 Found 168 hits with Last Name = 'tseng' and Initial = 't'
Found 168 hits with Last Name = 'tseng' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50056445
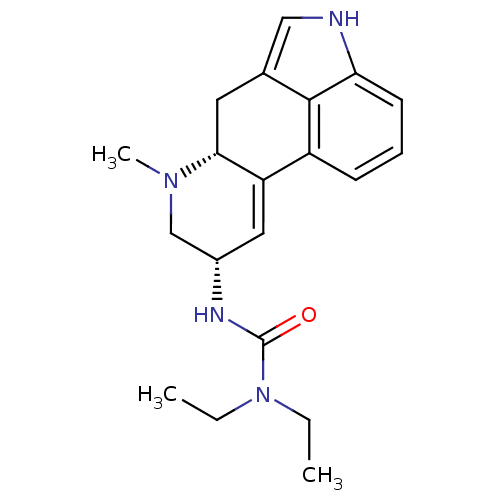
(1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...)Show SMILES CCN(CC)C(=O)N[C@@H]1CN(C)[C@@H]2Cc3c[nH]c4cccc(C2=C1)c34 |c:23| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50067719

((6aR,9R)-5-Bromo-9-carbamoyl-7-methyl-4,6,6a,7,8,9...)Show SMILES C[N@@H+]1C[C@@H](C=C2[C@H]1Cc1c(Br)[nH]c3cccc2c13)C(N)=O |c:4| Show InChI InChI=1S/C16H16BrN3O/c1-20-7-8(16(18)21)5-10-9-3-2-4-12-14(9)11(6-13(10)20)15(17)19-12/h2-5,8,13,19H,6-7H2,1H3,(H2,18,21)/p+1/t8-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004921
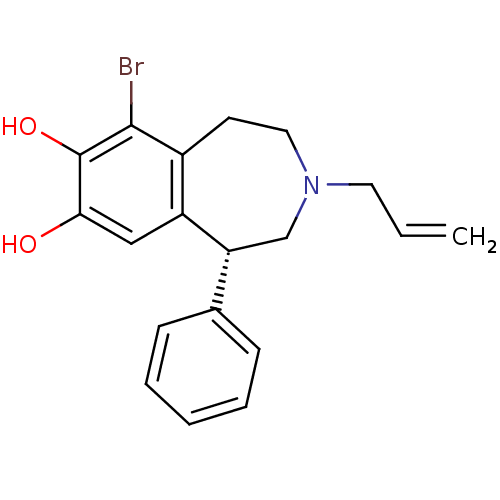
((R)-3-Allyl-6-bromo-7,8-dihydroxy-1-phenyl-2,3,4,5...)Show InChI InChI=1S/C19H20BrNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602543
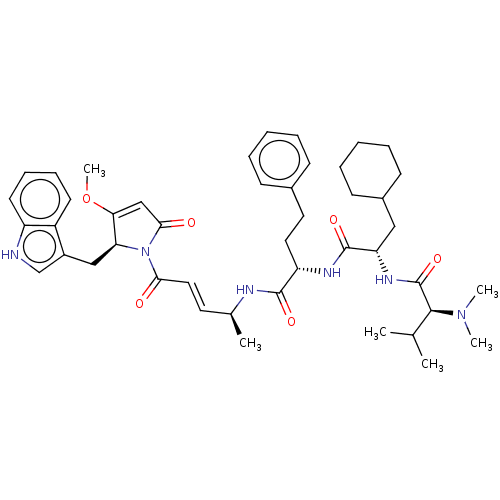
(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50049048

