

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM50129200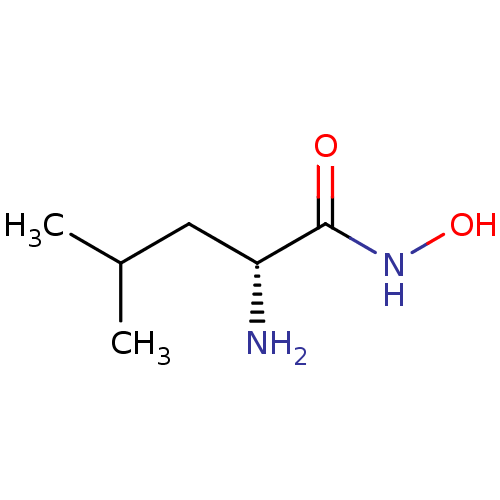 ((R)-2-Amino-4-methyl-pentanoic acid hydroxyamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidase | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50117763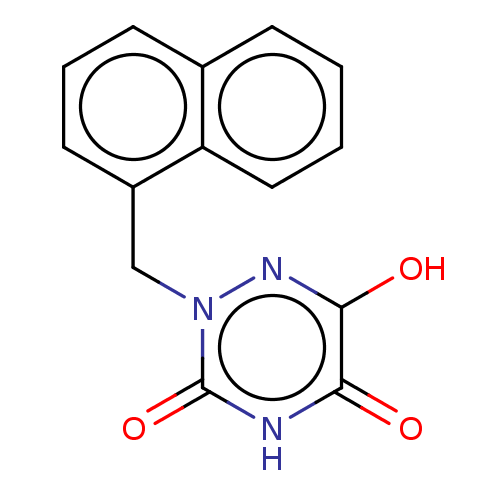 (CHEMBL3613921 | US9505753, 5u) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human DAAO expressed in HEK cells by double reciprocal plot analysis in presence of D-serine | J Med Chem 58: 7258-72 (2015) Article DOI: 10.1021/acs.jmedchem.5b00482 BindingDB Entry DOI: 10.7270/Q2SF2XZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM50129202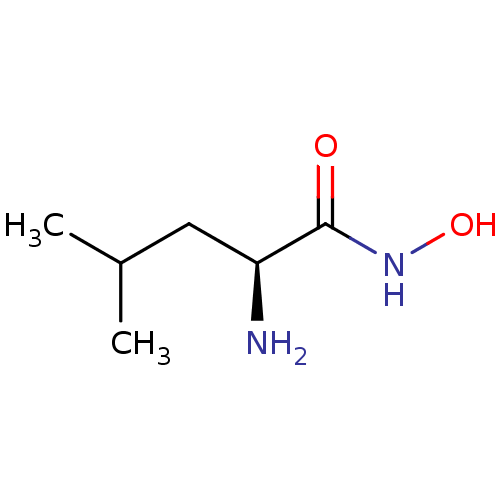 ((S)-2-Amino-4-methyl-pentanoic acid hydroxyamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidase | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM108460 (CHEMBL2178393 | US11191732, Example 1 | US8604016,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysis | J Med Chem 55: 10551-63 (2012) Article DOI: 10.1021/jm301191p BindingDB Entry DOI: 10.7270/Q2VD70M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50432188 (CHEMBL2346976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from full length androgen receptor in human LNCAP cells | Bioorg Med Chem Lett 23: 1945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.02.056 BindingDB Entry DOI: 10.7270/Q21J9C5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50432188 (CHEMBL2346976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to rat androgen receptor ligand binding domain by fluorescence polarization assay | Bioorg Med Chem Lett 23: 1945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.02.056 BindingDB Entry DOI: 