 Found 121 hits with Last Name = 'tsukada' and Initial = 'j'
Found 121 hits with Last Name = 'tsukada' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(RAT) | BDBM50108501
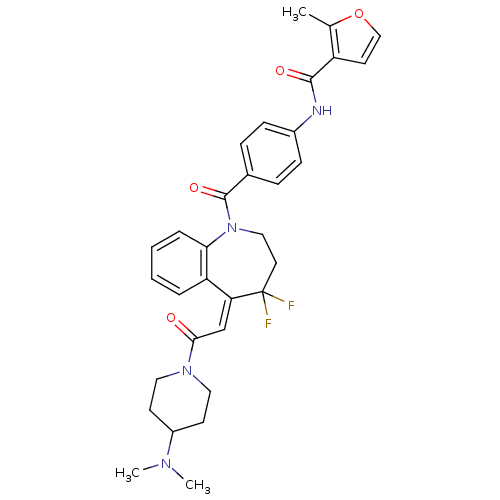
(2-Methyl-furan-3-carboxylic acid (4-{5-[2-(4-dimet...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1/c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccoc2C)cc1 Show InChI InChI=1S/C32H34F2N4O4/c1-21-25(14-19-42-21)30(40)35-23-10-8-22(9-11-23)31(41)38-18-15-32(33,34)27(26-6-4-5-7-28(26)38)20-29(39)37-16-12-24(13-17-37)36(2)3/h4-11,14,19-20,24H,12-13,15-18H2,1-3H3,(H,35,40)/b27-20+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108498
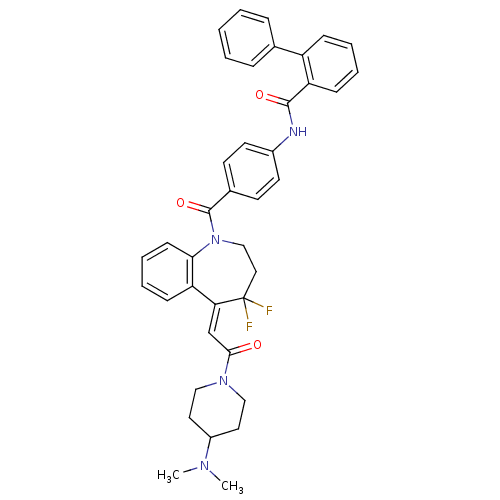
(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V1a receptor, performed using [3H]-AVP on rat liver |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095
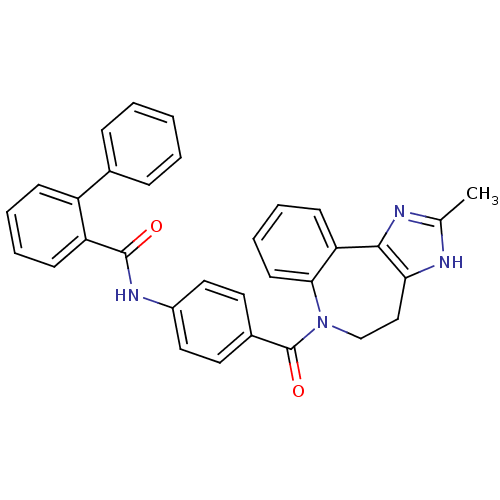
(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85096
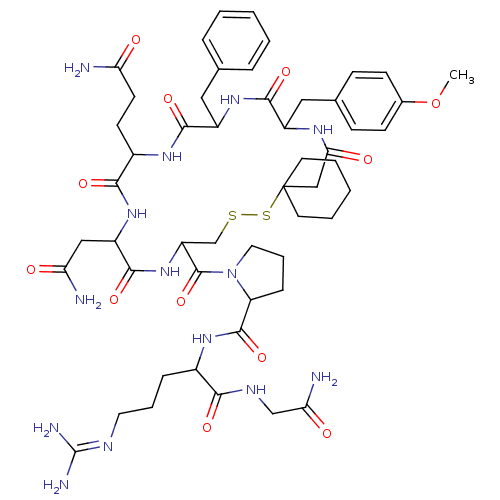
(CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114031
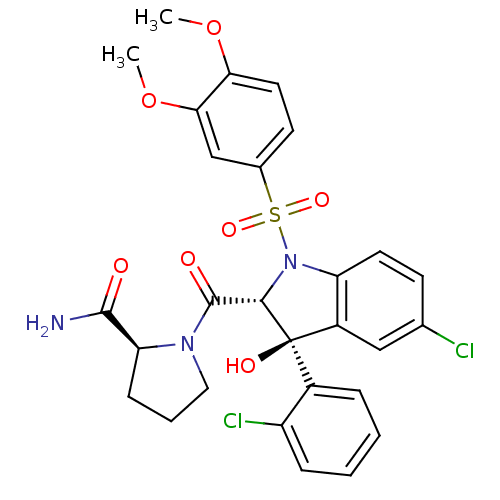
((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V2 receptor, performed using [3H]-AVP on rat kidney |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50013775