((R)-3-Allyl-6-chloro-1-phenyl-2,3,4,5-tetrahydro-1...)Show InChI InChI=1S/C19H20ClNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50010289
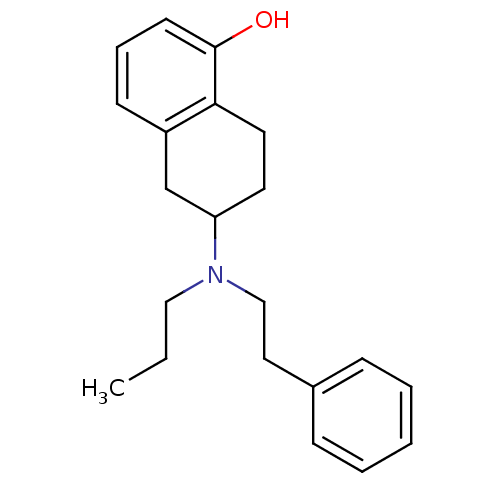
((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C21H27NO/c1-2-14-22(15-13-17-7-4-3-5-8-17)19-11-12-20-18(16-19)9-6-10-21(20)23/h3-10,19,23H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Mus musculus) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50067726
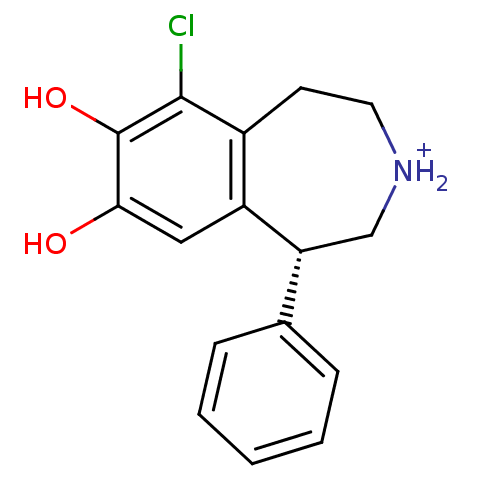
((R)-6-Chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrah...)Show InChI InChI=1S/C16H16ClNO2/c17-15-11-6-7-18-9-13(10-4-2-1-3-5-10)12(11)8-14(19)16(15)20/h1-5,8,13,18-20H,6-7,9H2/p+1/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM60917
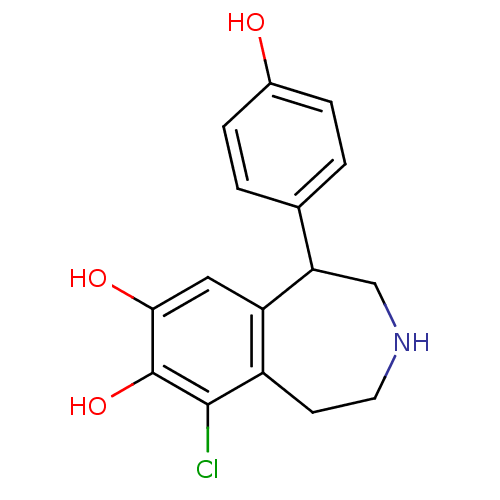
(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50017543
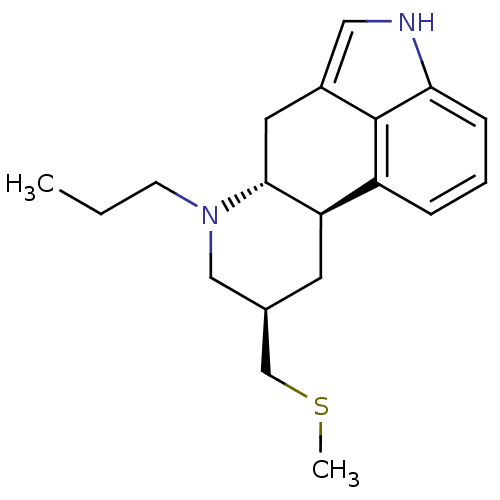
((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...)Show SMILES CCCN1C[C@H](CSC)C[C@H]2[C@H]1Cc1c[nH]c3cccc2c13 |r| Show InChI InChI=1S/C19H26N2S/c1-3-7-21-11-13(12-22-2)8-16-15-5-4-6-17-19(15)14(10-20-17)9-18(16)21/h4-6,10,13,16,18,20H,3,7-9,11-12H2,1-2H3/t13-,16-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50007422
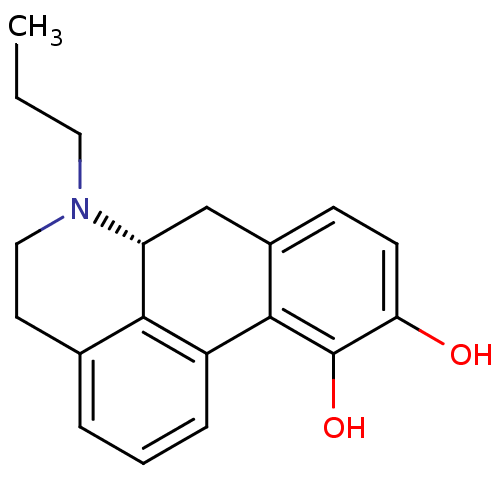
((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...)Show SMILES CCCN1CCc2cccc-3c2[C@H]1Cc1ccc(O)c(O)c-31 |r| Show InChI InChI=1S/C19H21NO2/c1-2-9-20-10-8-12-4-3-5-14-17(12)15(20)11-13-6-7-16(21)19(22)18(13)14/h3-7,15,21-22H,2,8-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233

