1nc(N)c2ncn(C3CCN(CC3)C(=O)c3ccc(cc3)S(F)(=O)=O)c2n1 Show InChI InChI=1S/C18H19FN6O3S/c1-11-22-16(20)15-17(23-11)25(10-21-15)13-6-8-24(9-7-13)18(26)12-2-4-14(5-3-12)29(19,27)28/h2-5,10,13H,6-9H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50292495
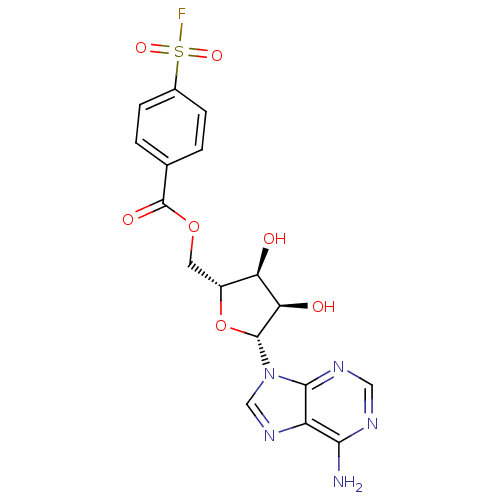
(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COC(=O)c2ccc(cc2)S(F)(=O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H16FN5O7S/c18-31(27,28)9-3-1-8(2-4-9)17(26)29-5-10-12(24)13(25)16(30-10)23-7-22-11-14(19)20-6-21-15(11)23/h1-4,6-7,10,12-13,16,24-25H,5H2,(H2,19,20,21)/t10-,12-,13-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50433336

(CHEMBL2376588)Show InChI InChI=1S/C19H15N3O3/c23-18(21-17-11-5-6-12-20-17)15-9-3-1-7-13(15)14-8-2-4-10-16(14)19(24)22-25/h1-12,25H,(H,22,24)(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain (1 to 429 amino acid) using Abz-Thr-dArg-Ile-Asp-Glu-Ala-Asn-Gln-Arg-Ala-Thr-Lys-Nle-Lys(Dnp)-... |
Bioorg Med Chem Lett 23: 2505-11 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.030
BindingDB Entry DOI: 10.7270/Q2PR7XC5 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50340758

((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-9 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452693
(CHEMBL4202426)Show SMILES Nc1ncnc2n(CCOC(=O)c3ccc(cc3)S(F)(=O)=O)cnc12 Show InChI InChI=1S/C14H12FN5O4S/c15-25(22,23)10-3-1-9(2-4-10)14(21)24-6-5-20-8-19-11-12(16)17-7-18-13(11)20/h1-4,7-8H,5-6H2,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452692

(CHEMBL4204554)Show SMILES Cc1nc(N)cc(OC2CCN(CC2)C(=O)c2ccc(cc2)S(F)(=O)=O)n1 Show InChI InChI=1S/C17H19FN4O4S/c1-11-20-15(19)10-16(21-11)26-13-6-8-22(9-7-13)17(23)12-2-4-14(5-3-12)27(18,24)25/h2-5,10,13H,6-9H2,1H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50340758

((S)-6-(4-fluorobenzylamino)-2-((R)-2-(4-fluorophen...)Show SMILES CO[C@H](C[C@H](CCCCNCc1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H28F2N2O3/c1-29-21(17-7-11-20(24)12-8-17)14-18(22(27)26-28)4-2-3-13-25-15-16-5-9-19(23)10-6-16/h5-12,18,21,25,28H,2-4,13-15H2,1H3,(H,26,27)/t18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-1 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452694

(CHEMBL4203961)Show SMILES Cc1nc(N)c2cnn(CCCNC(=O)c3ccc(cc3)S(F)(=O)=O)c2n1 Show InChI InChI=1S/C16H17FN6O3S/c1-10-21-14(18)13-9-20-23(15(13)22-10)8-2-7-19-16(24)11-3-5-12(6-4-11)27(17,25)26/h3-6,9H,2,7-8H2,1H3,(H,19,24)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452679

(CHEMBL4217046)Show SMILES Nc1ncnc2n(CCCNC(=O)c3ccc(cc3)S(F)(=O)=O)cnc12 Show InChI InChI=1S/C15H15FN6O3S/c16-26(24,25)11-4-2-10(3-5-11)15(23)18-6-1-7-22-9-21-12-13(17)19-8-20-14(12)22/h2-5,8-9H,1,6-7H2,(H,18,23)(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452686
(CHEMBL4217363)Show SMILES Nc1cc(O[C@@H]2CCN(C2)C(=O)c2ccc(cc2)S(F)(=O)=O)ccn1 |r| Show InChI InChI=1S/C16H16FN3O4S/c17-25(22,23)14-3-1-11(2-4-14)16(21)20-8-6-13(10-20)24-12-5-7-19-15(18)9-12/h1-5,7,9,13H,6,8,10H2,(H2,18,19)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452697