((oxytocin-OT) cyclo[Cys-Tyr-Ile-Gln-Asn-Cys]-Pro-L...)Show SMILES CCC(C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](N)CSSCC(NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCCC1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22?,25-,26+,27+,28+,29+,30?,31?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114033
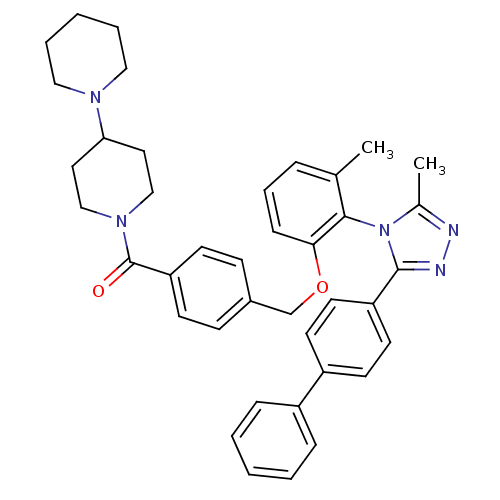
(CHEMBL86667 | {4-[2-(3-Biphenyl-4-yl-5-methyl-[1,2...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1c(C)cccc1OCc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCCC1 |(15.01,-.52,;13.48,-.29,;12.92,1.14,;11.39,1.05,;10.99,-.44,;9.57,-1,;9.33,-2.53,;7.9,-3.09,;6.69,-2.13,;6.92,-.61,;8.35,-.03,;5.26,-2.69,;4.05,-1.71,;2.62,-2.27,;2.38,-3.81,;3.58,-4.77,;5.02,-4.21,;12.29,-1.27,;12.45,-2.79,;11.2,-3.7,;9.8,-3.07,;11.36,-5.22,;12.77,-5.85,;14.02,-4.94,;13.86,-3.42,;15.1,-2.51,;16.53,-3.05,;16.79,-4.56,;18.23,-5.09,;18.49,-6.6,;17.32,-7.6,;15.87,-7.06,;15.59,-5.55,;17.57,-9.12,;19.02,-9.65,;16.39,-10.1,;17.53,-11.13,;17.18,-12.65,;15.71,-13.11,;14.59,-12.07,;14.92,-10.56,;15.48,-14.61,;16.67,-15.57,;16.44,-17.09,;15.01,-17.63,;13.82,-16.67,;14.05,-15.17,)| Show InChI InChI=1S/C40H43N5O2/c1-29-10-9-13-37(38(29)45-30(2)41-42-39(45)34-20-18-33(19-21-34)32-11-5-3-6-12-32)47-28-31-14-16-35(17-15-31)40(46)44-26-22-36(23-27-44)43-24-7-4-8-25-43/h3,5-6,9-21,36H,4,7-8,22-28H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114028

(1-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES CN1CCN(CCCCCCOc2ccccc2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)CC1 |(27.25,-2.68,;25.78,-2.25,;24.66,-3.31,;23.19,-2.88,;22.83,-1.38,;21.58,-2.29,;20.18,-1.64,;18.92,-2.53,;17.52,-1.89,;16.26,-2.77,;14.86,-2.13,;13.62,-3.02,;13.76,-4.55,;15.14,-5.18,;15.28,-6.72,;14.02,-7.59,;12.64,-6.96,;12.51,-5.42,;11.12,-4.78,;10.9,-3.25,;11.88,-2.08,;9.38,-2.99,;8.66,-4.36,;9.75,-5.46,;9.49,-6.98,;8.05,-7.52,;7.79,-9.03,;8.98,-10,;10.41,-9.48,;10.68,-7.96,;8.71,-11.52,;7.28,-12.06,;7,-13.57,;8.19,-14.56,;9.64,-14.02,;9.91,-12.51,;23.93,-.31,;25.4,-.75,)| Show InChI InChI=1S/C32H39N5O/c1-26-33-34-32(29-18-16-28(17-19-29)27-12-6-5-7-13-27)37(26)30-14-8-9-15-31(30)38-25-11-4-3-10-20-36-23-21-35(2)22-24-36/h5-9,12-19H,3-4,10-11,20-25H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114034
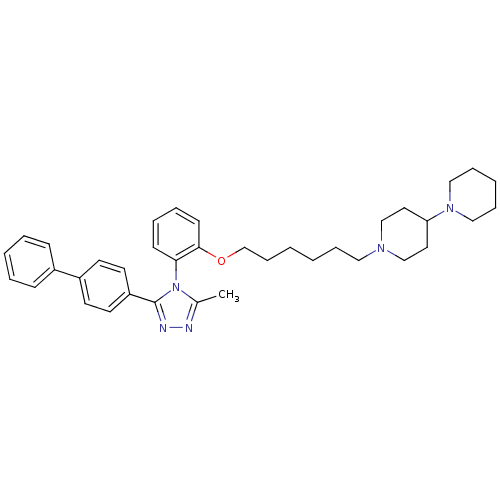
(1'-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1ccccc1OCCCCCCN1CCC(CC1)N1CCCCC1 |(6.93,-1.75,;5.55,-2.43,;4.25,-1.61,;3.06,-2.57,;3.62,-4.01,;2.78,-5.3,;1.24,-5.23,;.4,-6.52,;1.1,-7.87,;2.64,-7.96,;3.48,-6.68,;.26,-9.17,;.96,-10.54,;.12,-11.84,;-1.42,-11.74,;-2.11,-10.37,;-1.28,-9.08,;5.16,-3.93,;6.17,-5.07,;5.68,-6.54,;6.69,-7.68,;8.2,-7.38,;8.69,-5.91,;7.66,-4.77,;8.15,-3.3,;9.52,-2.62,;10.8,-3.48,;12.18,-2.78,;13.46,-3.65,;14.84,-2.95,;16.13,-3.81,;17.5,-3.13,;19.04,-3.27,;19.93,-2.04,;19.3,-.63,;17.76,-.47,;16.87,-1.73,;20.09,.67,;19.39,2.01,;20.21,3.3,;21.73,3.24,;22.45,1.91,;21.63,.61,)| Show InChI InChI=1S/C37H47N5O/c1-30-38-39-37(33-20-18-32(19-21-33)31-14-6-4-7-15-31)42(30)35-16-8-9-17-36(35)43-29-13-3-2-10-24-40-27-22-34(23-28-40)41-25-11-5-12-26-41/h4,6-9,14-21,34H,2-3,5,10-13,22-29H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V2 receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50108498

(Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)\C=C1\c2ccccc2N(CCC1(F)F)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C39H38F2N4O3/c1-43(2)30-20-23-44(24-21-30)36(46)26-34-33-14-8-9-15-35(33)45(25-22-39(34,40)41)38(48)28-16-18-29(19-17-28)42-37(47)32-13-7-6-12-31(32)27-10-4-3-5-11-27/h3-19,26,30H,20-25H2,1-2H3,(H,42,47)/b34-26- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V2) receptor in rat kidney membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50114031

((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V1a receptor, performed using [3H]-AVP on rat liver |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85096

(CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85094
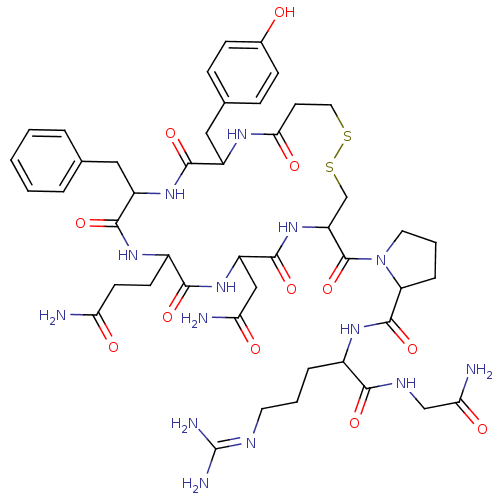
(CAS_62357-86-2 | NSC_64759 | dDAVP)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6]-1-[#7]-[#6](=O)-[#6](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114025

(4-{4-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1ccccc1OCCCCC1CCNCC1 |(10.8,-.03,;9.42,-.73,;8.13,.11,;6.93,-.86,;7.49,-2.29,;6.65,-3.59,;5.11,-3.5,;4.27,-4.8,;4.97,-6.16,;6.51,-6.25,;7.35,-4.96,;4.13,-7.44,;4.83,-8.82,;3.99,-10.11,;2.45,-10.03,;1.76,-8.65,;2.59,-7.36,;9.02,-2.22,;10.03,-3.36,;9.54,-4.82,;10.56,-5.97,;12.07,-5.66,;12.55,-4.19,;11.54,-3.05,;12.01,-1.59,;13.38,-.92,;14.67,-1.76,;16.04,-1.08,;17.32,-1.93,;17.23,-3.46,;18.55,-4.27,;18.49,-5.81,;17.14,-6.53,;15.83,-5.73,;15.88,-4.2,)| Show InChI InChI=1S/C30H34N4O/c1-23-32-33-30(27-16-14-26(15-17-27)25-10-3-2-4-11-25)34(23)28-12-5-6-13-29(28)35-22-8-7-9-24-18-20-31-21-19-24/h2-6,10-17,24,31H,7-9,18-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108493
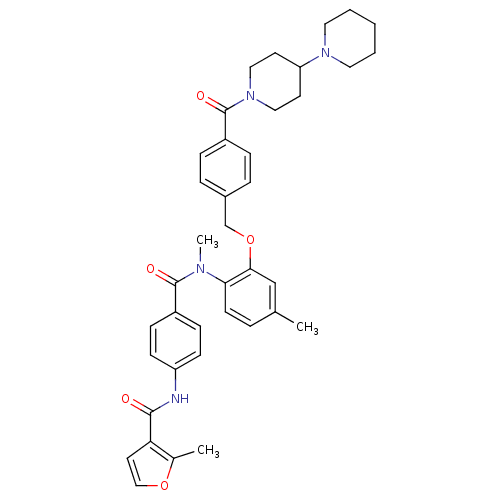
(2-Methyl-furan-3-carboxylic acid [4-({2-[4-([1,4']...)Show SMILES CN(C(=O)c1ccc(NC(=O)c2ccoc2C)cc1)c1ccc(C)cc1OCc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C39H44N4O5/c1-27-7-16-35(41(3)38(45)30-12-14-32(15-13-30)40-37(44)34-19-24-47-28(34)2)36(25-27)48-26-29-8-10-31(11-9-29)39(46)43-22-17-33(18-23-43)42-20-5-4-6-21-42/h7-16,19,24-25,33H,4-6,17-18,20-23,26H2,1-3H3,(H,40,44) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114032

