 Found 137 hits with Last Name = 'tsou' and Initial = 'lk'
Found 137 hits with Last Name = 'tsou' and Initial = 'lk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin reductase 2
(Human) | BDBM616841
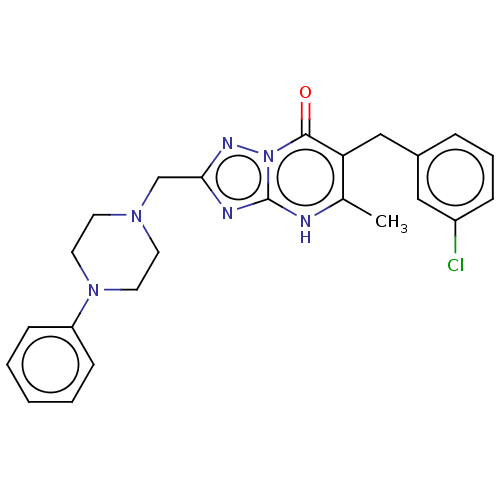
(6-(3-chlorobenzyl)-5-methyl-2-((4-phenylpiperazin-...)Show SMILES Cc1[nH]c2nc(CN3CCN(CC3)c3ccccc3)nn2c(=O)c1Cc1cccc(Cl)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616837
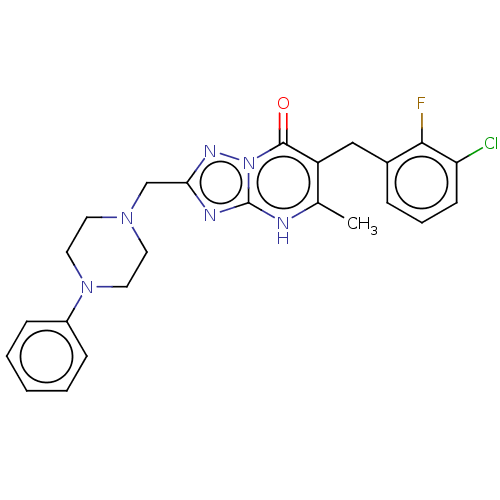
(6-(3-chloro-2-fluorobenzyl)-5-methyl-2-((4-phenylp...)Show SMILES Cc1[nH]c2nc(CN3CCN(CC3)c3ccccc3)nn2c(=O)c1Cc1cccc(Cl)c1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616833
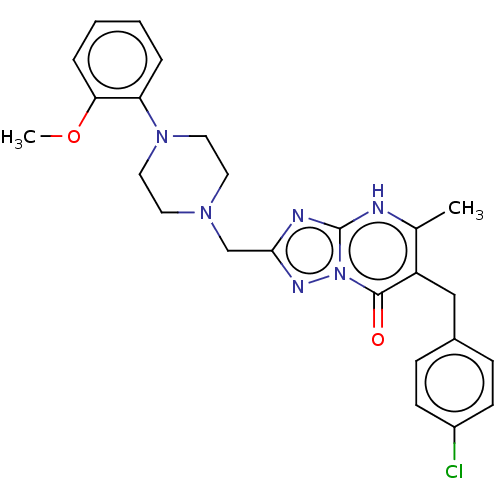
(6-(4-chlorobenzyl)-2-((4-(2-methoxyphenyl)piperazi...)Show SMILES COc1ccccc1N1CCN(Cc2nc3[nH]c(C)c(Cc4ccc(Cl)cc4)c(=O)n3n2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616851
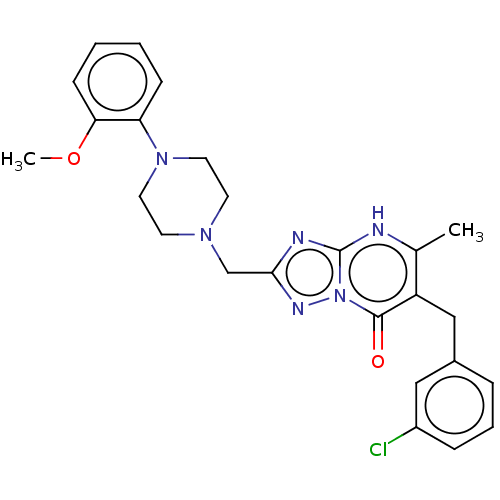
(6-(3-chlorobenzyl)-2-((4-(2-methoxyphenyl)piperazi...)Show SMILES COc1ccccc1N1CCN(Cc2nc3[nH]c(C)c(Cc4cccc(Cl)c4)c(=O)n3n2)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616846
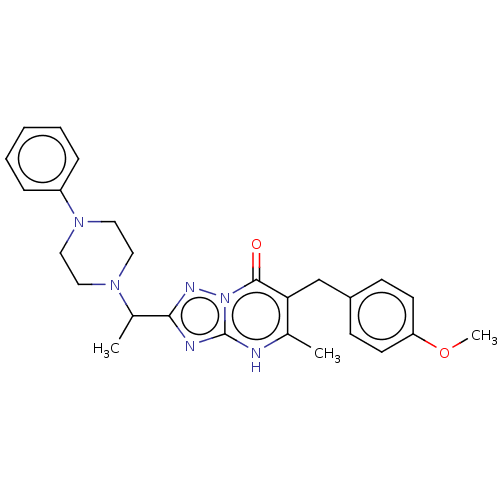
(6-(4-methoxybenzyl)-5-methyl-2-(1-(4-phenylpiperaz...)Show SMILES COc1ccc(Cc2c(C)[nH]c3nc(nn3c2=O)C(C)N2CCN(CC2)c2ccccc2)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616854
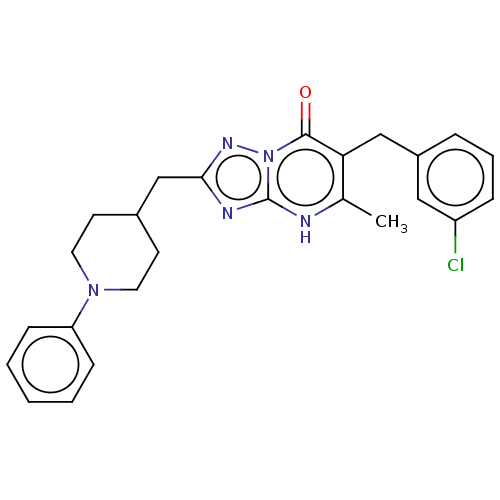
(6-(3-chlorobenzyl)-5-methyl-2-((1-phenylpiperidin-...)Show SMILES Cc1[nH]c2nc(CC3CCN(CC3)c3ccccc3)nn2c(=O)c1Cc1cccc(Cl)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616838
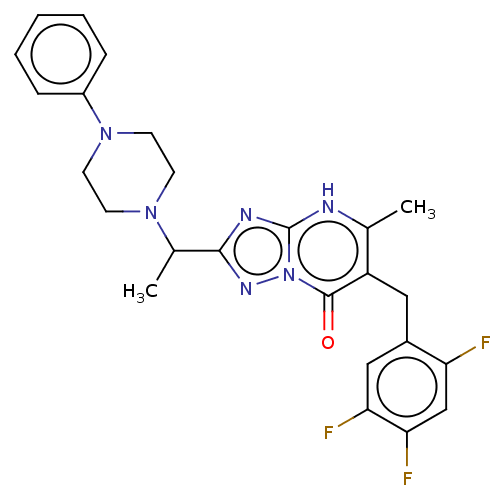
(5-methyl-2-(1-(4-phenylpiperazin-1-yl)ethyl)-6-(2,...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3cc(F)c(F)cc3F)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616839
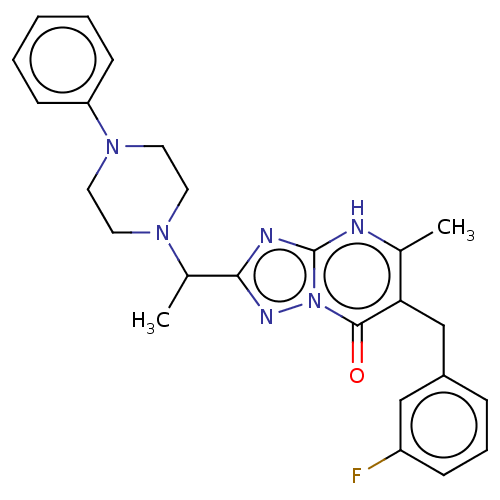
(6-(3-fluorobenzyl)-5-methyl-2-(1-(4-phenylpiperazi...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3cccc(F)c3)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616847
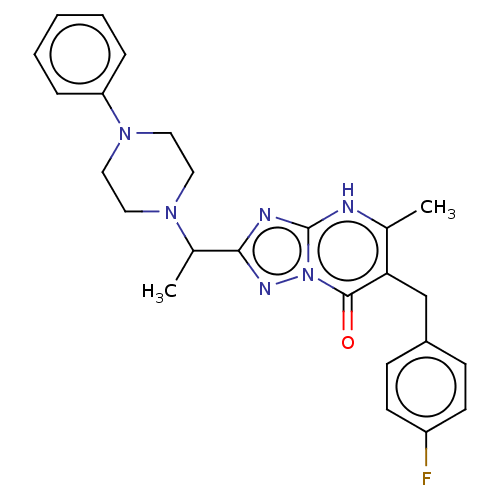
(6-(4-fluorobenzyl)-5-methyl-2-(1-(4-phenylpiperazi...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3ccc(F)cc3)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616845
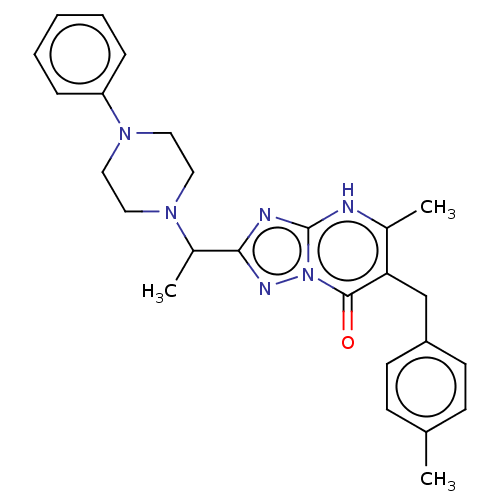