(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES C[C@H](OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O |r| Show InChI InChI=1S/C32H48N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h6,9-10,13-14,19,21-22,24-27H,5,7-8,11-12,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232
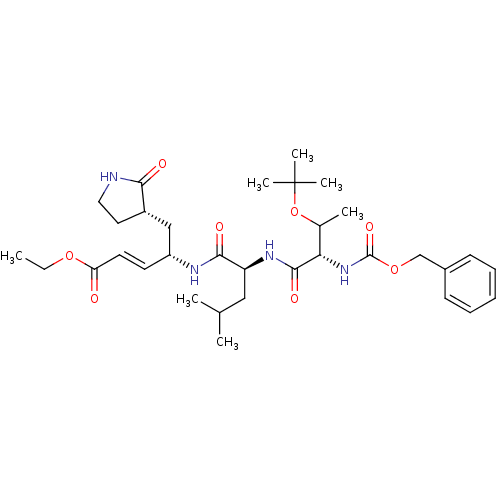
(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)OC(C)(C)C |r| Show InChI InChI=1S/C33H50N4O8/c1-8-43-27(38)15-14-25(19-24-16-17-34-29(24)39)35-30(40)26(18-21(2)3)36-31(41)28(22(4)45-33(5,6)7)37-32(42)44-20-23-12-10-9-11-13-23/h9-15,21-22,24-26,28H,8,16-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b15-14+/t22?,24-,25+,26-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50056445

(1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroerg...)Show SMILES CCN(CC)C(=O)N[C@@H]1CN(C)[C@@H]2Cc3c[nH]c4cccc(C2=C1)c34 |c:23| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50033762

(Gallinamide A)Show SMILES CC[C@H](C)[C@H](N(C)C)C(=O)O[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)\C=C\C(=O)N1[C@@H](C)C(OC)=CC1=O |r,c:38| Show InChI InChI=1S/C31H52N4O7/c1-12-20(6)28(34(9)10)31(40)42-25(16-19(4)5)30(39)33-23(15-18(2)3)29(38)32-21(7)13-14-26(36)35-22(8)24(41-11)17-27(35)37/h13-14,17-23,25,28H,12,15-16H2,1-11H3,(H,32,38)(H,33,39)/b14-13+/t20-,21-,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160665
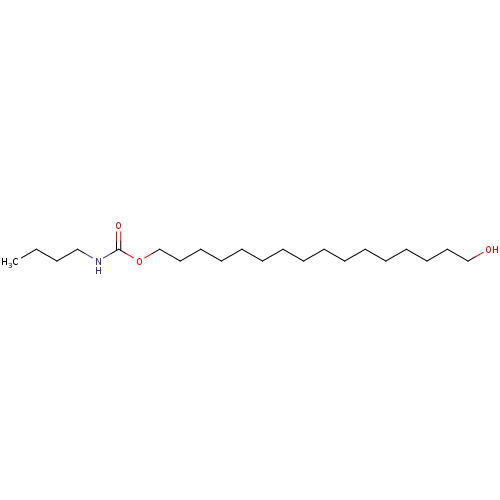
(Butyl-carbamic acid 16-hydroxy-hexadecyl ester | C...)Show InChI InChI=1S/C21H43NO3/c1-2-3-18-22-21(24)25-20-17-15-13-11-9-7-5-4-6-8-10-12-14-16-19-23/h23H,2-20H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160664
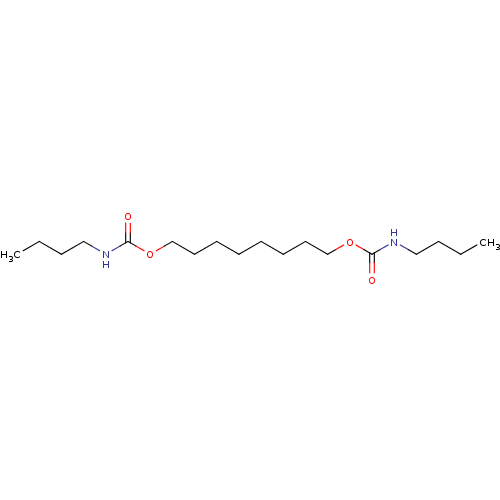
(Butyl-carbamic acid 8-butylcarbamoyloxy-octyl este...)Show InChI InChI=1S/C18H36N2O4/c1-3-5-13-19-17(21)23-15-11-9-7-8-10-12-16-24-18(22)20-14-6-4-2/h3-16H2,1-2H3,(H,19,21)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160660
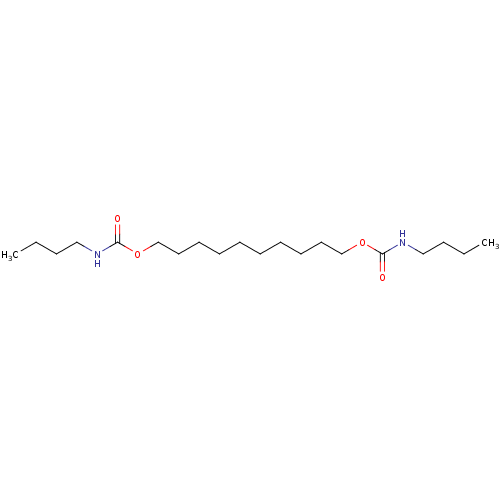
(Butyl-carbamic acid 10-butylcarbamoyloxy-decyl est...)Show InChI InChI=1S/C20H40N2O4/c1-3-5-15-21-19(23)25-17-13-11-9-7-8-10-12-14-18-26-20(24)22-16-6-4-2/h3-18H2,1-2H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160667