(CHEMBL86577 | {4-[2-(3-Biphenyl-4-yl-5-methyl-[1,2...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(COc2ccccc2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)cc1 |(15.94,-10.56,;16.17,-9.05,;17.64,-8.58,;17.99,-7.08,;16.85,-6.05,;15.38,-6.5,;15.04,-8,;18.02,-5.05,;19.47,-5.59,;17.76,-3.54,;16.31,-3,;16.06,-1.48,;17.25,-.5,;16.99,1.02,;15.54,1.54,;14.3,.65,;14.47,-.89,;13.23,-1.8,;11.82,-1.17,;11.66,.37,;12.9,1.26,;12.74,2.8,;13.93,3.78,;15.46,3.54,;13.37,5.2,;11.84,5.11,;11.45,3.61,;10.02,3.05,;8.81,4.02,;7.38,3.45,;7.16,1.93,;8.35,.97,;9.79,1.53,;5.71,1.37,;5.47,-.16,;4.04,-.71,;2.83,.25,;3.08,1.79,;4.52,2.34,;18.69,-1.04,;18.95,-2.55,)| Show InChI InChI=1S/C34H33N5O2/c1-25-35-36-33(29-18-16-28(17-19-29)27-8-4-3-5-9-27)39(25)31-10-6-7-11-32(31)41-24-26-12-14-30(15-13-26)34(40)38-22-20-37(2)21-23-38/h3-19H,20-24H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114045
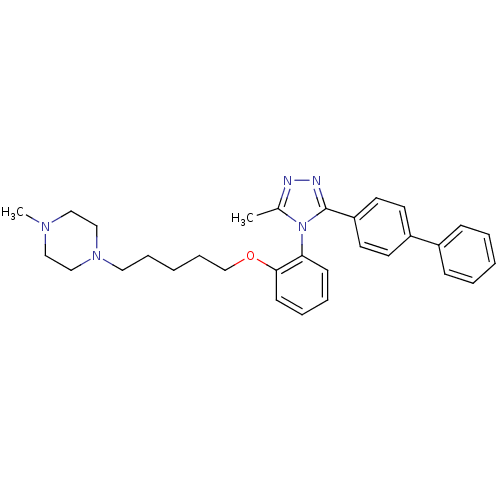
(1-{5-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES CN1CCN(CCCCCOc2ccccc2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)CC1 |(24.7,-5.65,;23.67,-4.52,;22.16,-4.86,;21.11,-3.74,;21.58,-2.29,;20.18,-1.64,;18.92,-2.53,;17.52,-1.89,;16.26,-2.78,;14.86,-2.13,;13.62,-3.02,;13.76,-4.55,;15.14,-5.19,;15.28,-6.72,;14.02,-7.59,;12.64,-6.96,;12.51,-5.42,;11.12,-4.78,;10.9,-3.25,;11.88,-2.08,;9.38,-2.99,;8.66,-4.36,;9.75,-5.46,;9.49,-6.98,;8.05,-7.52,;7.79,-9.03,;8.98,-10,;10.41,-9.48,;10.68,-7.96,;8.71,-11.52,;9.91,-12.51,;9.64,-14.02,;8.19,-14.56,;7,-13.57,;7.28,-12.06,;23.07,-1.92,;24.12,-3.06,)| Show InChI InChI=1S/C31H37N5O/c1-25-32-33-31(28-17-15-27(16-18-28)26-11-5-3-6-12-26)36(25)29-13-7-8-14-30(29)37-24-10-4-9-19-35-22-20-34(2)21-23-35/h3,5-8,11-18H,4,9-10,19-24H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114038
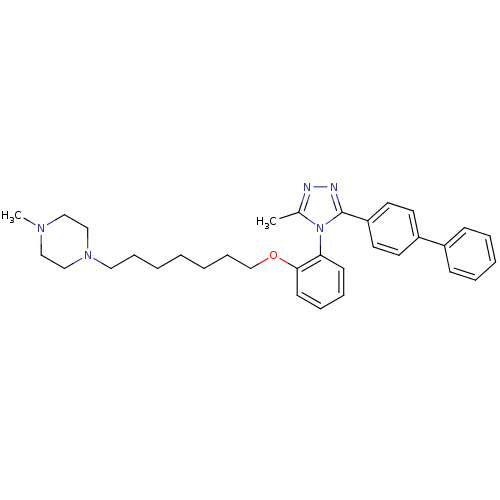
(1-{7-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES CN1CCN(CCCCCCCOc2ccccc2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)CC1 |(27.36,-5.41,;26.31,-4.28,;24.82,-4.63,;23.77,-3.51,;24.24,-2.04,;22.84,-1.4,;21.58,-2.29,;20.18,-1.64,;18.92,-2.53,;17.52,-1.89,;16.26,-2.77,;14.86,-2.13,;13.62,-3.02,;13.76,-4.55,;15.14,-5.18,;15.28,-6.72,;14.02,-7.59,;12.64,-6.96,;12.51,-5.42,;11.12,-4.78,;10.9,-3.25,;11.88,-2.08,;9.38,-2.99,;8.66,-4.36,;9.75,-5.46,;9.49,-6.98,;8.05,-7.52,;7.79,-9.03,;8.98,-10,;10.41,-9.48,;10.68,-7.96,;8.71,-11.52,;9.91,-12.51,;9.64,-14.02,;8.19,-14.56,;7,-13.57,;7.28,-12.06,;25.73,-1.68,;26.78,-2.81,)| Show InChI InChI=1S/C33H41N5O/c1-27-34-35-33(30-19-17-29(18-20-30)28-13-7-6-8-14-28)38(27)31-15-9-10-16-32(31)39-26-12-5-3-4-11-21-37-24-22-36(2)23-25-37/h6-10,13-20H,3-5,11-12,21-26H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108494