(5-methyl-6-(4-methylbenzyl)-2-(1-(4-phenylpiperazi...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3ccc(C)cc3)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616836
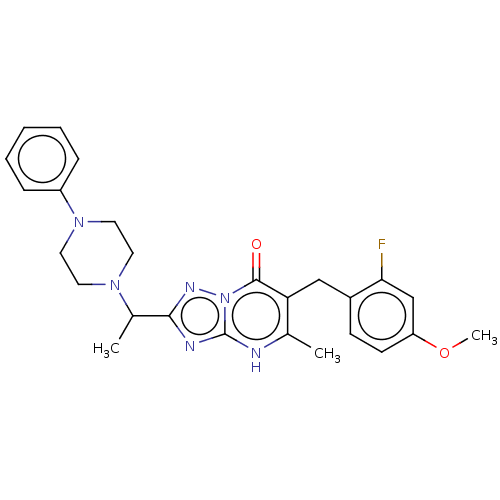
(6-(2-fluoro-4-methoxybenzyl)-5-methyl-2-(1-(4-phen...)Show SMILES COc1ccc(Cc2c(C)[nH]c3nc(nn3c2=O)C(C)N2CCN(CC2)c2ccccc2)c(F)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616848
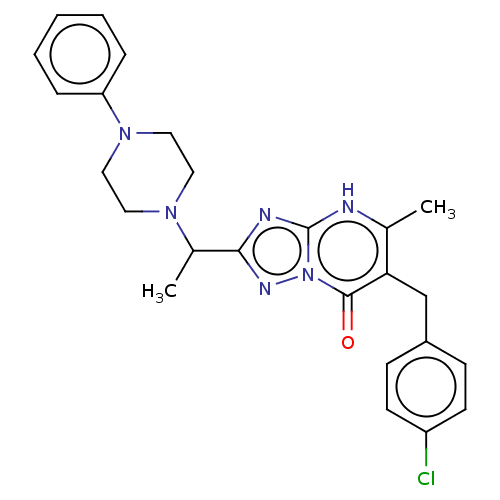
(6-(4-chlorobenzyl)-5-methyl-2-(1-(4-phenylpiperazi...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3ccc(Cl)cc3)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616849
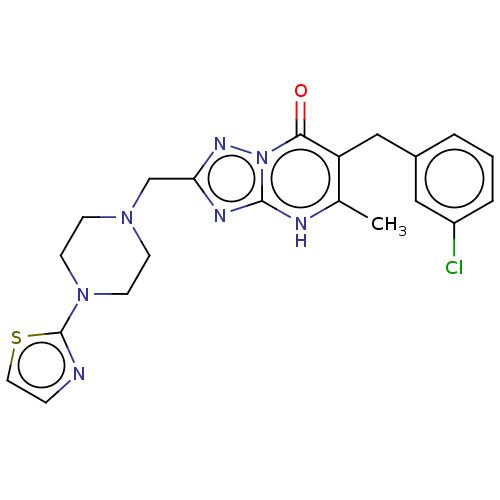
(6-(3-chlorobenzyl)-5-methyl-2-((4-(thiazol-2-yl)pi...)Show SMILES Cc1[nH]c2nc(CN3CCN(CC3)c3nccs3)nn2c(=O)c1Cc1cccc(Cl)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 23.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247630
(CHEMBL4068500)Show SMILES Cc1cc(NC2CCNCC2)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C25H44N10/c1-20-17-24(31-22-9-14-27-15-10-22)32-25(30-20)29-18-23-19-35(34-33-23)16-6-12-26-11-5-13-28-21-7-3-2-4-8-21/h17,19,21-22,26-28H,2-16,18H2,1H3,(H2,29,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616835
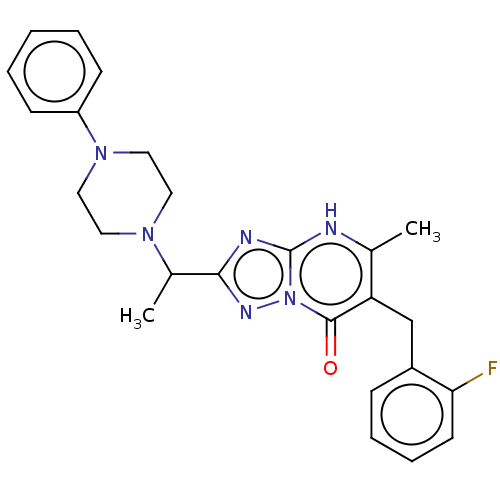
(6-(2-fluorobenzyl)-5-methyl-2-(1-(4-phenylpiperazi...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3ccccc3F)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247631
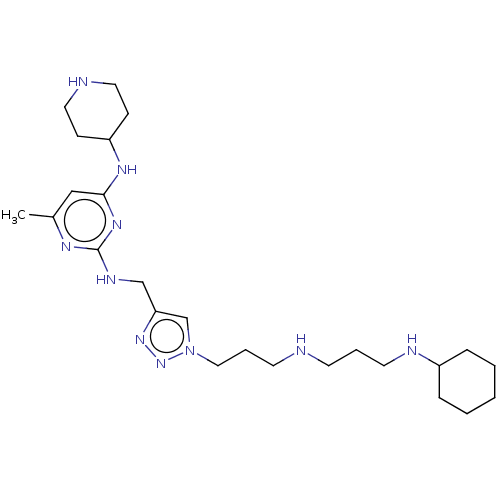
(CHEMBL4060426)Show SMILES Cc1cc(NC2CCN(CCC(N)=O)CC2)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C28H49N11O/c1-22-19-27(34-24-9-16-38(17-10-24)18-11-26(29)40)35-28(33-22)32-20-25-21-39(37-36-25)15-6-13-30-12-5-14-31-23-7-3-2-4-8-23/h19,21,23-24,30-31H,2-18,20H2,1H3,(H2,29,40)(H2,32,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616857
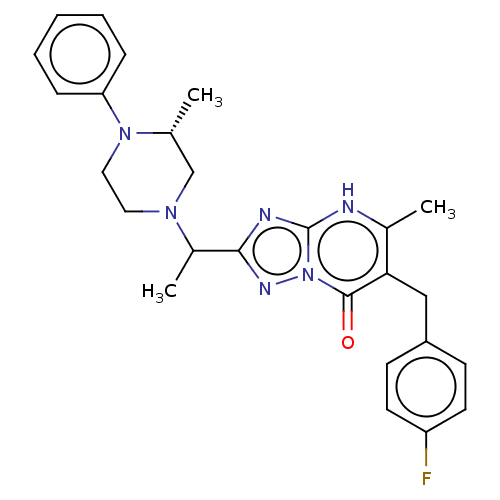
(6-(4-fluorobenzyl)-5-methyl-2-(1-((R)-3-methyl-4-p...)Show SMILES CC(N1CCN([C@H](C)C1)c1ccccc1)c1nc2[nH]c(C)c(Cc3ccc(F)cc3)c(=O)n2n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 31.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616843
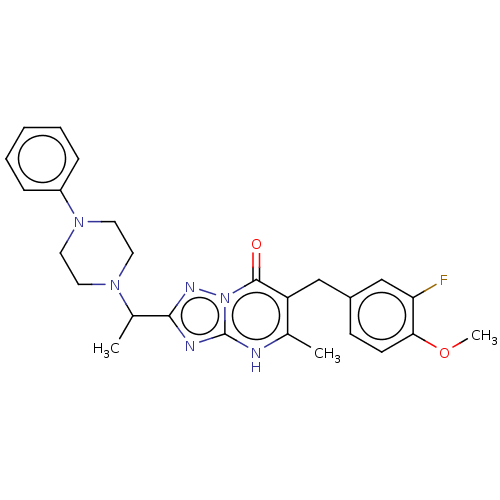