(Butyl-carbamic acid 14-butylcarbamoyloxy-tetradecy...)Show InChI InChI=1S/C24H48N2O4/c1-3-5-19-25-23(27)29-21-17-15-13-11-9-7-8-10-12-14-16-18-22-30-24(28)26-20-6-4-2/h3-22H2,1-2H3,(H,25,27)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160659
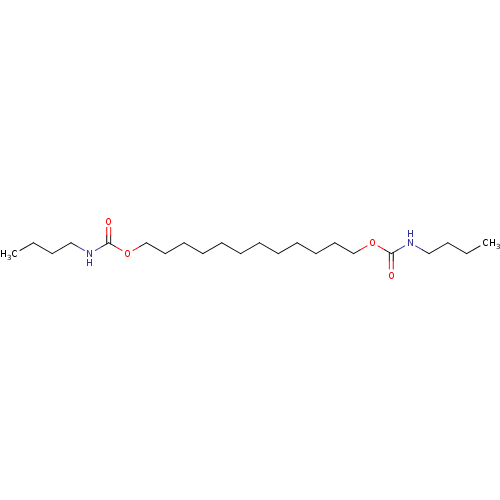
(Butyl-carbamic acid 12-butylcarbamoyloxy-dodecyl e...)Show InChI InChI=1S/C22H44N2O4/c1-3-5-17-23-21(25)27-19-15-13-11-9-7-8-10-12-14-16-20-28-22(26)24-18-6-4-2/h3-20H2,1-2H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160661
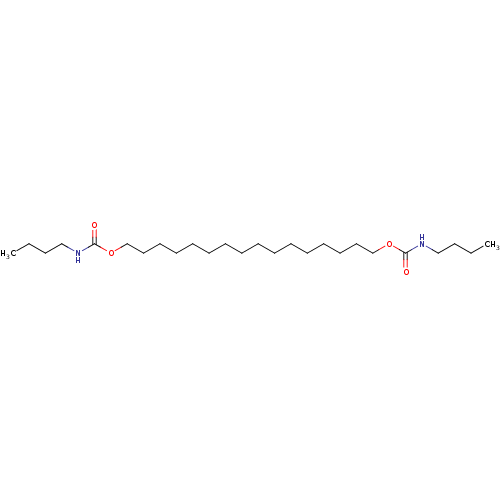
(Butyl-carbamic acid 16-butylcarbamoyloxy-hexadecyl...)Show InChI InChI=1S/C26H52N2O4/c1-3-5-21-27-25(29)31-23-19-17-15-13-11-9-7-8-10-12-14-16-18-20-24-32-26(30)28-22-6-4-2/h3-24H2,1-2H3,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160657