10.7270/Q21J9C5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Sus scrofa (pig)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Competitive inhibition of pig kidney DAAO using D-Alanine as substrate by Michaelis-Menten plot analysis | Bioorg Med Chem Lett 23: 3910-3 (2013) Article DOI: 10.1016/j.bmcl.2013.04.062 BindingDB Entry DOI: 10.7270/Q2K35W2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099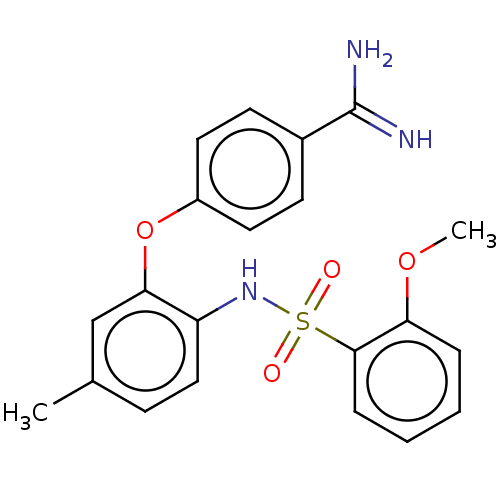 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.43E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509101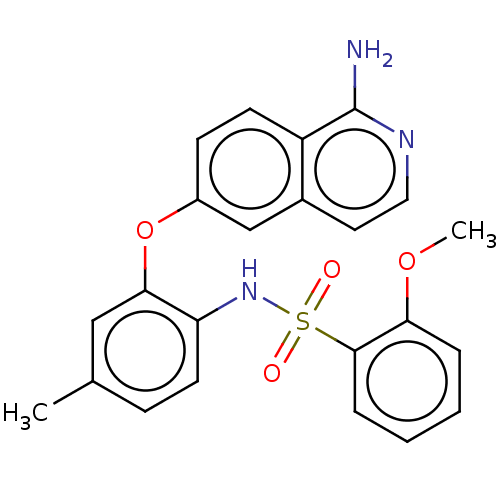 (CHEMBL4460098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509101 (CHEMBL4460098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 3.73E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509101 (CHEMBL4460098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.21E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 [44-750] (Homo sapiens (Human)) | BDBM17759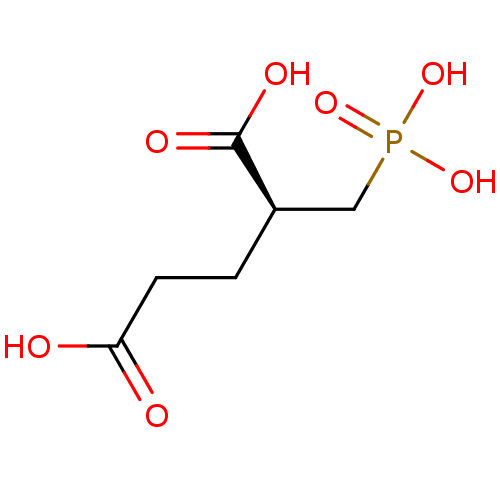 ((2S)-2-(phosphonomethyl)pentanedioic acid | (S)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Guilford | Assay Description GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ... | J Med Chem 48: 2319-24 (2005) Article DOI: 10.1021/jm049258g BindingDB Entry DOI: 10.7270/Q2D50K77 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 [44-750] (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Guilford | Assay Description GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ... | J Med Chem 48: 2319-24 (2005) Article DOI: 10.1021/jm049258g BindingDB Entry DOI: 10.7270/Q2D50K77 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459018 (CHEMBL4166405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535970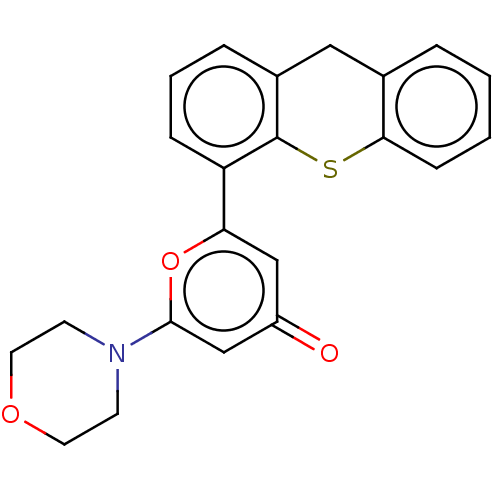 (CHEMBL4569967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459007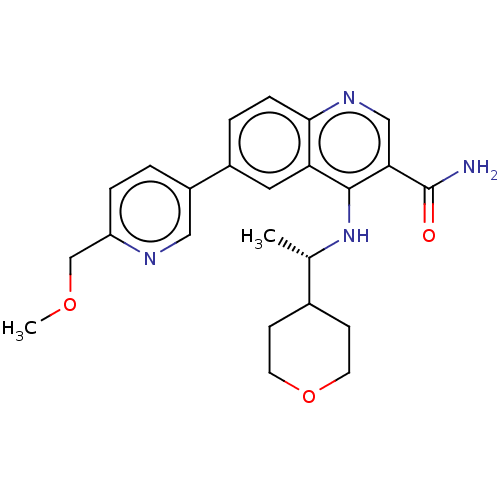 (CHEMBL4218734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535972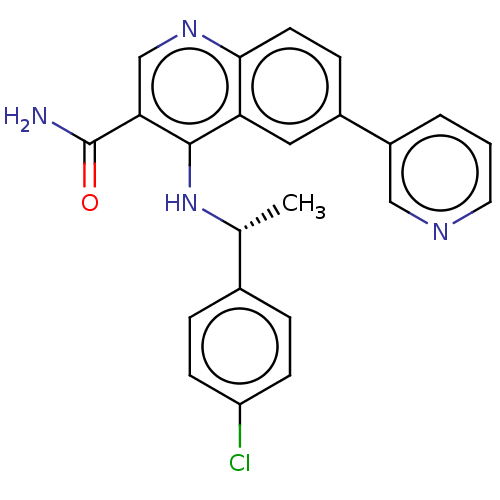 (CHEMBL4565988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50208517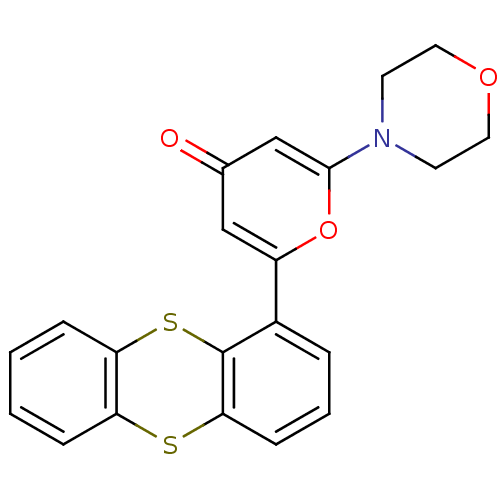 (2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cystine/glutamate transporter (Homo sapiens (Human)) | BDBM50126160 (CHEMBL3629669) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of Xct in human CCF-STTG1 cells assessed as glutamate release after 2 hrs by fluorometry | Bioorg Med Chem Lett 25: 4787-92 (2015) Article DOI: 10.1016/j.bmcl.2015.07.018 BindingDB Entry DOI: 10.7270/Q27H1MD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DNAPK (unknown origin) using p53-based peptide substrate preincubated for 5 min prior to ATP addition | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kalpha using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535971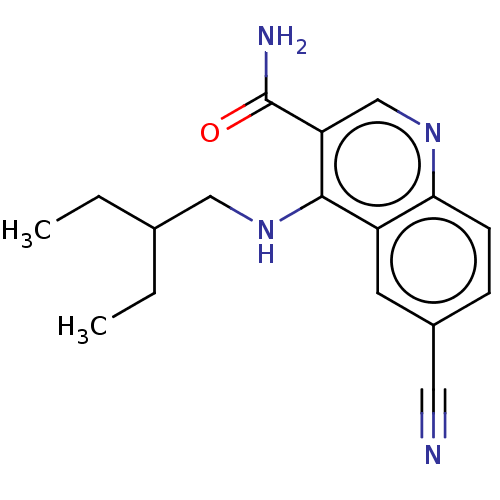 (CHEMBL4593348) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521402 (CHEMBL4471834) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser... | Eur J Med Chem 170: 276-289 (2019) Article DOI: 10.1016/j.ejmech.2019.03.015 BindingDB Entry DOI: 10.7270/Q27H1NZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459011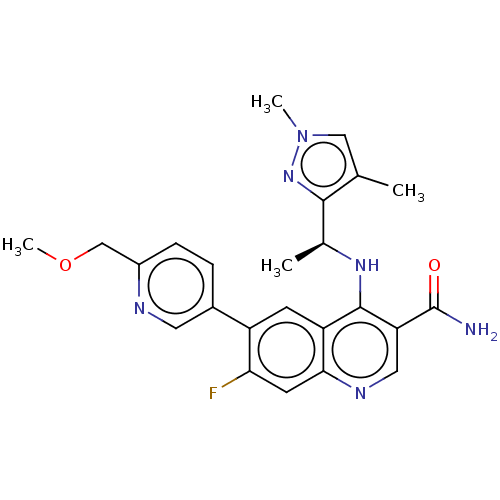 (CHEMBL4215266) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystine/glutamate transporter (Homo sapiens (Human)) | BDBM50126159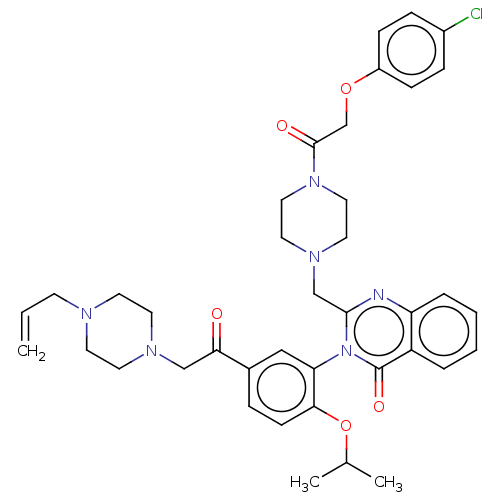 (CHEMBL3629668) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of Xct in human CCF-STTG1 