(6-(3-fluoro-4-methoxybenzyl)-5-methyl-2-(1-(4-phen...)Show SMILES COc1ccc(Cc2c(C)[nH]c3nc(nn3c2=O)C(C)N2CCN(CC2)c2ccccc2)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 33.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616844
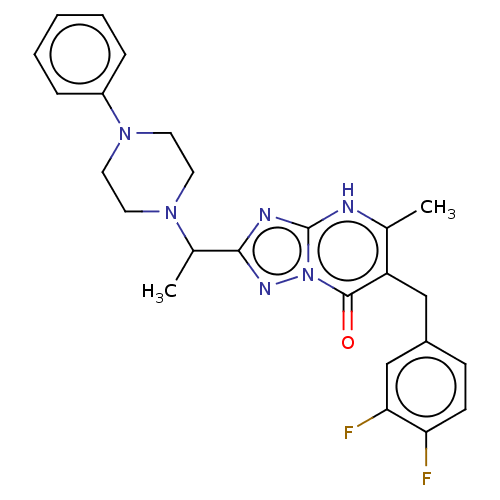
(6-(3,4-difluorobenzyl)-5-methyl-2-(1-(4-phenylpipe...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3ccc(F)c(F)c3)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 33.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247595

(CHEMBL4064397)Show SMILES Cc1cc(NC2CCN(CC2)C(=O)CCNCC(O)=O)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C30H51N11O3/c1-23-19-27(36-25-10-17-40(18-11-25)28(42)9-15-32-21-29(43)44)37-30(35-23)34-20-26-22-41(39-38-26)16-6-13-31-12-5-14-33-24-7-3-2-4-8-24/h19,22,24-25,31-33H,2-18,20-21H2,1H3,(H,43,44)(H2,34,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247610

(CHEMBL4070263)Show SMILES C(CNCCCn1cc(CNc2nccc(NC3CCNCC3)n2)nn1)CNC1CCCCC1 Show InChI InChI=1S/C24H42N10/c1-2-6-20(7-3-1)27-13-4-11-25-12-5-17-34-19-22(32-33-34)18-29-24-28-16-10-23(31-24)30-21-8-14-26-15-9-21/h10,16,19-21,25-27H,1-9,11-15,17-18H2,(H2,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238197
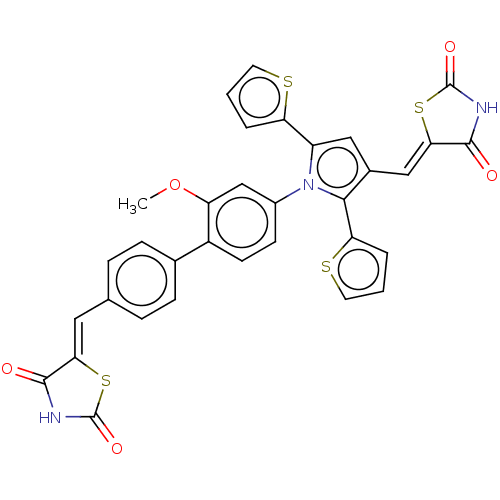
(CHEMBL4082885)Show SMILES COc1cc(ccc1-c1ccc(\C=C2/SC(=O)NC2=O)cc1)-n1c(cc(\C=C2/SC(=O)NC2=O)c1-c1cccs1)-c1cccs1 Show InChI InChI=1S/C33H21N3O5S4/c1-41-24-17-21(10-11-22(24)19-8-6-18(7-9-19)14-27-30(37)34-32(39)44-27)36-23(25-4-2-12-42-25)15-20(29(36)26-5-3-13-43-26)16-28-31(38)35-33(40)45-28/h2-17H,1H3,(H,34,37,39)(H,35,38,40)/b27-14-,28-16- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238209
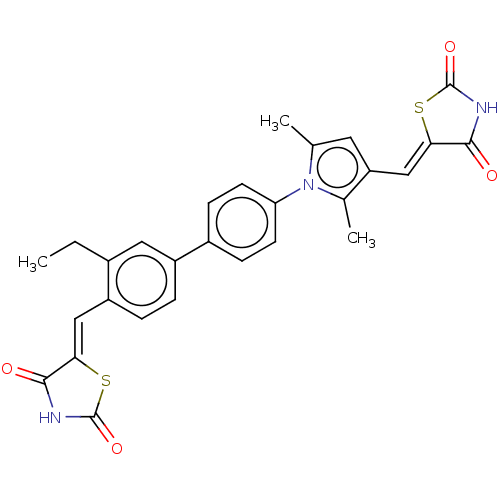
(CHEMBL4072390)Show SMILES CCc1cc(ccc1\C=C1/SC(=O)NC1=O)-c1ccc(cc1)-n1c(C)cc(\C=C2/SC(=O)NC2=O)c1C Show InChI InChI=1S/C28H23N3O4S2/c1-4-17-12-19(5-6-20(17)13-23-25(32)29-27(34)36-23)18-7-9-22(10-8-18)31-15(2)11-21(16(31)3)14-24-26(33)30-28(35)37-24/h5-14H,4H2,1-3H3,(H,29,32,34)(H,30,33,35)/b23-13-,24-14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616834
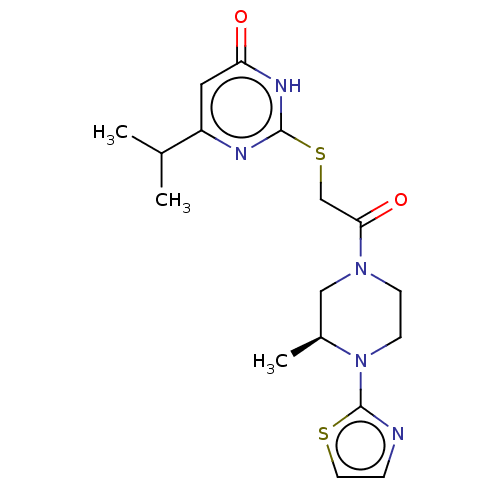
((S)-6-isopropyl-2-((2-(3-methyl-4-(thiazol-2-yl)pi...)Show SMILES CC(C)c1cc(=O)[nH]c(SCC(=O)N2CCN([C@@H](C)C2)c2nccs2)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238195