(Butyl-carbamic acid 14-hydroxy-tetradecyl ester | ...)Show InChI InChI=1S/C19H39NO3/c1-2-3-16-20-19(22)23-18-15-13-11-9-7-5-4-6-8-10-12-14-17-21/h21H,2-18H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160656
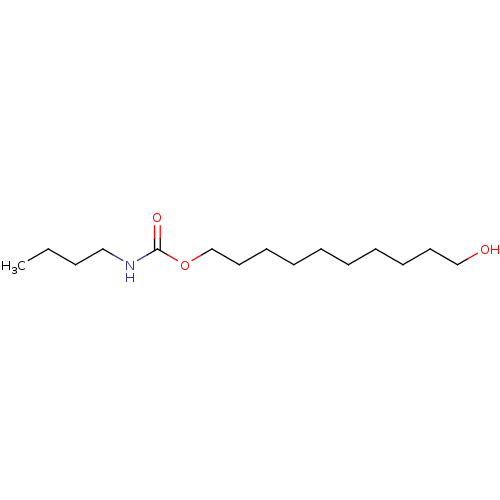
(Butyl-carbamic acid 10-hydroxy-decyl ester | CHEMB...)Show InChI InChI=1S/C15H31NO3/c1-2-3-12-16-15(18)19-14-11-9-7-5-4-6-8-10-13-17/h17H,2-14H2,1H3,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602545

(CHEMBL4596647)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160666
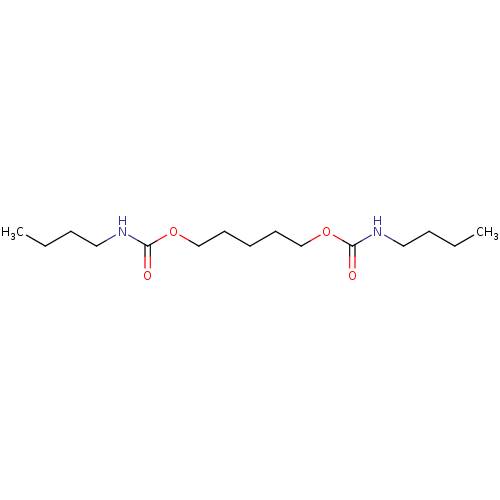
(Butyl-carbamic acid 5-butylcarbamoyloxy-pentyl est...)Show InChI InChI=1S/C15H30N2O4/c1-3-5-10-16-14(18)20-12-8-7-9-13-21-15(19)17-11-6-4-2/h3-13H2,1-2H3,(H,16,18)(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160654
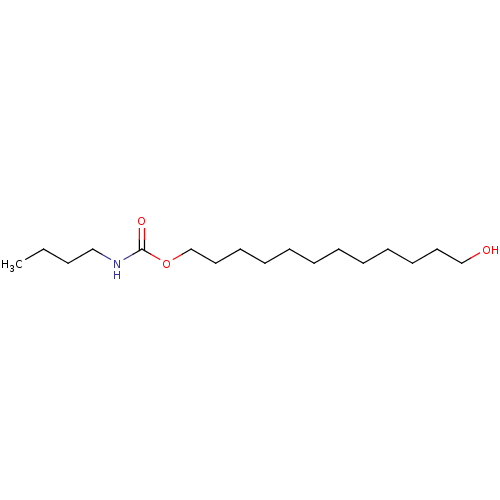
(Butyl-carbamic acid 12-hydroxy-dodecyl ester | CHE...)Show InChI InChI=1S/C17H35NO3/c1-2-3-14-18-17(20)21-16-13-11-9-7-5-4-6-8-10-12-15-19/h19H,2-16H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004822
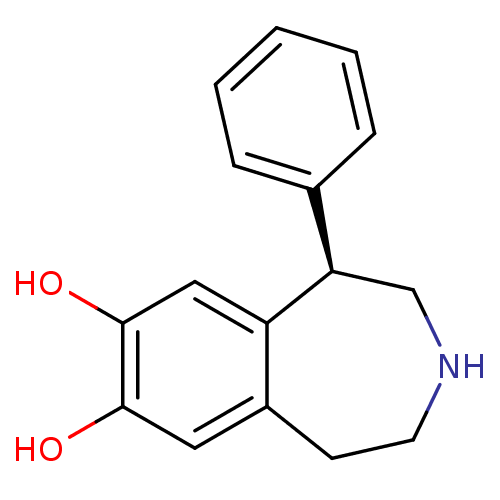
((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50025206
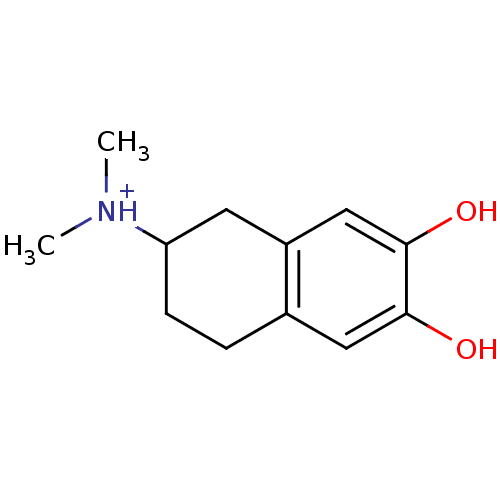
((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...)Show InChI InChI=1S/C12H17NO2/c1-13(2)10-4-3-8-6-11(14)12(15)7-9(8)5-10/h6-7,10,14-15H,3-5H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602543