(2-Methyl-furan-3-carboxylic acid (4-{[2-(6-[1,4']b...)Show SMILES CN(C(=O)c1ccc(NC(=O)c2ccoc2C)cc1)c1ccc(C)cc1OCCCCCC(=O)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C37H48N4O5/c1-27-11-16-33(39(3)37(44)29-12-14-30(15-13-29)38-36(43)32-19-25-45-28(32)2)34(26-27)46-24-9-4-6-10-35(42)41-22-17-31(18-23-41)40-20-7-5-8-21-40/h11-16,19,25-26,31H,4-10,17-18,20-24H2,1-3H3,(H,38,43) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108495
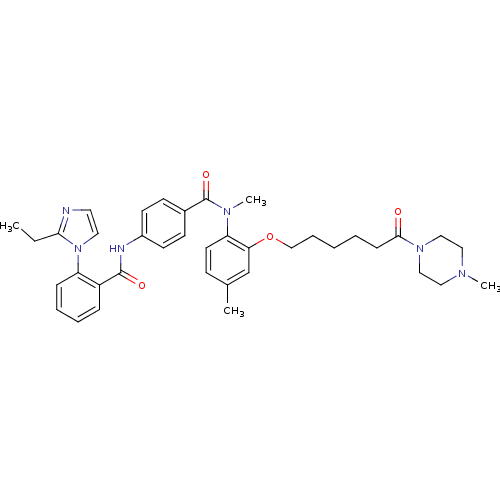
(1N-methyl-1N-{4-methyl-2-[6-(4-methylhexahydro-1-p...)Show SMILES CCc1nccn1-c1ccccc1C(=O)Nc1ccc(cc1)C(=O)N(C)c1ccc(C)cc1OCCCCCC(=O)N1CCN(C)CC1 Show InChI InChI=1S/C38H46N6O4/c1-5-35-39-20-21-44(35)32-12-9-8-11-31(32)37(46)40-30-17-15-29(16-18-30)38(47)42(4)33-19-14-28(2)27-34(33)48-26-10-6-7-13-36(45)43-24-22-41(3)23-25-43/h8-9,11-12,14-21,27H,5-7,10,13,22-26H2,1-4H3,(H,40,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108499
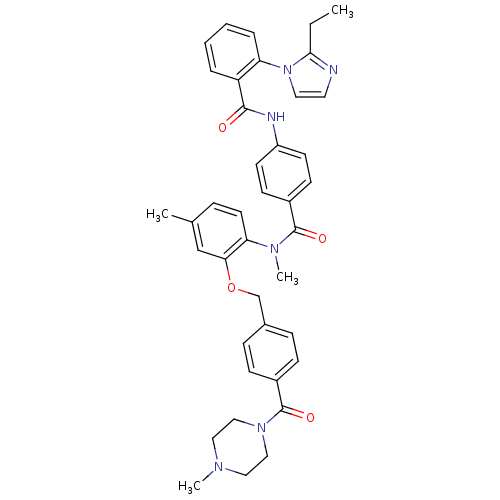
(1N-methyl-1N-{4-methyl-2-[4-(4-methylhexahydro-1-p...)Show SMILES CCc1nccn1-c1ccccc1C(=O)Nc1ccc(cc1)C(=O)N(C)c1ccc(C)cc1OCc1ccc(cc1)C(=O)N1CCN(C)CC1 Show InChI InChI=1S/C40H42N6O4/c1-5-37-41-20-21-46(37)34-9-7-6-8-33(34)38(47)42-32-17-15-30(16-18-32)39(48)44(4)35-19-10-28(2)26-36(35)50-27-29-11-13-31(14-12-29)40(49)45-24-22-43(3)23-25-45/h6-21,26H,5,22-25,27H2,1-4H3,(H,42,47) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114037
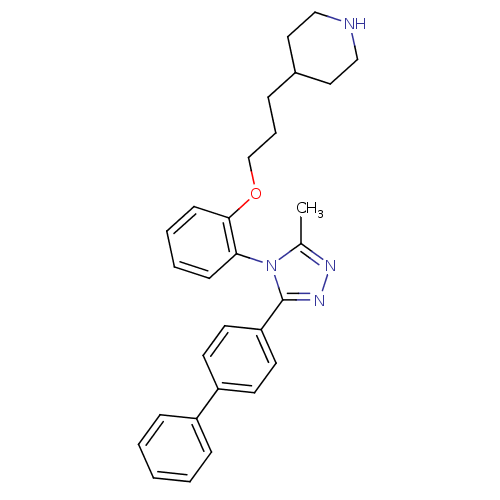
(4-{3-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1ccccc1OCCCC1CCNCC1 |(9.43,-1.75,;8.05,-2.43,;6.76,-1.61,;5.56,-2.57,;6.12,-4.01,;5.28,-5.3,;3.74,-5.22,;2.9,-6.51,;3.6,-7.87,;5.14,-7.96,;5.98,-6.68,;2.76,-9.17,;3.46,-10.54,;2.62,-11.83,;1.08,-11.74,;.38,-10.36,;1.22,-9.08,;7.65,-3.93,;8.66,-5.07,;8.17,-6.54,;9.2,-7.68,;10.71,-7.38,;11.18,-5.91,;10.17,-4.77,;10.64,-3.31,;12.02,-2.62,;13.3,-3.47,;14.68,-2.78,;15.96,-3.65,;17.31,-2.9,;18.63,-3.72,;18.57,-5.26,;17.22,-5.98,;15.91,-5.18,)| Show InChI InChI=1S/C29H32N4O/c1-22-31-32-29(26-15-13-25(14-16-26)24-9-3-2-4-10-24)33(22)27-11-5-6-12-28(27)34-21-7-8-23-17-19-30-20-18-23/h2-6,9-16,23,30H,7-8,17-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85094

