

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19854 (CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410611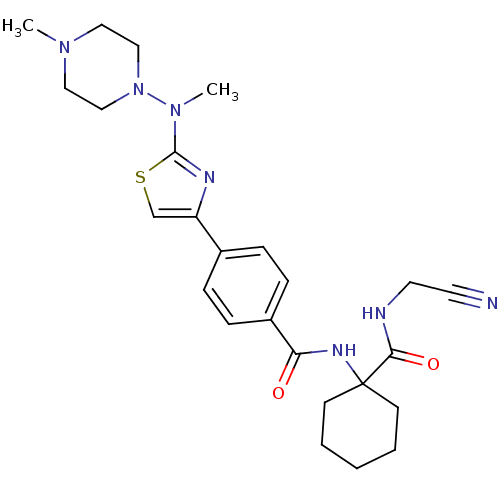 (CHEMBL414669) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410588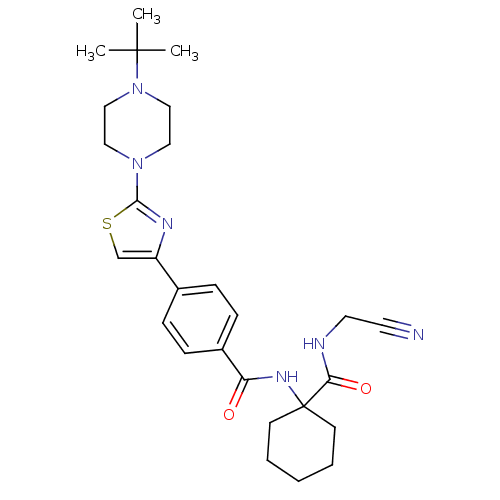 (CHEMBL200708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410609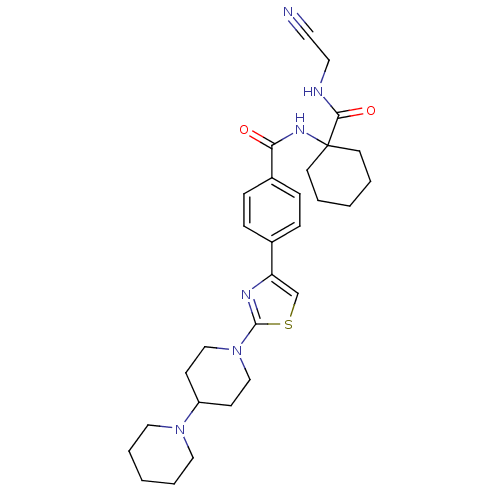 (CHEMBL198798) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410590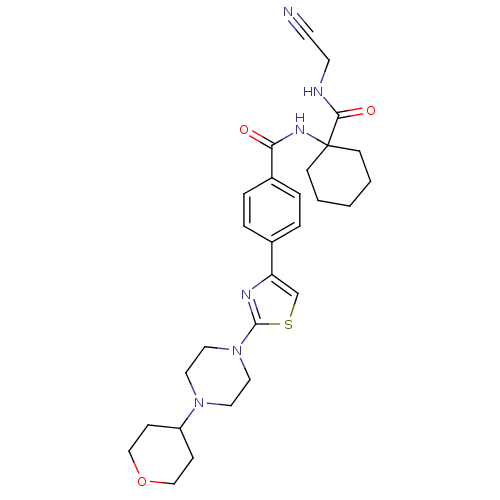 (CHEMBL200543) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410587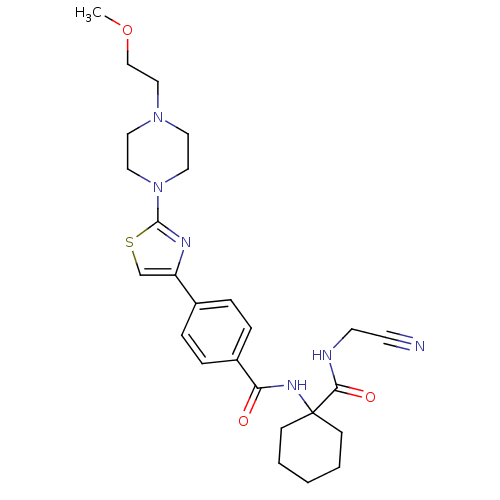 (CHEMBL200602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19854 (CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410607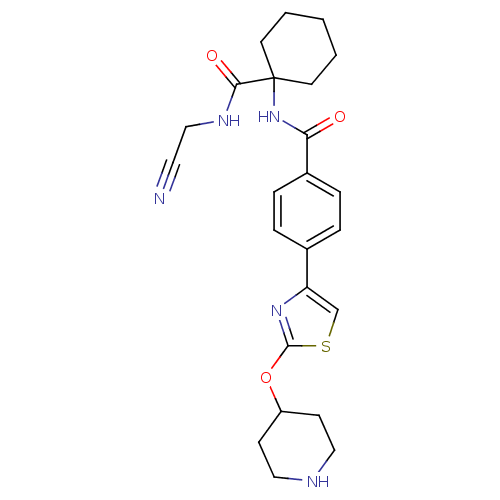 (CHEMBL200744) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410571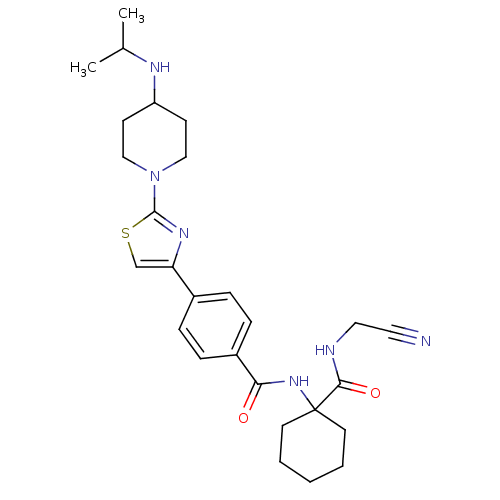 (CHEMBL200287) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410580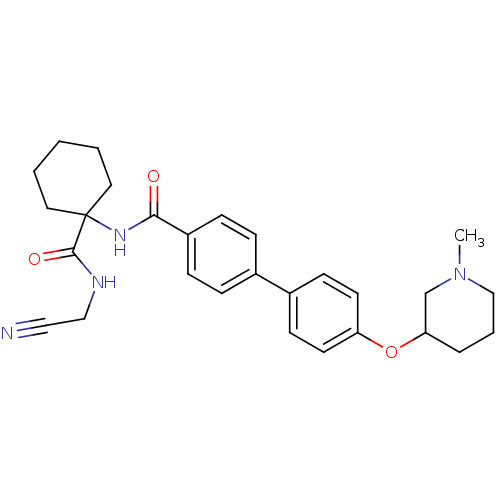 (CHEMBL435913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410575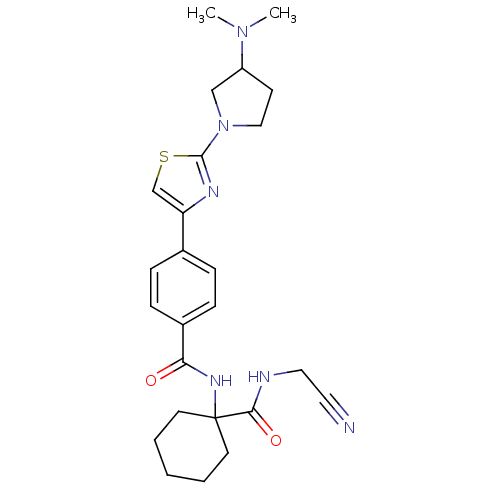 (CHEMBL199470) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410591 (CHEMBL200506) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410612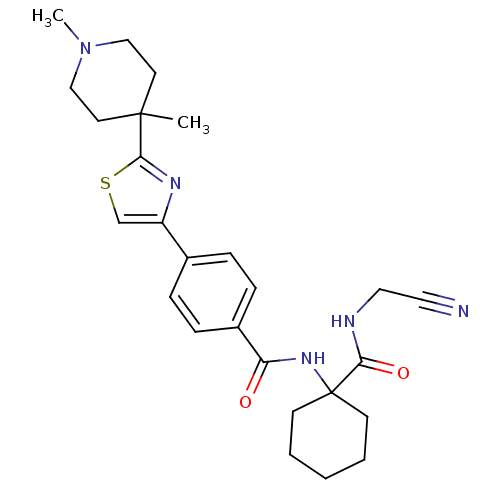 (CHEMBL200166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410595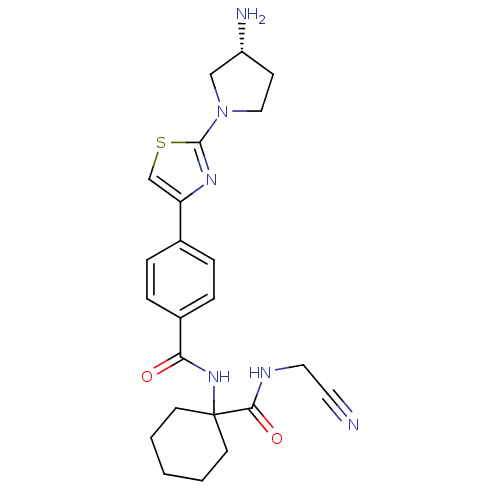 (CHEMBL200507) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19855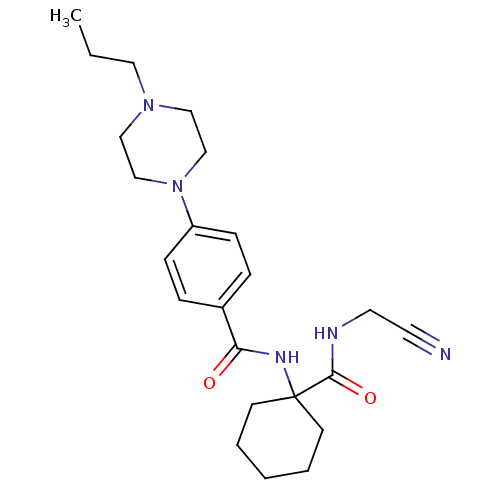 (Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410572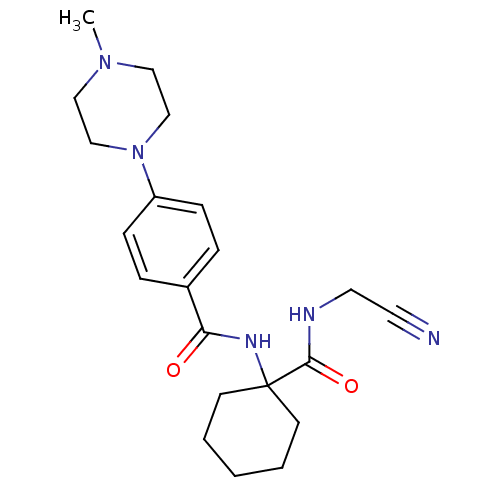 (CHEMBL440035) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410592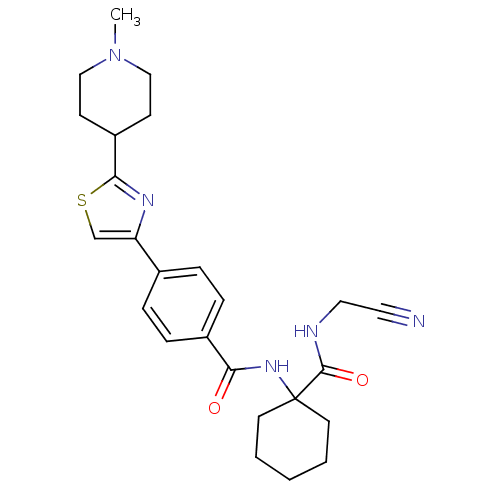 (CHEMBL200455) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410594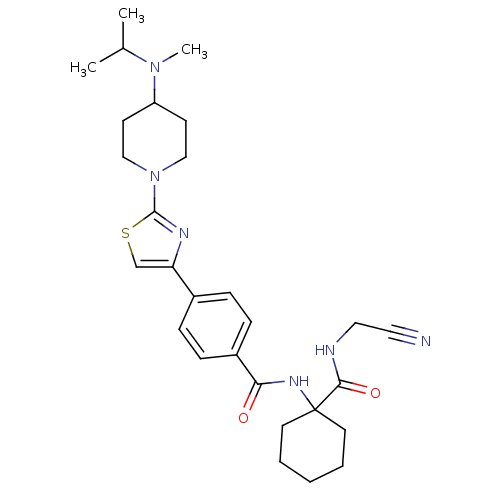 (CHEMBL200596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15494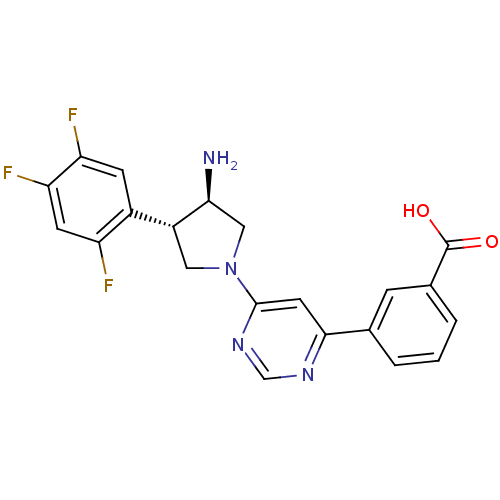 (3-{6-[(3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410606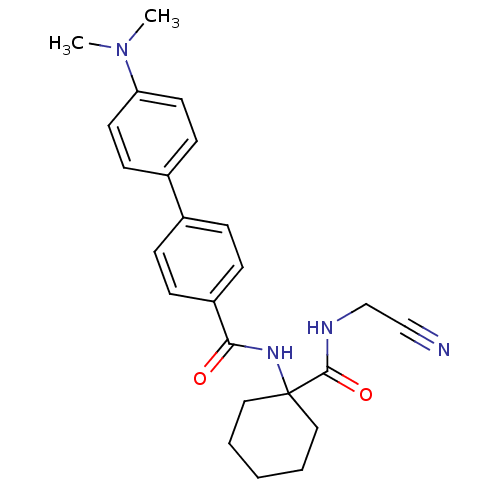 (CHEMBL383186) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15500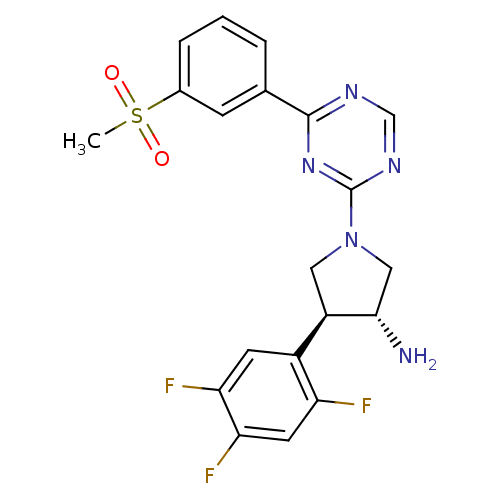 ((3R,4S)-1-[4-(3-methanesulfonylphenyl)-1,3,5-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19857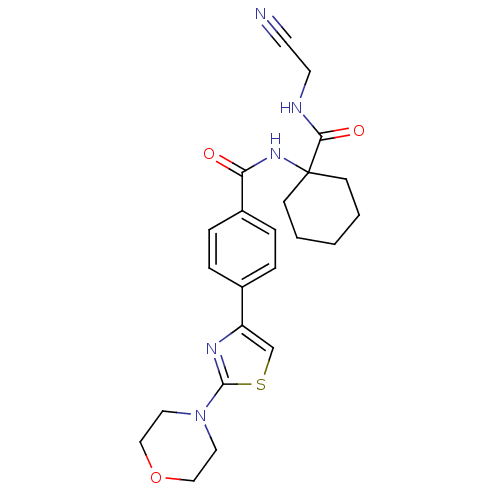 (N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-[2-(mor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15497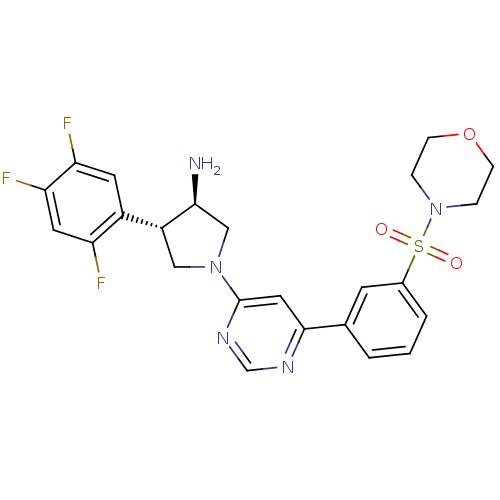 ((3R,4S)-1-{6-[3-(morpholine-4-sulfonyl)phenyl]pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410603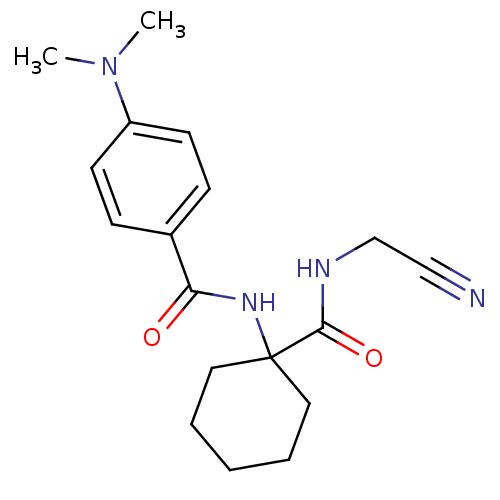 (CHEMBL198859) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15498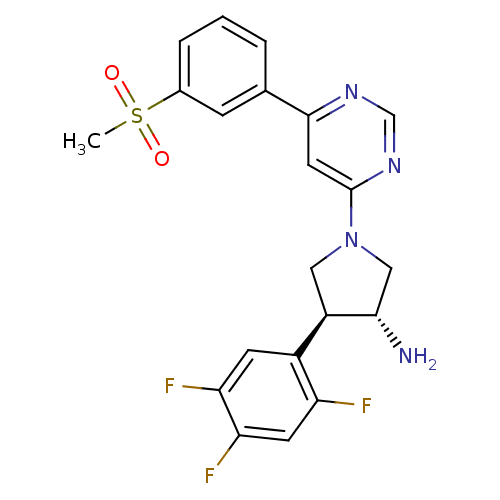 ((3R,4S)-1-[6-(3-methanesulfonylphenyl)pyrimidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410578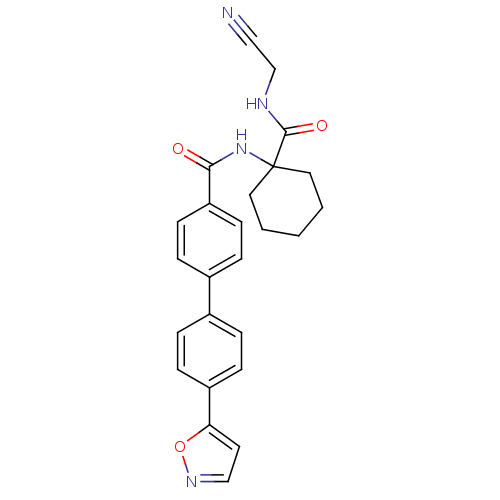 (CHEMBL199014) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15491 ((+/-) racemate | (3R,4S)-1-[6-(thiophen-3-yl)pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410593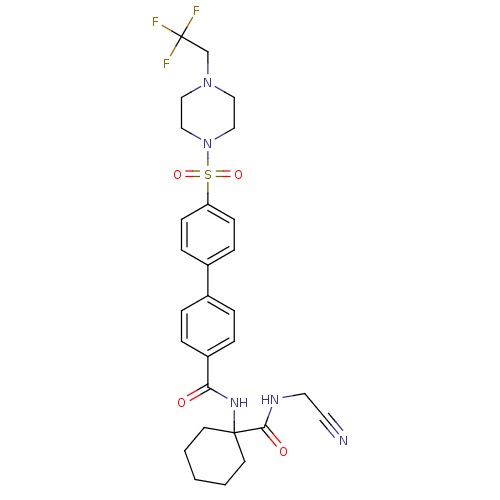 (CHEMBL197440) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410589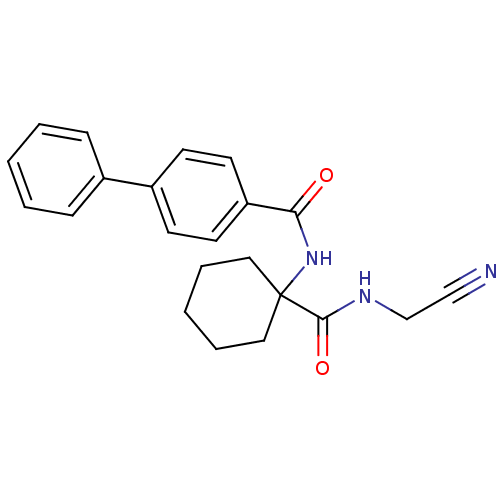 (CHEMBL200619) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15496 (3-{6-[(3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410574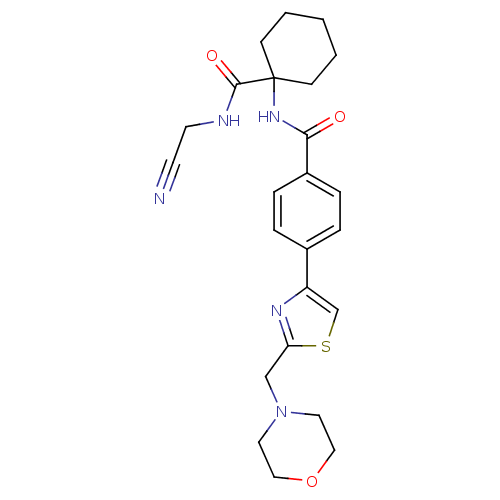 (CHEMBL381026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15499 ((3R,4S)-1-[6-(3-fluoro-5-methanesulfonylphenyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410583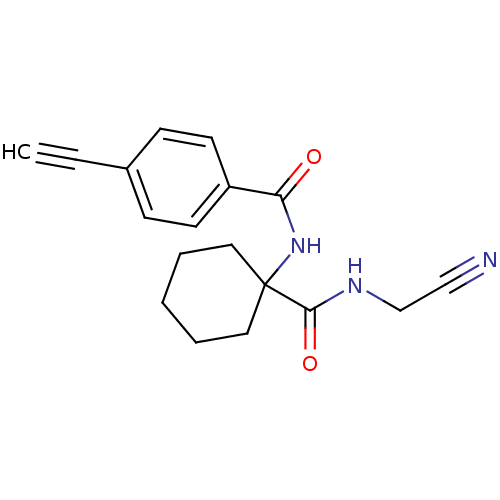 (CHEMBL200599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410597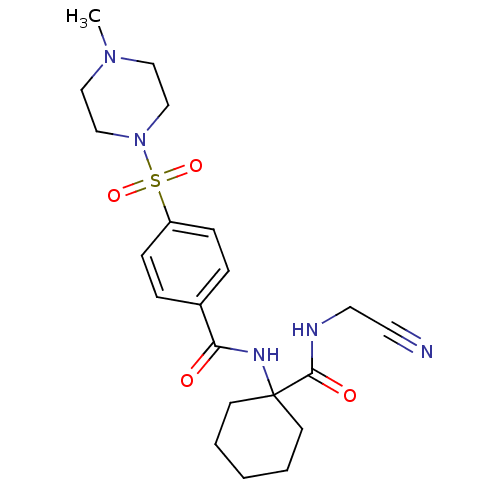 (CHEMBL199488) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410601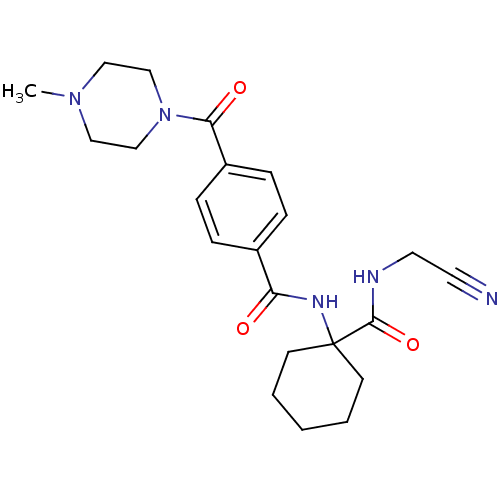 (CHEMBL199487) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15488 ((+/-) racemate | (3R,4S)-4-(2,4-dichlorophenyl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410600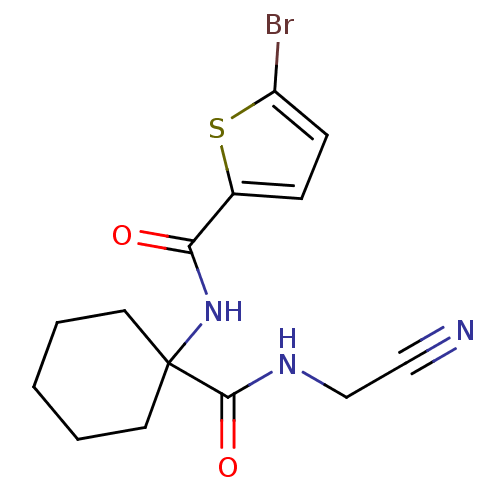 (CHEMBL200167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15491 ((+/-) racemate | (3R,4S)-1-[6-(thiophen-3-yl)pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410602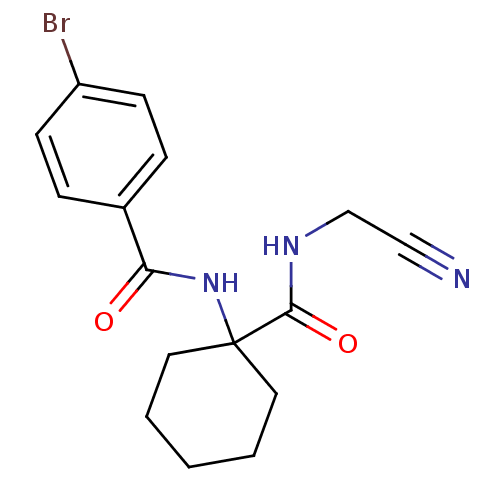 (CHEMBL370786) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19858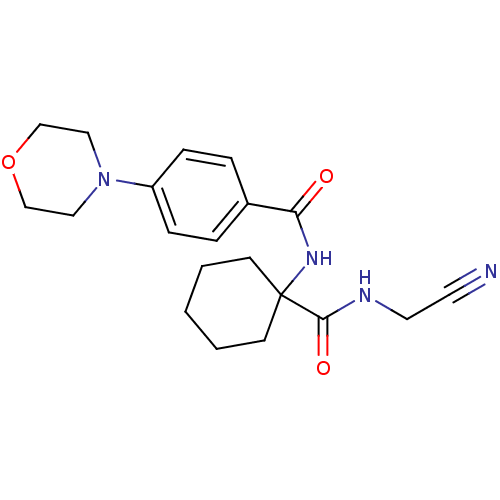 (N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-(morpho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15492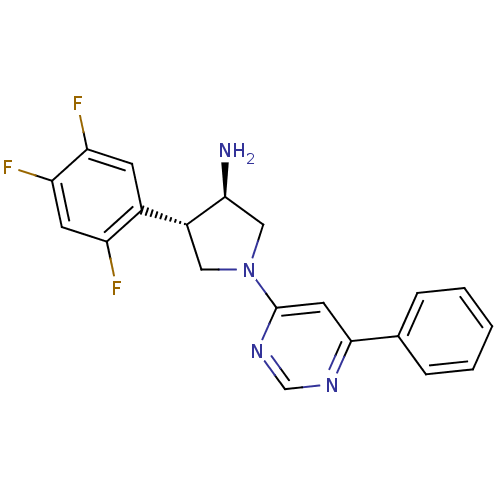 ((3R,4S)-1-(6-phenylpyrimidin-4-yl)-4-(2,4,5-triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410576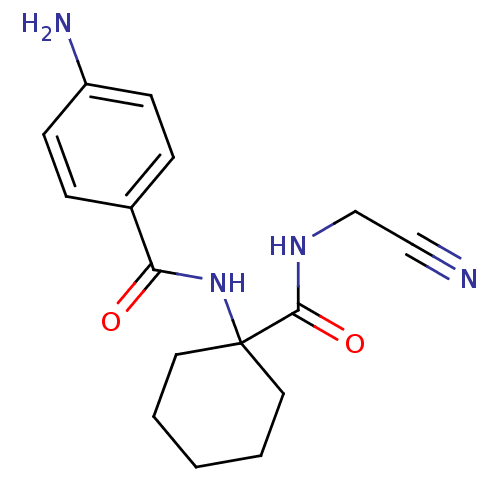 (CHEMBL200203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15489 ((+/-) racemate | (3R,4S)-4-(2-chloro-4-fluoropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19768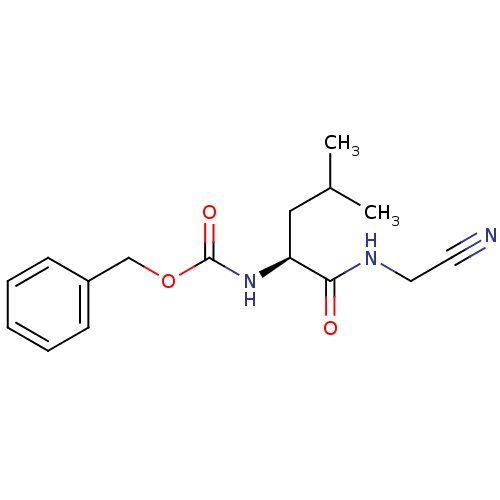 (Cbz-Leu-NH-CH2-CN | JMC487688 Compound 8 | benzyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 34.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15490 ((+/-) racemate | (3R,4S)-4-(2-chloro-4,5-difluorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15486 ((+/-) racemate | (3R,4S)-4-(2,4-dichlorophenyl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410608 (CHEMBL200151) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15495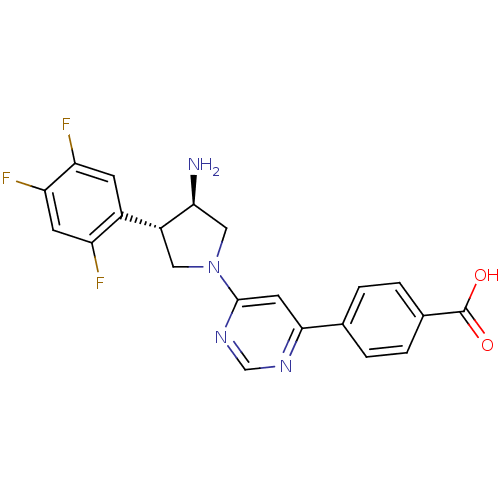 (4-{6-[(3R,4S)-3-amino-4-(2,4,5-trifluorophenyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM15485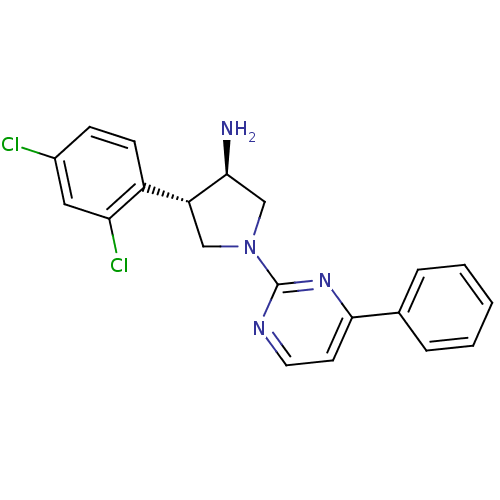 ((+/-) racemate | (3R,4S)-4-(2,4-dichlorophenyl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 140 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | Bioorg Med Chem Lett 17: 2005-12 (2007) Article DOI: 10.1016/j.bmcl.2007.01.026 BindingDB Entry DOI: 10.7270/Q20G3HCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM50410579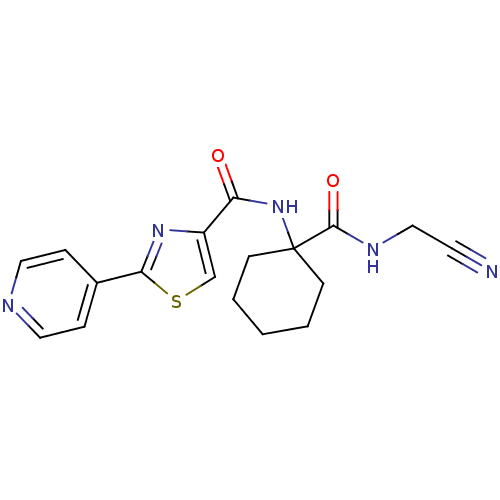 (CHEMBL371771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate | J Med Chem 48: 7520-34 (2005) Article DOI: 10.1021/jm058198r BindingDB Entry DOI: 10.7270/Q23T9GSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 679 total ) | Next | Last >> |