(CHEMBL4598338)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](C(C)C)N(C)C |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50004921

((R)-3-Allyl-6-bromo-7,8-dihydroxy-1-phenyl-2,3,4,5...)Show InChI InChI=1S/C19H20BrNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160663
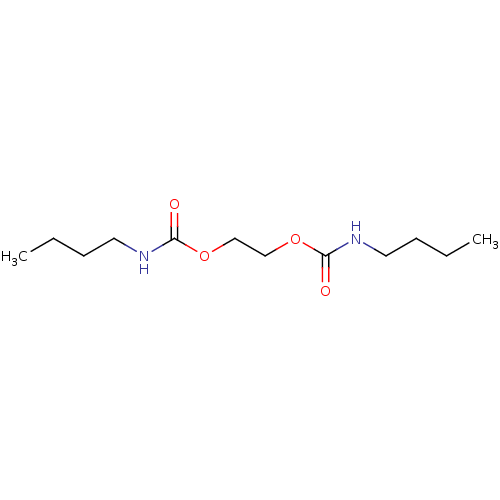
(Butyl-carbamic acid 2-butylcarbamoyloxy-ethyl este...)Show InChI InChI=1S/C12H24N2O4/c1-3-5-7-13-11(15)17-9-10-18-12(16)14-8-6-4-2/h3-10H2,1-2H3,(H,13,15)(H,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160662
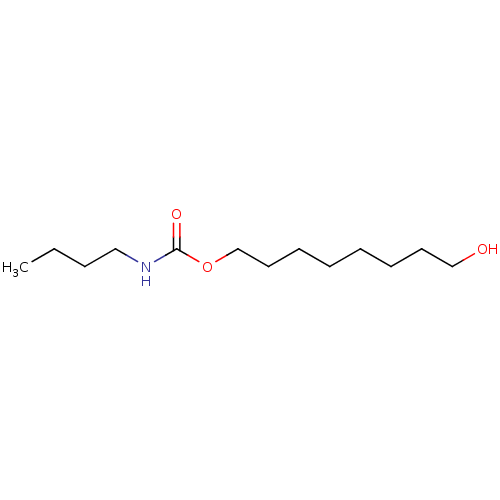
(Butyl-carbamic acid 8-hydroxy-octyl ester | CHEMBL...)Show InChI InChI=1S/C13H27NO3/c1-2-3-10-14-13(16)17-12-9-7-5-4-6-8-11-15/h15H,2-12H2,1H3,(H,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50049048

((R)-3-Allyl-6-chloro-1-phenyl-2,3,4,5-tetrahydro-1...)Show InChI InChI=1S/C19H20ClNO2/c1-2-9-21-10-8-14-15(11-17(22)19(23)18(14)20)16(12-21)13-6-4-3-5-7-13/h2-7,11,16,22-23H,1,8-10,12H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231

(N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C30H44N4O7/c1-6-40-25(35)13-12-23(17-22-14-15-31-27(22)36)32-28(37)24(16-19(2)3)33-29(38)26(20(4)5)34-30(39)41-18-21-10-8-7-9-11-21/h7-13,19-20,22-24,26H,6,14-18H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H,34,39)/b13-12+/t22-,23+,24-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602544