(CAS_62357-86-2 | NSC_64759 | dDAVP)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6]-1-[#7]-[#6](=O)-[#6](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114041
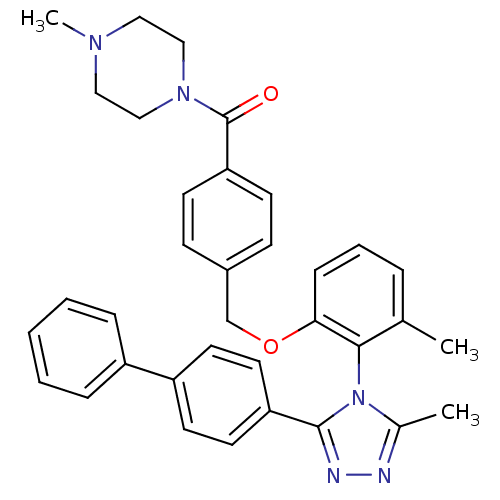
(CHEMBL316093 | {4-[2-(3-Biphenyl-4-yl-5-methyl-[1,...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(COc2cccc(C)c2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)cc1 |(15.47,-14.61,;15.71,-13.11,;14.58,-12.06,;14.91,-10.56,;16.38,-10.1,;17.53,-11.13,;17.18,-12.65,;17.57,-9.12,;19.02,-9.65,;17.31,-7.6,;18.49,-6.6,;18.23,-5.09,;16.78,-4.56,;16.52,-3.04,;15.09,-2.51,;13.85,-3.42,;14.02,-4.94,;12.76,-5.85,;11.36,-5.22,;11.2,-3.7,;9.8,-3.07,;12.45,-2.79,;12.29,-1.27,;13.48,-.29,;15.01,-.52,;12.92,1.14,;11.38,1.05,;10.99,-.44,;9.56,-1,;9.33,-2.53,;7.9,-3.09,;6.69,-2.13,;6.92,-.61,;8.35,-.03,;5.26,-2.69,;4.05,-1.71,;2.62,-2.27,;2.38,-3.81,;3.58,-4.77,;5.02,-4.21,;15.59,-5.54,;15.86,-7.06,)| Show InChI InChI=1S/C35H35N5O2/c1-25-8-7-11-32(42-24-27-12-14-31(15-13-27)35(41)39-22-20-38(3)21-23-39)33(25)40-26(2)36-37-34(40)30-18-16-29(17-19-30)28-9-5-4-6-10-28/h4-19H,20-24H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50108500
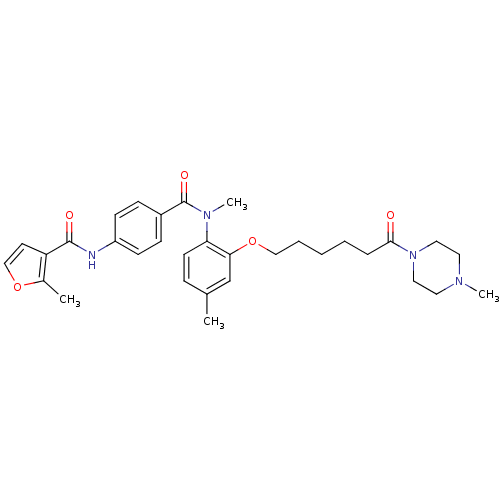
(2-Methyl-furan-3-carboxylic acid [4-(methyl-{4-met...)Show SMILES CN(C(=O)c1ccc(NC(=O)c2ccoc2C)cc1)c1ccc(C)cc1OCCCCCC(=O)N1CCN(C)CC1 Show InChI InChI=1S/C32H40N4O5/c1-23-9-14-28(29(22-23)41-20-7-5-6-8-30(37)36-18-16-34(3)17-19-36)35(4)32(39)25-10-12-26(13-11-25)33-31(38)27-15-21-40-24(27)2/h9-15,21-22H,5-8,16-20H2,1-4H3,(H,33,38) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards vasopressin (V1A) receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114043
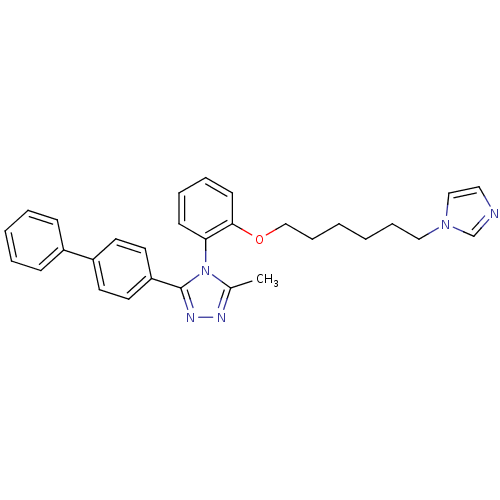
(3-Biphenyl-4-yl-4-[2-(6-imidazol-1-yl-hexyloxy)-ph...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1ccccc1OCCCCCCn1ccnc1 |(8.16,-1.75,;6.79,-2.43,;5.49,-1.61,;4.3,-2.57,;4.86,-4.01,;4.02,-5.3,;2.47,-5.22,;1.64,-6.51,;2.34,-7.87,;3.86,-7.96,;4.71,-6.68,;1.5,-9.16,;2.2,-10.54,;1.35,-11.83,;-.19,-11.74,;-.88,-10.36,;-.04,-9.08,;6.39,-3.93,;7.4,-5.07,;6.91,-6.54,;7.93,-7.68,;9.43,-7.38,;9.92,-5.91,;8.89,-4.77,;9.38,-3.3,;10.75,-2.62,;12.03,-3.47,;13.41,-2.78,;14.69,-3.65,;16.08,-2.95,;17.36,-3.81,;18.73,-3.12,;18.98,-1.61,;20.51,-1.38,;21.18,-2.76,;20.09,-3.83,)| Show InChI InChI=1S/C30H31N5O/c1-24-32-33-30(27-17-15-26(16-18-27)25-11-5-4-6-12-25)35(24)28-13-7-8-14-29(28)36-22-10-3-2-9-20-34-21-19-31-23-34/h4-8,11-19,21,23H,2-3,9-10,20,22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114040
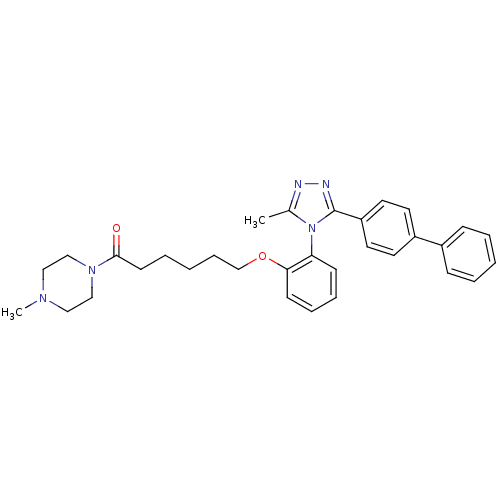
(6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4-yl...)Show SMILES CN1CCN(CC1)C(=O)CCCCCOc1ccccc1-n1c(C)nnc1-c1ccc(cc1)-c1ccccc1 |(17.72,4.65,;16.39,3.89,;16.5,2.35,;15.21,1.49,;13.84,2.17,;13.74,3.71,;15.01,4.57,;12.56,1.32,;12.65,-.22,;11.17,2,;9.89,1.16,;8.51,1.84,;7.22,.98,;5.84,1.67,;4.56,.82,;4.07,-.64,;5.1,-1.78,;4.61,-3.26,;3.11,-3.56,;2.09,-2.41,;2.58,-.96,;1.55,.19,;1.96,1.68,;3.34,2.38,;.66,2.52,;-.53,1.56,;.03,.11,;-.81,-1.18,;-2.36,-1.11,;-3.19,-2.39,;-2.49,-3.76,;-.96,-3.84,;-.12,-2.56,;-3.33,-5.06,;-2.63,-6.43,;-3.48,-7.72,;-5.02,-7.64,;-5.72,-6.25,;-4.88,-4.96,)| Show InChI InChI=1S/C32H37N5O2/c1-25-33-34-32(28-18-16-27(17-19-28)26-11-5-3-6-12-26)37(25)29-13-8-9-14-30(29)39-24-10-4-7-15-31(38)36-22-20-35(2)21-23-36/h3,5-6,8-9,11-14,16-19H,4,7,10,15,20-24H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50114034