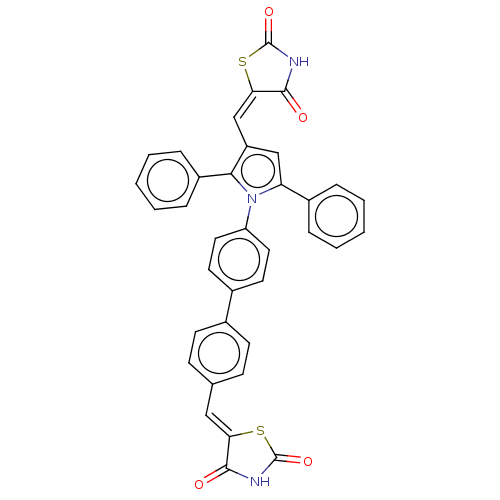
(CHEMBL4090687)Show SMILES O=C1NC(=O)\C(S1)=C\c1cc(-c2ccccc2)n(c1-c1ccccc1)-c1ccc(cc1)-c1ccc(\C=C2/SC(=O)NC2=O)cc1 Show InChI InChI=1S/C36H23N3O4S2/c40-33-30(44-35(42)37-33)19-22-11-13-23(14-12-22)24-15-17-28(18-16-24)39-29(25-7-3-1-4-8-25)20-27(21-31-34(41)38-36(43)45-31)32(39)26-9-5-2-6-10-26/h1-21H,(H,37,40,42)(H,38,41,43)/b30-19-,31-21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247609
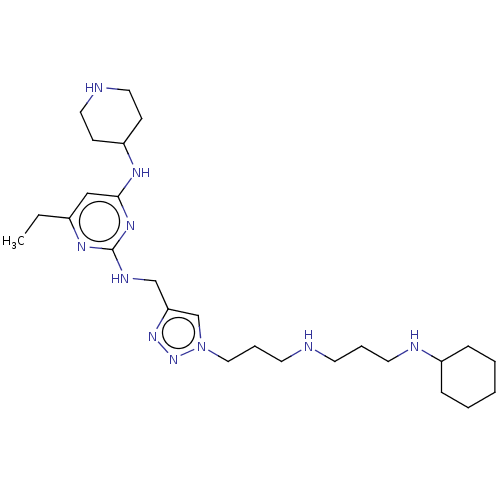
(CHEMBL4091201)Show SMILES CCc1cc(NC2CCNCC2)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C26H46N10/c1-2-21-18-25(31-23-10-15-28-16-11-23)33-26(32-21)30-19-24-20-36(35-34-24)17-7-13-27-12-6-14-29-22-8-4-3-5-9-22/h18,20,22-23,27-29H,2-17,19H2,1H3,(H2,30,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238198
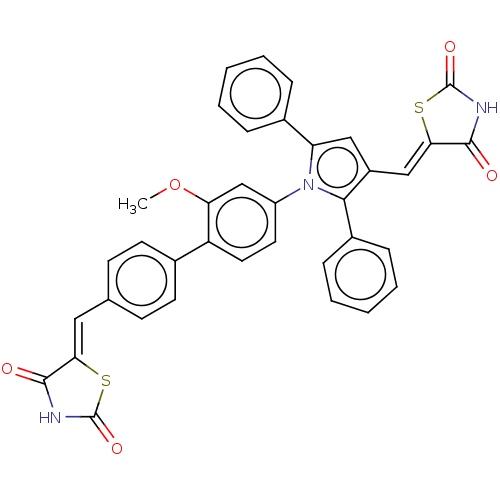
(CHEMBL4061000)Show SMILES COc1cc(ccc1-c1ccc(\C=C2/SC(=O)NC2=O)cc1)-n1c(cc(\C=C2/SC(=O)NC2=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C37H25N3O5S2/c1-45-30-21-27(16-17-28(30)23-14-12-22(13-15-23)18-31-34(41)38-36(43)46-31)40-29(24-8-4-2-5-9-24)19-26(20-32-35(42)39-37(44)47-32)33(40)25-10-6-3-7-11-25/h2-21H,1H3,(H,38,41,43)(H,39,42,44)/b31-18-,32-20- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247621

(CHEMBL4078499)Show SMILES CCCN1CCC(CC1)Nc1cc(C)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C28H50N10/c1-3-16-37-18-11-25(12-19-37)33-27-20-23(2)32-28(34-27)31-21-26-22-38(36-35-26)17-8-14-29-13-7-15-30-24-9-5-4-6-10-24/h20,22,24-25,29-30H,3-19,21H2,1-2H3,(H2,31,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238194
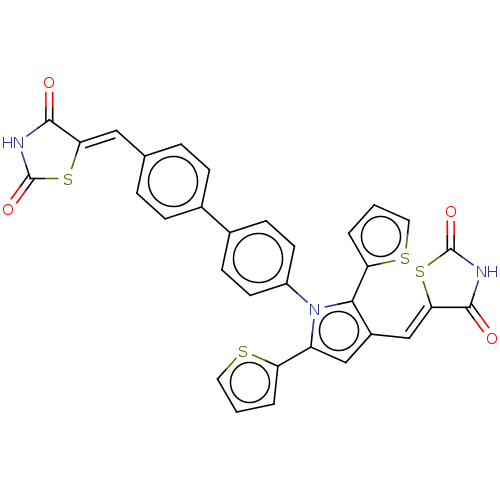
(CHEMBL4080388)Show SMILES O=C1NC(=O)\C(S1)=C\c1cc(-c2cccs2)n(c1-c1cccs1)-c1ccc(cc1)-c1ccc(\C=C2/SC(=O)NC2=O)cc1 Show InChI InChI=1S/C32H19N3O4S4/c36-29-26(42-31(38)33-29)15-18-5-7-19(8-6-18)20-9-11-22(12-10-20)35-23(24-3-1-13-40-24)16-21(28(35)25-4-2-14-41-25)17-27-30(37)34-32(39)43-27/h1-17H,(H,33,36,38)(H,34,37,39)/b26-15-,27-17- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238194

(CHEMBL4080388)Show SMILES O=C1NC(=O)\C(S1)=C\c1cc(-c2cccs2)n(c1-c1cccs1)-c1ccc(cc1)-c1ccc(\C=C2/SC(=O)NC2=O)cc1 Show InChI InChI=1S/C32H19N3O4S4/c36-29-26(42-31(38)33-29)15-18-5-7-19(8-6-18)20-9-11-22(12-10-20)35-23(24-3-1-13-40-24)16-21(28(35)25-4-2-14-41-25)17-27-30(37)34-32(39)43-27/h1-17H,(H,33,36,38)(H,34,37,39)/b26-15-,27-17- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616856