(CHEMBL4594757)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50602542

(CHEMBL4594870)Show SMILES COC1=CC(=O)N([C@H]1Cc1c[nH]c2ccccc12)C(=O)\C=C\[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCN(C)CC1 |r,t:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 861 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01494
BindingDB Entry DOI: 10.7270/Q2FR01PM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50010686
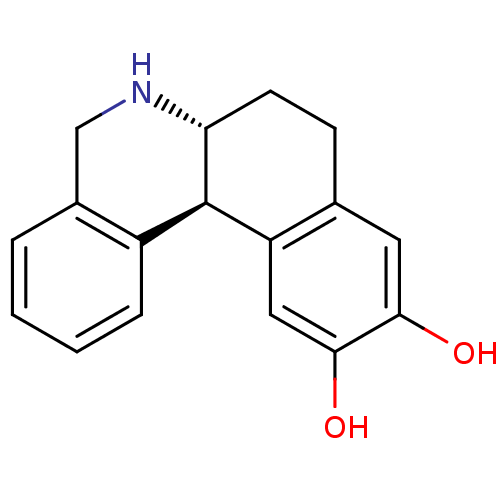
((6aR,12bS)-10,11-Dihydroxy-5,6,6a,7,8,12b-hexahydr...)Show InChI InChI=1S/C17H17NO2/c19-15-7-10-5-6-14-17(13(10)8-16(15)20)12-4-2-1-3-11(12)9-18-14/h1-4,7-8,14,17-20H,5-6,9H2/t14-,17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50010289

((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...)Show InChI InChI=1S/C21H27NO/c1-2-14-22(15-13-17-7-4-3-5-8-17)19-11-12-20-18(16-19)9-6-10-21(20)23/h3-10,19,23H,2,11-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50067719

((6aR,9R)-5-Bromo-9-carbamoyl-7-methyl-4,6,6a,7,8,9...)Show SMILES C[N@@H+]1C[C@@H](C=C2[C@H]1Cc1c(Br)[nH]c3cccc2c13)C(N)=O |c:4| Show InChI InChI=1S/C16H16BrN3O/c1-20-7-8(16(18)21)5-10-9-3-2-4-12-14(9)11(6-13(10)20)15(17)19-12/h2-5,8,13,19H,6-7H2,1H3,(H2,18,21)/p+1/t8-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160658
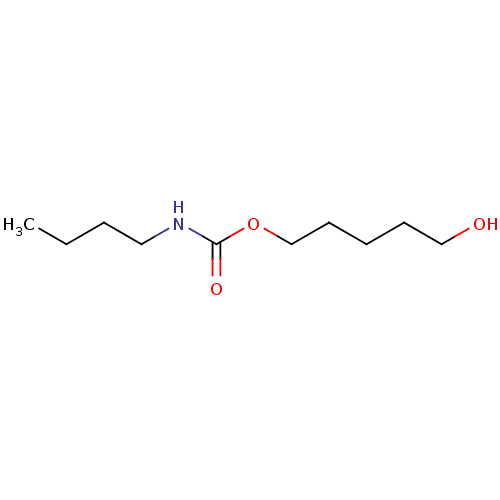
(Butyl-carbamic acid 5-hydroxy-pentyl ester | CHEMB...)Show InChI InChI=1S/C10H21NO3/c1-2-3-7-11-10(13)14-9-6-4-5-8-12/h12H,2-9H2,1H3,(H,11,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50160655
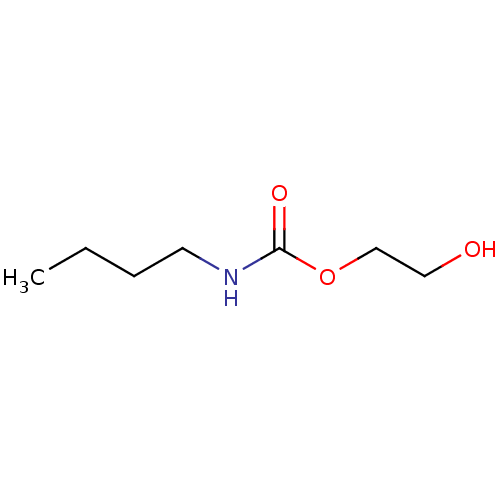
(Butyl-carbamic acid 2-hydroxy-ethyl ester | CHEMBL...)Show InChI InChI=1S/C7H15NO3/c1-2-3-4-8-7(10)11-6-5-9/h9H,2-6H2,1H3,(H,8,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University
Curated by ChEMBL
| Assay Description
Inhibition constant for acetylcholinesterase |
Bioorg Med Chem Lett 15: 951-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.058
BindingDB Entry DOI: 10.7270/Q2KW5FJ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data