(CHEMBL4204818)Show SMILES Cc1cc(OC2CCN(Cc3ccc(cc3)S(F)(=O)=O)CC2)cc(N)n1 Show InChI InChI=1S/C18H22FN3O3S/c1-13-10-16(11-18(20)21-13)25-15-6-8-22(9-7-15)12-14-2-4-17(5-3-14)26(19,23)24/h2-5,10-11,15H,6-9,12H2,1H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-1 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Calmodulin-sensitive adenylate cyclase
(Bacillus anthracis) | BDBM50452689

(CHEMBL4217944)Show InChI InChI=1S/C14H16FN3O3S/c15-22(19,20)13-3-1-11(2-4-13)10-17-7-8-21-12-5-6-18-14(16)9-12/h1-6,9,17H,7-8,10H2,(H2,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hawaii Biotech
Curated by ChEMBL
| Assay Description
Inactivation of Bacillus anthracis edema factor in presence of CaM incubated for 10 to 240 mins by EnzChek pyrophosphate assay |
Bioorg Med Chem Lett 28: 134-139 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.040
BindingDB Entry DOI: 10.7270/Q25D8VD2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50379533

(CHEMBL2012838)Show SMILES CCC(NCCCC[C@@H](C[C@@H](OC)c1ccc(F)cc1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O3/c1-3-22(17-7-11-20(25)12-8-17)27-15-5-4-6-19(24(29)28-30)16-23(31-2)18-9-13-21(26)14-10-18/h7-14,19,22-23,27,30H,3-6,15-16H2,1-2H3,(H,28,29)/t19-,22?,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50379534

(CHEMBL2012837)Show SMILES CCC(NCCCC[C@@H](Oc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O3/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(28)27-29)30-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26,29H,4-7,12H2,1-3H3,(H,27,28)/t20?,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50340768

((R)-2-(4-fluoro-3,5-dimethylphenoxy)-6-(4-fluorobe...)Show SMILES Cc1cc(O[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H26F2N2O3/c1-14-11-18(12-15(2)20(14)23)28-19(21(26)25-27)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24,27H,3-5,10,13H2,1-2H3,(H,25,26)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50379535

(CHEMBL2010824)Show SMILES CCC(NCCCC[C@@H](Cc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H32F2N2O2/c1-4-22(19-8-10-21(25)11-9-19)27-12-6-5-7-20(24(29)28-30)15-18-13-16(2)23(26)17(3)14-18/h8-11,13-14,20,22,27,30H,4-7,12,15H2,1-3H3,(H,28,29)/t20-,22?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50340754

((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...)Show SMILES Cc1cc(C[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C22H28F2N2O2/c1-15-11-18(12-16(2)21(15)24)13-19(22(27)26-28)5-3-4-10-25-14-17-6-8-20(23)9-7-17/h6-9,11-12,19,25,28H,3-5,10,13-14H2,1-2H3,(H,26,27)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50379536

(CHEMBL2012836)Show SMILES CCC(NCCCC[C@@H](Nc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H31F2N3O2/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(29)28-30)27-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26-27,30H,4-7,12H2,1-3H3,(H,28,29)/t20?,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50329265

((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...)Show SMILES Cc1cc(N[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H27F2N3O2/c1-14-11-18(12-15(2)20(14)23)25-19(21(27)26-28)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24-25,28H,3-5,10,13H2,1-2H3,(H,26,27)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-14 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50379534

(CHEMBL2012837)Show SMILES CCC(NCCCC[C@@H](Oc1cc(C)c(F)c(C)c1)C(=O)NO)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H30F2N2O3/c1-4-20(17-8-10-18(24)11-9-17)26-12-6-5-7-21(23(28)27-29)30-19-13-15(2)22(25)16(3)14-19/h8-11,13-14,20-21,26,29H,4-7,12H2,1-3H3,(H,27,28)/t20?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50340768

((R)-2-(4-fluoro-3,5-dimethylphenoxy)-6-(4-fluorobe...)Show SMILES Cc1cc(O[C@H](CCCCNCc2ccc(F)cc2)C(=O)NO)cc(C)c1F |r| Show InChI InChI=1S/C21H26F2N2O3/c1-14-11-18(12-15(2)20(14)23)28-19(21(26)25-27)5-3-4-10-24-13-16-6-8-17(22)9-7-16/h6-9,11-12,19,24,27H,3-5,10,13H2,1-2H3,(H,25,26)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC
Curated by ChEMBL
| Assay Description
Inhibition of MMP-12 using OmniMMP as substrate |
Bioorg Med Chem Lett 22: 2242-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.095
BindingDB Entry DOI: 10.7270/Q2MC912Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data