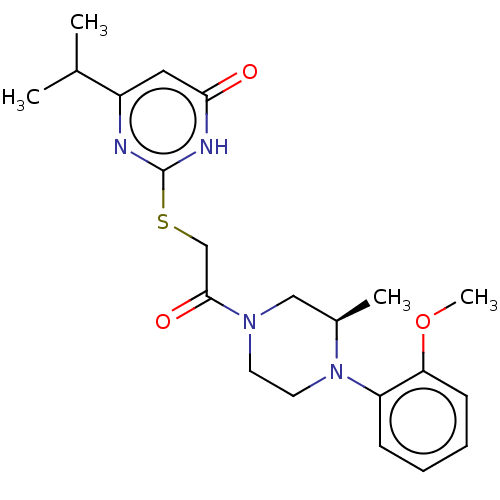
((R)-6-isopropyl-2-((2-(4-(2-methoxyphenyl)-3-methy...)Show SMILES COc1ccccc1N1CCN(C[C@H]1C)C(=O)CSc1nc(cc(=O)[nH]1)C(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 52.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238197

(CHEMBL4082885)Show SMILES COc1cc(ccc1-c1ccc(\C=C2/SC(=O)NC2=O)cc1)-n1c(cc(\C=C2/SC(=O)NC2=O)c1-c1cccs1)-c1cccs1 Show InChI InChI=1S/C33H21N3O5S4/c1-41-24-17-21(10-11-22(24)19-8-6-18(7-9-19)14-27-30(37)34-32(39)44-27)36-23(25-4-2-12-42-25)15-20(29(36)26-5-3-13-43-26)16-28-31(38)35-33(40)45-28/h2-17H,1H3,(H,34,37,39)(H,35,38,40)/b27-14-,28-16- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238199
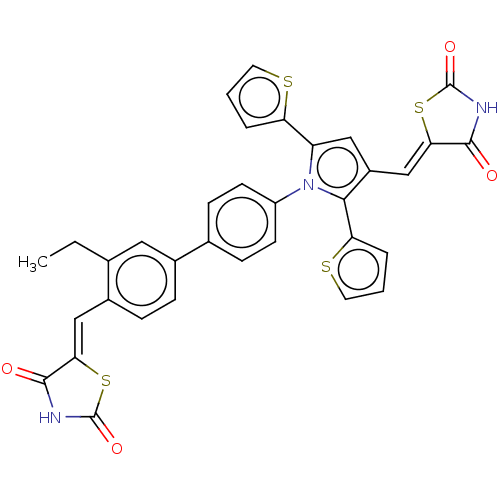
(CHEMBL4099331)Show SMILES CCc1cc(ccc1\C=C1/SC(=O)NC1=O)-c1ccc(cc1)-n1c(cc(\C=C2/SC(=O)NC2=O)c1-c1cccs1)-c1cccs1 Show InChI InChI=1S/C34H23N3O4S4/c1-2-19-15-21(7-8-22(19)17-28-31(38)35-33(40)44-28)20-9-11-24(12-10-20)37-25(26-5-3-13-42-26)16-23(30(37)27-6-4-14-43-27)18-29-32(39)36-34(41)45-29/h3-18H,2H2,1H3,(H,35,38,40)(H,36,39,41)/b28-17-,29-18- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616840
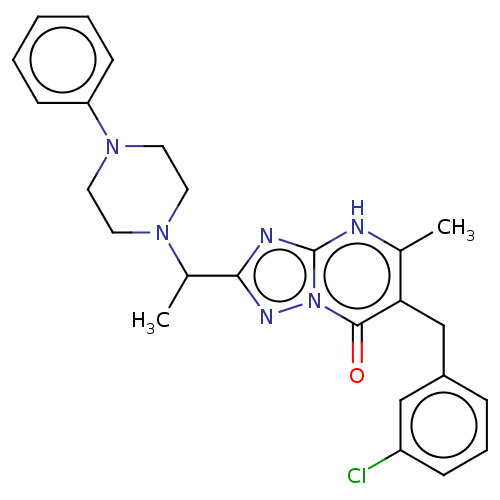
(6-(3-chlorobenzyl)-5-methyl-2-(1-(4-phenylpiperazi...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3cccc(Cl)c3)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 54.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238200
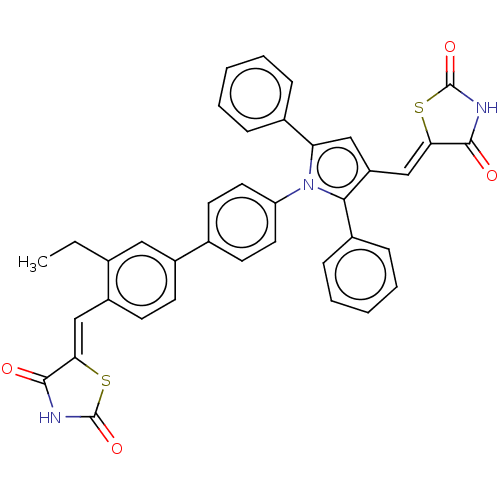
(CHEMBL4087349)Show SMILES CCc1cc(ccc1\C=C1/SC(=O)NC1=O)-c1ccc(cc1)-n1c(cc(\C=C2/SC(=O)NC2=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C38H27N3O4S2/c1-2-23-19-27(13-14-28(23)21-32-35(42)39-37(44)46-32)24-15-17-30(18-16-24)41-31(25-9-5-3-6-10-25)20-29(22-33-36(43)40-38(45)47-33)34(41)26-11-7-4-8-12-26/h3-22H,2H2,1H3,(H,39,42,44)(H,40,43,45)/b32-21-,33-22- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238203
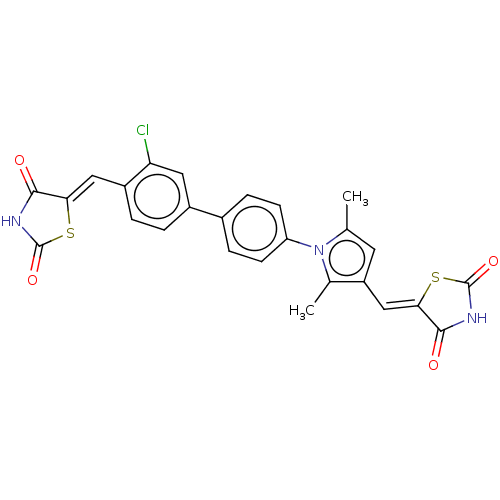