cells assessed as glutamate release after 2 hrs by fluorometry | Bioorg Med Chem Lett 25: 4787-92 (2015) Article DOI: 10.1016/j.bmcl.2015.07.018 BindingDB Entry DOI: 10.7270/Q27H1MD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystine/glutamate transporter (Homo sapiens (Human)) | BDBM50126156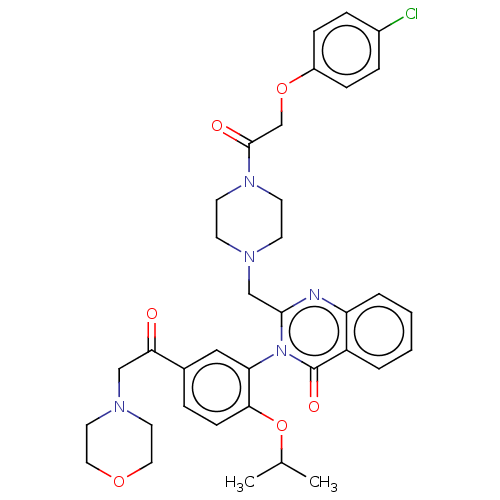 (CHEMBL3629579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of Xct in human CCF-STTG1 cells assessed as glutamate release after 2 hrs by fluorometry | Bioorg Med Chem Lett 25: 4787-92 (2015) Article DOI: 10.1016/j.bmcl.2015.07.018 BindingDB Entry DOI: 10.7270/Q27H1MD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50535995 (CHEMBL4517663) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521405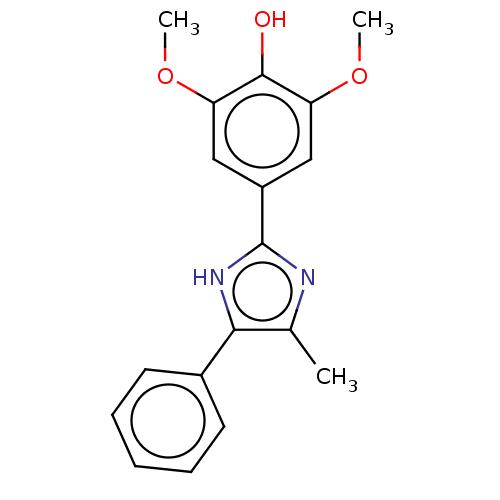 (CHEMBL4551018) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser... | Eur J Med Chem 170: 276-289 (2019) Article DOI: 10.1016/j.ejmech.2019.03.015 BindingDB Entry DOI: 10.7270/Q27H1NZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521425 (CHEMBL4449305) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser... | Eur J Med Chem 170: 276-289 (2019) Article DOI: 10.1016/j.ejmech.2019.03.015 BindingDB Entry DOI: 10.7270/Q27H1NZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM67506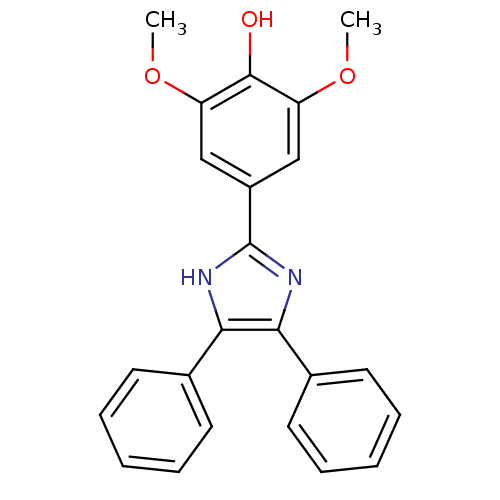 (4-(4,5-diphenyl-1,3-dihydroimidazol-2-ylidene)-2,6...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser... | Eur J Med Chem 170: 276-289 (2019) Article DOI: 10.1016/j.ejmech.2019.03.015 BindingDB Entry DOI: 10.7270/Q27H1NZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystine/glutamate transporter (Homo sapiens (Human)) | BDBM50126155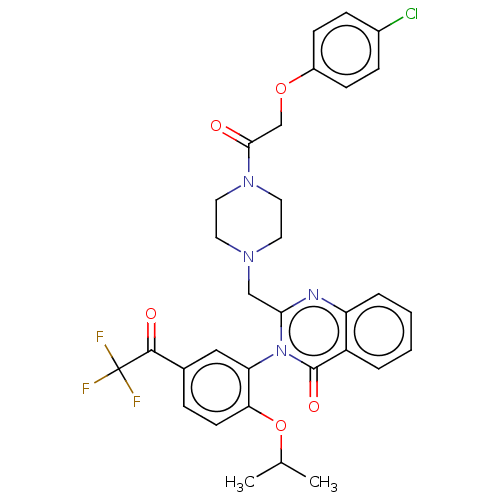 (CHEMBL3629578) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of Xct in human CCF-STTG1 cells assessed as glutamate release after 2 hrs by fluorometry | Bioorg Med Chem Lett 25: 4787-92 (2015) Article DOI: 10.1016/j.bmcl.2015.07.018 BindingDB Entry DOI: 10.7270/Q27H1MD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521404 (CHEMBL4449307) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser... | Eur J Med Chem 170: 276-289 (2019) Article DOI: 10.1016/j.ejmech.2019.03.015 BindingDB Entry DOI: 10.7270/Q27H1NZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521420 (CHEMBL4467223) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser... | Eur J Med Chem 170: 276-289 (2019) Article DOI: 10.1016/j.ejmech.2019.03.015 BindingDB Entry DOI: 10.7270/Q27H1NZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50145038 (CHEMBL2143829) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate incubated for 30 mins followed by substrate addition measured after 2 hrs in presence of AT... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50535972 (CHEMBL4565988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kbeta using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 [44-750] (Homo sapiens (Human)) | BDBM17758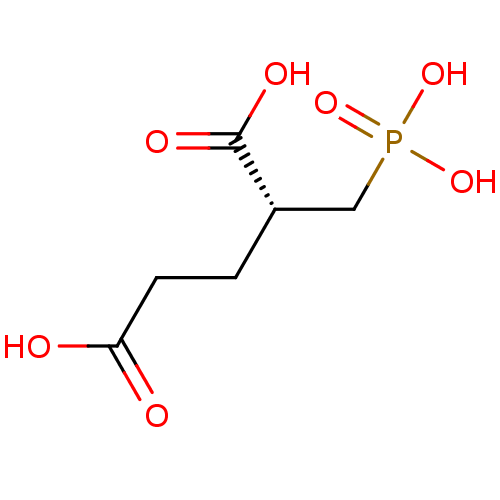 ((2R)-2-(phosphonomethyl)pentanedioic acid | (R)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Guilford | Assay Description GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ... | J Med Chem 48: 2319-24 (2005) Article DOI: 10.1021/jm049258g BindingDB Entry DOI: 10.7270/Q2D50K77 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521419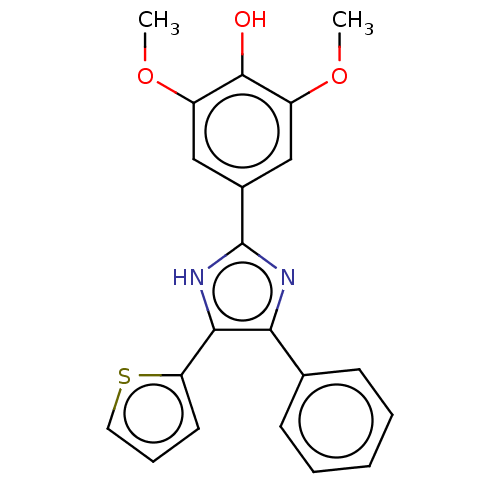 (CHEMBL1342201) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser... | Eur