(1'-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1ccccc1OCCCCCCN1CCC(CC1)N1CCCCC1 |(6.93,-1.75,;5.55,-2.43,;4.25,-1.61,;3.06,-2.57,;3.62,-4.01,;2.78,-5.3,;1.24,-5.23,;.4,-6.52,;1.1,-7.87,;2.64,-7.96,;3.48,-6.68,;.26,-9.17,;.96,-10.54,;.12,-11.84,;-1.42,-11.74,;-2.11,-10.37,;-1.28,-9.08,;5.16,-3.93,;6.17,-5.07,;5.68,-6.54,;6.69,-7.68,;8.2,-7.38,;8.69,-5.91,;7.66,-4.77,;8.15,-3.3,;9.52,-2.62,;10.8,-3.48,;12.18,-2.78,;13.46,-3.65,;14.84,-2.95,;16.13,-3.81,;17.5,-3.13,;19.04,-3.27,;19.93,-2.04,;19.3,-.63,;17.76,-.47,;16.87,-1.73,;20.09,.67,;19.39,2.01,;20.21,3.3,;21.73,3.24,;22.45,1.91,;21.63,.61,)| Show InChI InChI=1S/C37H47N5O/c1-30-38-39-37(33-20-18-32(19-21-33)31-14-6-4-7-15-31)42(30)35-16-8-9-17-36(35)43-29-13-3-2-10-24-40-27-22-34(23-28-40)41-25-11-5-12-26-41/h4,6-9,14-21,34H,2-3,5,10-13,22-29H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V1a receptor, performed using [3H]-AVP on rat liver |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114029

(1-{8-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES CN1CCN(CCCCCCCCOc2ccccc2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)CC1 |(24.67,-8.17,;24.58,-6.63,;23.19,-5.95,;23.09,-4.42,;24.38,-3.58,;24.24,-2.04,;22.84,-1.4,;21.58,-2.29,;20.18,-1.64,;18.92,-2.53,;17.52,-1.89,;16.26,-2.77,;14.86,-2.13,;13.62,-3.02,;13.76,-4.55,;15.14,-5.18,;15.28,-6.72,;14.02,-7.59,;12.64,-6.96,;12.51,-5.42,;11.12,-4.78,;10.9,-3.25,;11.88,-2.08,;9.38,-2.99,;8.66,-4.36,;9.75,-5.46,;9.49,-6.98,;8.05,-7.52,;7.79,-9.03,;8.98,-10,;10.41,-9.48,;10.68,-7.96,;8.71,-11.52,;9.91,-12.51,;9.64,-14.02,;8.19,-14.56,;7,-13.57,;7.28,-12.06,;25.75,-4.24,;25.85,-5.78,)| Show InChI InChI=1S/C34H43N5O/c1-28-35-36-34(31-20-18-30(19-21-31)29-14-8-7-9-15-29)39(28)32-16-10-11-17-33(32)40-27-13-6-4-3-5-12-22-38-25-23-37(2)24-26-38/h7-11,14-21H,3-6,12-13,22-27H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114039

(1-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES Cc1nnc(-c2ccc(cc2)-c2ccccc2)n1-c1ccccc1OCCCCCCN1CCCCC1 |(9.29,-1.39,;7.83,-1.97,;6.55,-1.15,;5.37,-2.08,;5.92,-3.51,;5.02,-4.76,;5.68,-6.17,;4.76,-7.42,;3.23,-7.27,;2.58,-5.86,;3.49,-4.61,;2.33,-8.52,;2.97,-9.93,;2.07,-11.18,;.52,-11.06,;-.12,-9.64,;.78,-8.39,;7.45,-3.44,;8.55,-4.53,;8.14,-5.99,;9.24,-7.08,;10.72,-6.68,;11.12,-5.22,;10.03,-4.13,;10.44,-2.63,;11.94,-2.23,;12.33,-.75,;13.82,-.36,;14.22,1.13,;15.68,1.53,;16.09,3.01,;17.57,3.39,;18.67,2.32,;20.12,2.71,;20.53,4.19,;19.44,5.28,;17.96,4.88,)| Show InChI InChI=1S/C32H38N4O/c1-26-33-34-32(29-20-18-28(19-21-29)27-14-6-4-7-15-27)36(26)30-16-8-9-17-31(30)37-25-13-3-2-10-22-35-23-11-5-12-24-35/h4,6-9,14-21H,2-3,5,10-13,22-25H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50029644

(CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...)Show SMILES CC(=O)NCCCOc1ccc(cc1)C(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C26H31N3O4/c1-19(30)27-15-4-18-33-23-10-7-21(8-11-23)26(32)28-16-13-22(14-17-28)29-24-6-3-2-5-20(24)9-12-25(29)31/h2-3,5-8,10-11,22H,4,9,12-18H2,1H3,(H,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50114028

(1-{6-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES CN1CCN(CCCCCCOc2ccccc2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)CC1 |(27.25,-2.68,;25.78,-2.25,;24.66,-3.31,;23.19,-2.88,;22.83,-1.38,;21.58,-2.29,;20.18,-1.64,;18.92,-2.53,;17.52,-1.89,;16.26,-2.77,;14.86,-2.13,;13.62,-3.02,;13.76,-4.55,;15.14,-5.18,;15.28,-6.72,;14.02,-7.59,;12.64,-6.96,;12.51,-5.42,;11.12,-4.78,;10.9,-3.25,;11.88,-2.08,;9.38,-2.99,;8.66,-4.36,;9.75,-5.46,;9.49,-6.98,;8.05,-7.52,;7.79,-9.03,;8.98,-10,;10.41,-9.48,;10.68,-7.96,;8.71,-11.52,;7.28,-12.06,;7,-13.57,;8.19,-14.56,;9.64,-14.02,;9.91,-12.51,;23.93,-.31,;25.4,-.75,)| Show InChI InChI=1S/C32H39N5O/c1-26-33-34-32(29-18-16-28(17-19-29)27-12-6-5-7-13-27)37(26)30-14-8-9-15-31(30)38-25-11-4-3-10-20-36-23-21-35(2)22-24-36/h5-9,12-19H,3-4,10-11,20-25H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V1a receptor, performed using [3H]-AVP on rat liver |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50029644

(CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...)Show SMILES CC(=O)NCCCOc1ccc(cc1)C(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C26H31N3O4/c1-19(30)27-15-4-18-33-23-10-7-21(8-11-23)26(32)28-16-13-22(14-17-28)29-24-6-3-2-5-20(24)9-12-25(29)31/h2-3,5-8,10-11,22H,4,9,12-18H2,1H3,(H,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50029644

(CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...)Show SMILES CC(=O)NCCCOc1ccc(cc1)C(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C26H31N3O4/c1-19(30)27-15-4-18-33-23-10-7-21(8-11-23)26(32)28-16-13-22(14-17-28)29-24-6-3-2-5-20(24)9-12-25(29)31/h2-3,5-8,10-11,22H,4,9,12-18H2,1H3,(H,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at Vasopressin V1a receptor, performed using [3H]-AVP on rat liver |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50029644

(CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...)Show SMILES CC(=O)NCCCOc1ccc(cc1)C(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C26H31N3O4/c1-19(30)27-15-4-18-33-23-10-7-21(8-11-23)26(32)28-16-13-22(14-17-28)29-24-6-3-2-5-20(24)9-12-25(29)31/h2-3,5-8,10-11,22H,4,9,12-18H2,1H3,(H,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for the binding affinity towards Vasopressin V1a receptor in rat liver membrane using [3H]AVP as radioligand |
Bioorg Med Chem Lett 12: 229-32 (2001)
BindingDB Entry DOI: 10.7270/Q2R78FQP |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM85094

(CAS_62357-86-2 | NSC_64759 | dDAVP)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6]-1-[#7]-[#6](=O)-[#6](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114026
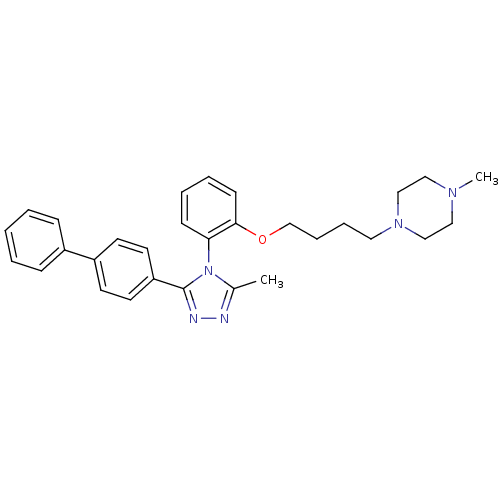
(1-{4-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...)Show SMILES CN1CCN(CCCCOc2ccccc2-n2c(C)nnc2-c2ccc(cc2)-c2ccccc2)CC1 |(24.65,-.56,;23.17,-.92,;22.72,-2.39,;21.23,-2.76,;20.18,-1.64,;18.92,-2.53,;17.52,-1.89,;16.26,-2.78,;14.86,-2.13,;13.62,-3.02,;13.76,-4.55,;15.14,-5.19,;15.28,-6.72,;14.02,-7.59,;12.64,-6.96,;12.51,-5.42,;11.12,-4.78,;10.9,-3.25,;11.88,-2.08,;9.38,-2.99,;8.66,-4.36,;9.75,-5.46,;9.49,-6.98,;8.05,-7.52,;7.79,-9.03,;8.98,-10.01,;10.41,-9.48,;10.68,-7.96,;8.71,-11.52,;9.91,-12.51,;9.64,-14.02,;8.19,-14.56,;7,-13.57,;7.28,-12.06,;20.6,-.16,;22.09,.2,)| Show InChI InChI=1S/C30H35N5O/c1-24-31-32-30(27-16-14-26(15-17-27)25-10-4-3-5-11-25)35(24)28-12-6-7-13-29(28)36-23-9-8-18-34-21-19-33(2)20-22-34/h3-7,10-17H,8-9,18-23H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity at cloned human Vasopressin V1a receptor stably expressed in CHO cells, using [3H]-AVP as radioligand |
J Med Chem 45: 2589-98 (2002)
BindingDB Entry DOI: 10.7270/Q2NC60H9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