(CHEMBL4074218)Show SMILES Cc1cc(\C=C2/SC(=O)NC2=O)c(C)n1-c1ccc(cc1)-c1ccc(\C=C2/SC(=O)NC2=O)c(Cl)c1 Show InChI InChI=1S/C26H18ClN3O4S2/c1-13-9-18(12-22-24(32)29-26(34)36-22)14(2)30(13)19-7-5-15(6-8-19)16-3-4-17(20(27)10-16)11-21-23(31)28-25(33)35-21/h3-12H,1-2H3,(H,28,31,33)(H,29,32,34)/b21-11-,22-12- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238210
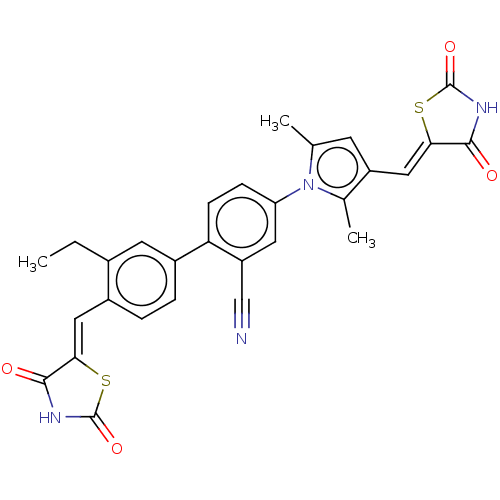
(CHEMBL4065595)Show SMILES CCc1cc(ccc1\C=C1/SC(=O)NC1=O)-c1ccc(cc1C#N)-n1c(C)cc(\C=C2/SC(=O)NC2=O)c1C Show InChI InChI=1S/C29H22N4O4S2/c1-4-17-10-19(6-5-18(17)12-24-26(34)31-28(36)38-24)23-8-7-22(11-21(23)14-30)33-15(2)9-20(16(33)3)13-25-27(35)32-29(37)39-25/h5-13H,4H2,1-3H3,(H,31,34,36)(H,32,35,37)/b24-12-,25-13- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616852
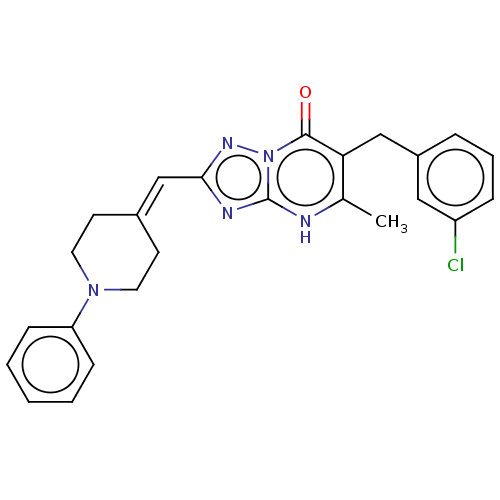
(6-(3-chlorobenzyl)-5-methyl-2-((1-phenylpiperidin-...)Show SMILES Cc1[nH]c2nc(C=C3CCN(CC3)c3ccccc3)nn2c(=O)c1Cc1cccc(Cl)c1 |(-2.99,-18.46,;-1.65,-17.69,;-.32,-18.46,;1.01,-17.69,;2.48,-18.17,;3.38,-16.92,;4.92,-16.92,;5.69,-15.59,;7.23,-15.59,;8,-14.25,;7.23,-12.92,;5.69,-12.92,;4.92,-14.25,;8,-11.59,;9.54,-11.59,;10.31,-10.25,;9.54,-8.92,;8,-8.92,;7.23,-10.25,;2.48,-15.68,;1.01,-16.15,;-.32,-15.38,;-.32,-13.84,;-1.65,-16.15,;-2.99,-15.38,;-4.32,-16.15,;-4.32,-17.69,;-5.66,-18.46,;-6.99,-17.69,;-6.99,-16.15,;-8.32,-15.38,;-5.66,-15.38,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 60.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247601
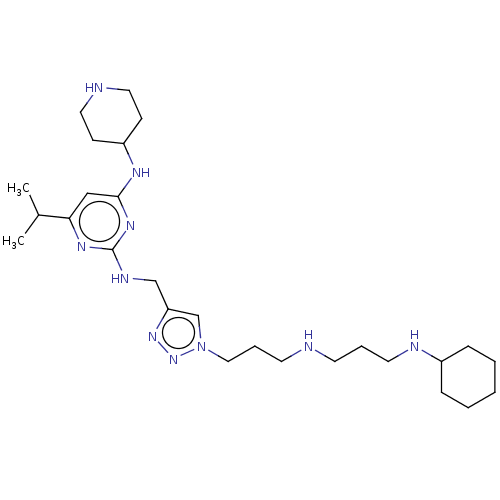
(CHEMBL4075454)Show SMILES CC(C)c1cc(NC2CCNCC2)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C27H48N10/c1-21(2)25-18-26(32-23-10-15-29-16-11-23)34-27(33-25)31-19-24-20-37(36-35-24)17-7-13-28-12-6-14-30-22-8-4-3-5-9-22/h18,20-23,28-30H,3-17,19H2,1-2H3,(H2,31,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
Prostaglandin reductase 2
(Human) | BDBM616842
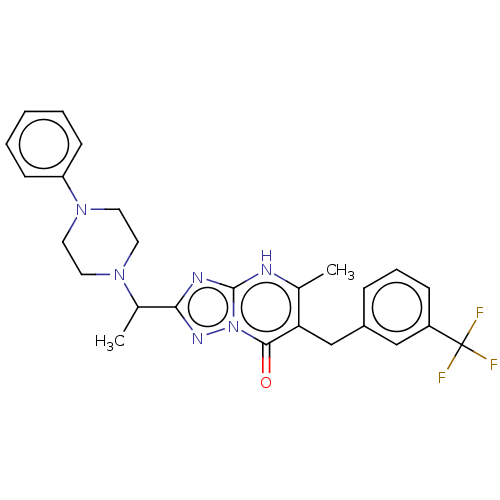
(5-methyl-2-(1-(4-phenylpiperazin-1-yl)ethyl)-6-(3-...)Show SMILES CC(N1CCN(CC1)c1ccccc1)c1nc2[nH]c(C)c(Cc3cccc(c3)C(F)(F)F)c(=O)n2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 61.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50238201
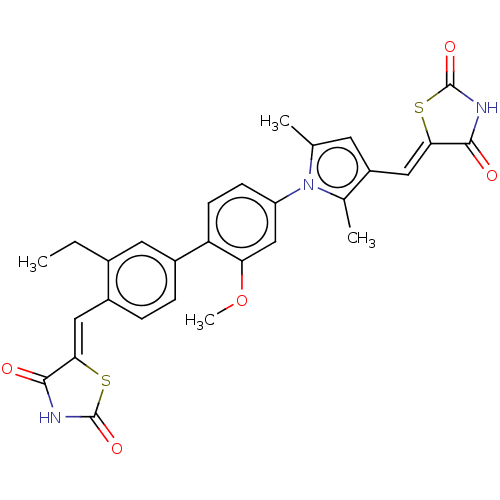