J Med Chem 170: 276-289 (2019) Article DOI: 10.1016/j.ejmech.2019.03.015 BindingDB Entry DOI: 10.7270/Q27H1NZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50547684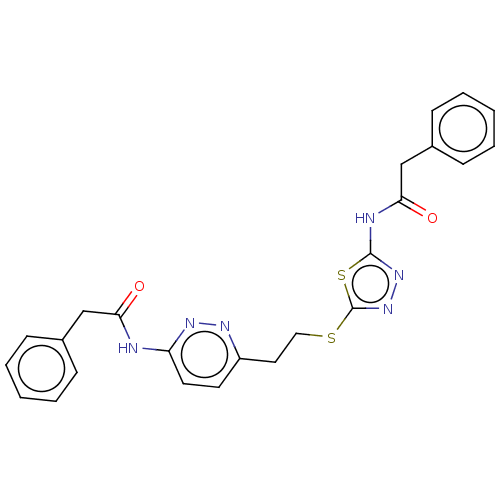 (CHEMBL4740067) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human kidney glutaminase using [3H]-Glutamine as substrate in presence of inhibitor incubated for 45 mins by Perkin Elmer ba... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115698 BindingDB Entry DOI: 10.7270/Q2KW5KMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystine/glutamate transporter (Homo sapiens (Human)) | BDBM50126162 (CHEMBL3629671) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of Xct in human CCF-STTG1 cells assessed as glutamate release after 2 hrs by fluorometry | Bioorg Med Chem Lett 25: 4787-92 (2015) Article DOI: 10.1016/j.bmcl.2015.07.018 BindingDB Entry DOI: 10.7270/Q27H1MD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521419 (CHEMBL1342201) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length nSMase expressed in HEK293 cells using sphingomyelin as substrate measured after 1 hr by alkaline phospha... | J Med Chem 63: 6028-6056 (2020) Article DOI: 10.1021/acs.jmedchem.0c00278 BindingDB Entry DOI: 10.7270/Q2862M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystine/glutamate transporter (Homo sapiens (Human)) | BDBM50126153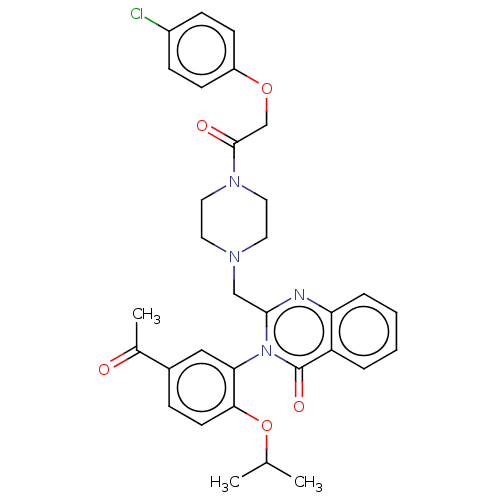 (CHEMBL3629576) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of Xct in human CCF-STTG1 cells assessed as glutamate release after 2 hrs by fluorometry | Bioorg Med Chem Lett 25: 4787-92 (2015) Article DOI: 10.1016/j.bmcl.2015.07.018 BindingDB Entry DOI: 10.7270/Q27H1MD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459018 (CHEMBL4166405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 [44-750] (Homo sapiens (Human)) | BDBM17754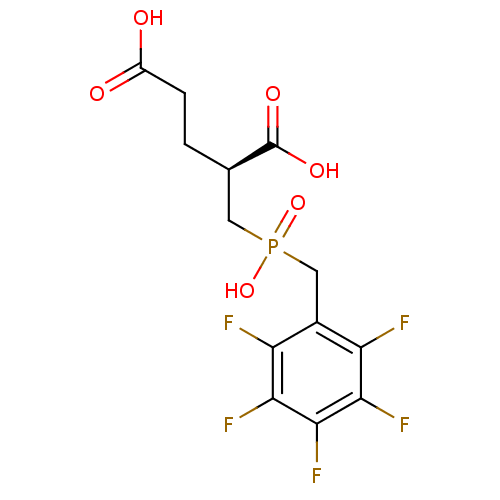 ((2S)-2-({hydroxy[(2,3,4,5,6-pentafluorophenyl)meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Guilford | Assay Description GCPII activity in vitro is monitored through the hydrolysis [3H]NAAG to NAA and [3H]Glu. The radioactivity-based assay was miniaturized to a 96-well ... | J Med Chem 48: 2319-24 (2005) Article DOI: 10.1021/jm049258g BindingDB Entry DOI: 10.7270/Q2D50K77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50467718 (CHEMBL4287507) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokushima University Curated by ChEMBL | Assay Description Inhibition of human DAO using D-serine as substrate after 20 mins in presence of FAD | Eur J Med Chem 159: 23-34 (2018) Article DOI: 10.1016/j.ejmech.2018.09.040 BindingDB Entry DOI: 10.7270/Q28918KH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50535990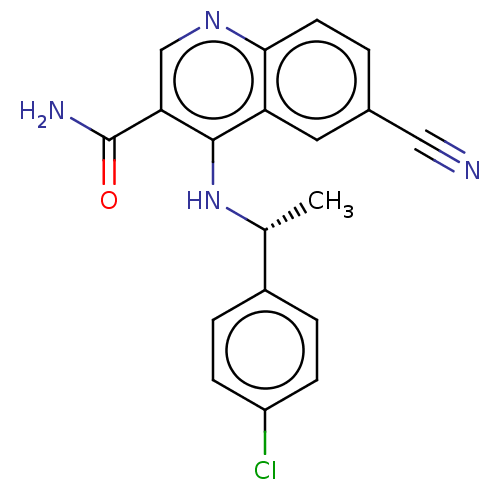 (CHEMBL4527822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant PI3Kalpha using PIP2/ATP as substrate incubated for 20 mins followed by substrate addition by Kinase Glo reagent base... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50536001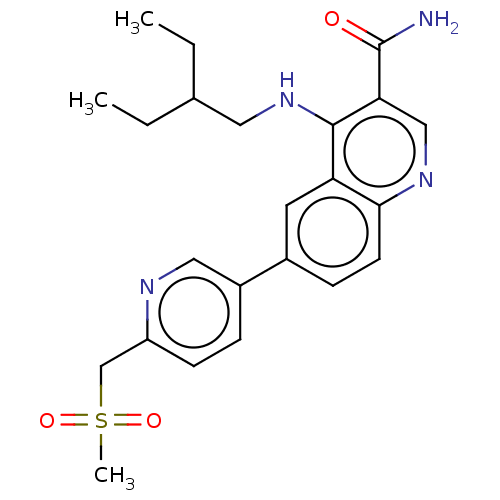 (CHEMBL4576302) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM phosphorylation at Ser-1981 residue in human HT-29 cells incubated for 1 hr followed by X-ray irradiation by Hoechst staining based... | J Med Chem 59: 6281-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00519 BindingDB Entry DOI: 10.7270/Q2NV9NR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 749 total ) | Next | Last >> |