(CHEMBL4065936)Show SMILES CCc1cc(ccc1\C=C1/SC(=O)NC1=O)-c1ccc(cc1OC)-n1c(C)cc(\C=C2/SC(=O)NC2=O)c1C Show InChI InChI=1S/C29H25N3O5S2/c1-5-17-11-19(7-6-18(17)12-24-26(33)30-28(35)38-24)22-9-8-21(14-23(22)37-4)32-15(2)10-20(16(32)3)13-25-27(34)31-29(36)39-25/h6-14H,5H2,1-4H3,(H,30,33,35)(H,31,34,36)/b24-12-,25-13- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247617
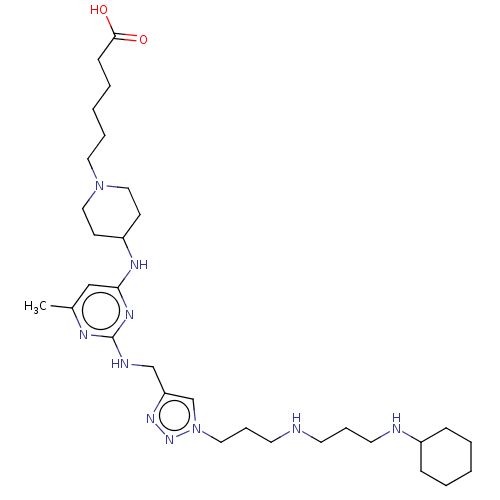
(CHEMBL4090175)Show SMILES Cc1cc(NC2CCN(CCCCCC(O)=O)CC2)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C31H54N10O2/c1-25-22-29(36-27-13-20-40(21-14-27)18-7-3-6-12-30(42)43)37-31(35-25)34-23-28-24-41(39-38-28)19-9-16-32-15-8-17-33-26-10-4-2-5-11-26/h22,24,26-27,32-33H,2-21,23H2,1H3,(H,42,43)(H2,34,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247623

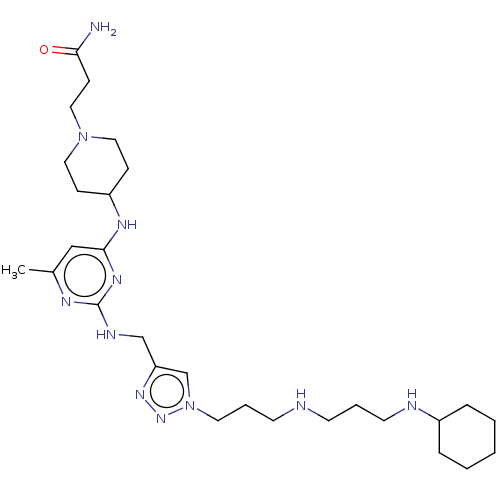
(CHEMBL4098404)Show SMILES Cc1cc(NC2CCN(CC2)C(=O)CC[C@H](N)C(O)=O)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 |r| Show InChI InChI=1S/C30H51N11O3/c1-22-19-27(36-24-11-17-40(18-12-24)28(42)10-9-26(31)29(43)44)37-30(35-22)34-20-25-21-41(39-38-25)16-6-14-32-13-5-15-33-23-7-3-2-4-8-23/h19,21,23-24,26,32-33H,2-18,20,31H2,1H3,(H,43,44)(H2,34,35,36,37)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247626
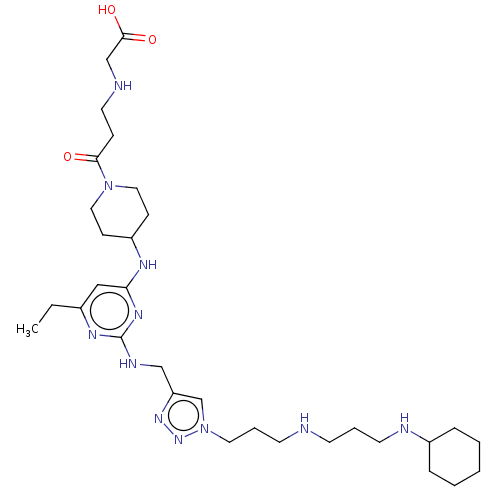
(CHEMBL4068647)Show SMILES CCc1cc(NC2CCN(CC2)C(=O)CCNCC(O)=O)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C31H53N11O3/c1-2-24-20-28(36-26-11-18-41(19-12-26)29(43)10-16-33-22-30(44)45)38-31(37-24)35-21-27-23-42(40-39-27)17-7-14-32-13-6-15-34-25-8-4-3-5-9-25/h20,23,25-26,32-34H,2-19,21-22H2,1H3,(H,44,45)(H2,35,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247620
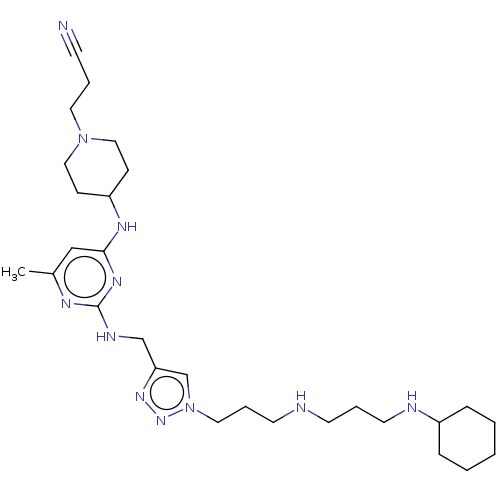
(CHEMBL4102780)Show SMILES Cc1cc(NC2CCN(CCC#N)CC2)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C28H47N11/c1-23-20-27(34-25-10-18-38(19-11-25)16-5-12-29)35-28(33-23)32-21-26-22-39(37-36-26)17-7-14-30-13-6-15-31-24-8-3-2-4-9-24/h20,22,24-25,30-31H,2-11,13-19,21H2,1H3,(H2,32,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247627
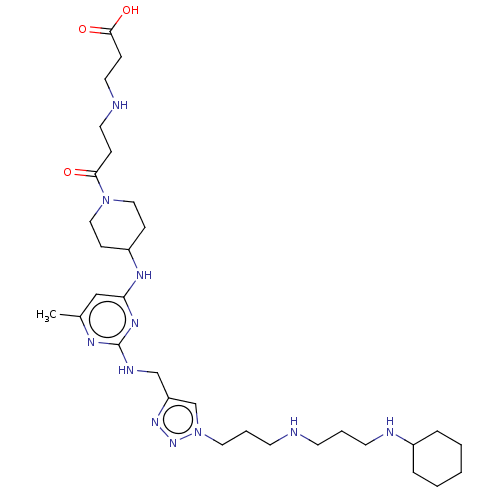
(CHEMBL4089763)Show SMILES Cc1cc(NC2CCN(CC2)C(=O)CCNCCC(O)=O)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C31H53N11O3/c1-24-21-28(37-26-11-19-41(20-12-26)29(43)9-16-33-17-10-30(44)45)38-31(36-24)35-22-27-23-42(40-39-27)18-6-14-32-13-5-15-34-25-7-3-2-4-8-25/h21,23,25-26,32-34H,2-20,22H2,1H3,(H,44,45)(H2,35,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50400050
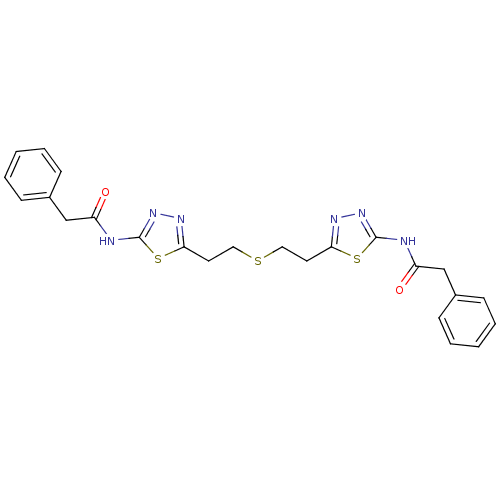
(CHEMBL2177757 | US10793535, Cmpd ID 1 | US11191732...)Show SMILES O=C(Cc1ccccc1)Nc1nnc(CCSCCc2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C24H24N6O2S3/c31-19(15-17-7-3-1-4-8-17)25-23-29-27-21(34-23)11-13-33-14-12-22-28-30-24(35-22)26-20(32)16-18-9-5-2-6-10-18/h1-10H,11-16H2,(H,25,29,31)(H,26,30,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged KGA (L123 to L669 residues) expressed in Escherichia coli BL21(DE3)pLysS using glutamine substrate preincu... |
J Med Chem 60: 5599-5612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00282
BindingDB Entry DOI: 10.7270/Q26H4KP5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin reductase 2
(Human) | BDBM616858
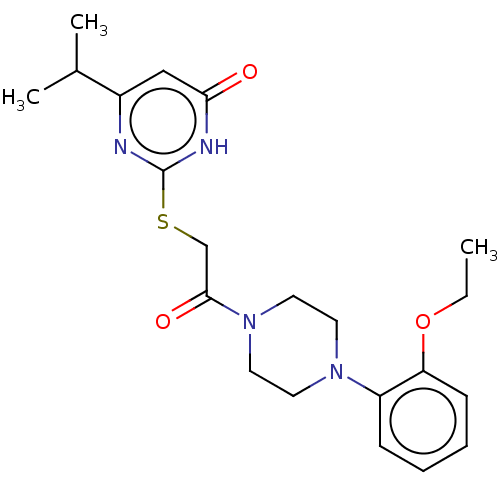
(2-((2-(4-(2-ethoxyphenyl)piperazin-1-yl)-2-oxoethy...)Show SMILES CCOc1ccccc1N1CCN(CC1)C(=O)CSc1nc(cc(=O)[nH]1)C(C)C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 81.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27P93JH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247619
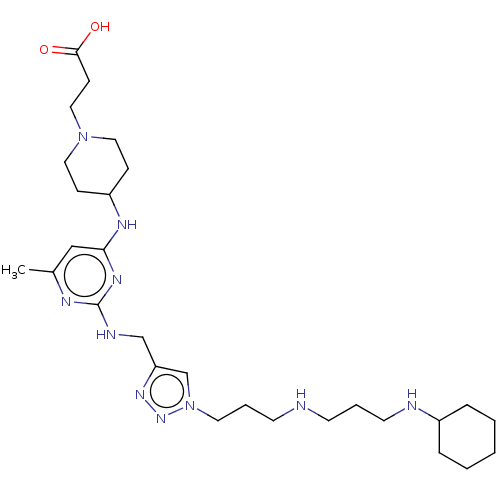
(CHEMBL4082328)Show SMILES Cc1cc(NC2CCN(CCC(O)=O)CC2)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C28H48N10O2/c1-22-19-26(33-24-9-16-37(17-10-24)18-11-27(39)40)34-28(32-22)31-20-25-21-38(36-35-25)15-6-13-29-12-5-14-30-23-7-3-2-4-8-23/h19,21,23-24,29-30H,2-18,20H2,1H3,(H,39,40)(H2,31,32,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50247594
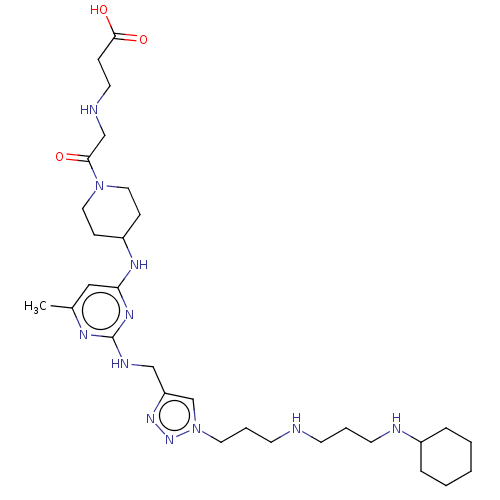
(CHEMBL4104222)Show SMILES Cc1cc(NC2CCN(CC2)C(=O)CNCCC(O)=O)nc(NCc2cn(CCCNCCCNC3CCCCC3)nn2)n1 Show InChI InChI=1S/C30H51N11O3/c1-23-19-27(36-25-10-17-40(18-11-25)28(42)21-32-15-9-29(43)44)37-30(35-23)34-20-26-22-41(39-38-26)16-6-13-31-12-5-14-33-24-7-3-2-4-8-24/h19,22,24-25,31-33H,2-18,20-21H2,1H3,(H,43,44)(H2,34,35,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL12 from human CXCR4 expressed in HEK293 cell membranes after 1.5 hrs by Topcount method |
J Med Chem 61: 818-833 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01322
BindingDB Entry DOI: 10.7270/Q2KH0QRM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data