

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273137 ((3-{[(3-Carbamimidoyl-phenyl)-({4-[1-(1-imino-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078977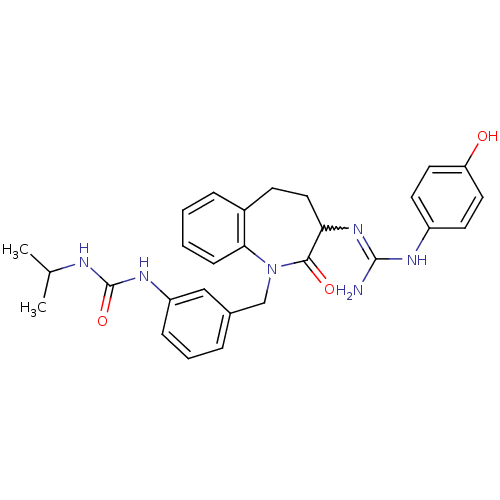 (1-(3-{3-[N'-(4-Hydroxy-phenyl)-guanidino]-2-oxo-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273136 (CHEMBL455932 | CHEMBL463179 | {4-[2-(5-Carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078965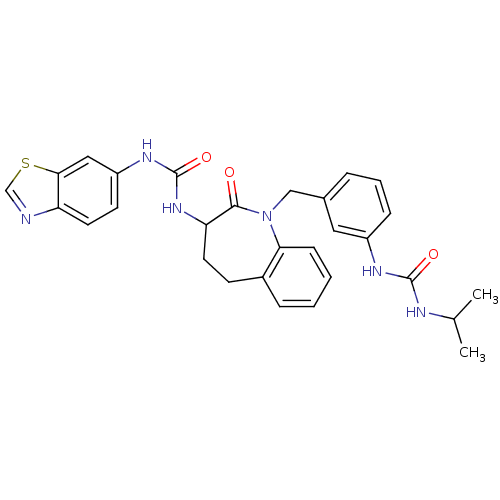 (1-Benzothiazol-6-yl-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273299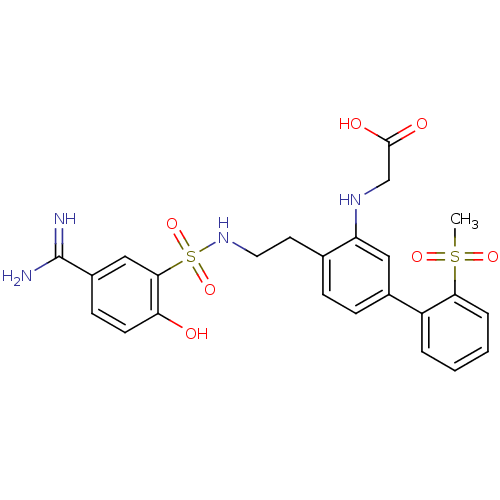 (CHEMBL455933 | {4-[2-(5-Carbamimidoyl-2-hydroxy-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079009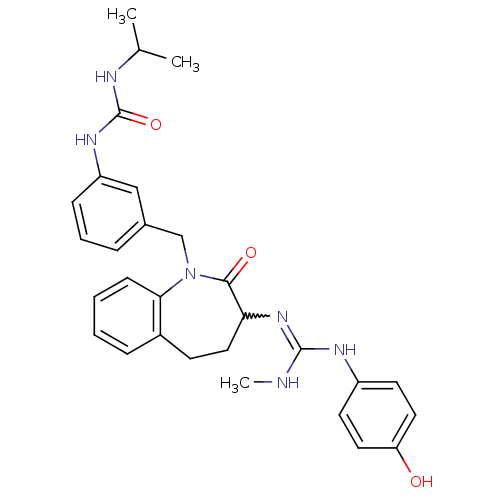 (1-(3-{3-[N'-(4-Hydroxy-phenyl)-N''-methyl-guanidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078970 (1-(2-Fluoro-phenyl)-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079012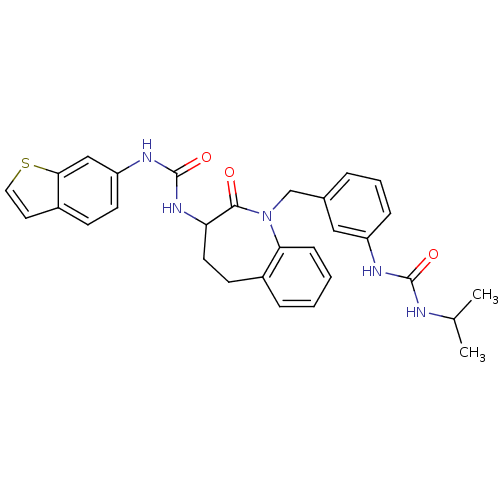 (1-Benzo[b]thiophen-6-yl-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079015 (1-(2,4-Difluoro-phenyl)-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079016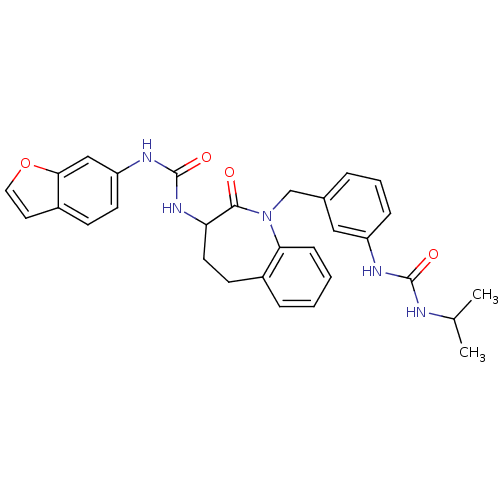 (1-Benzofuran-6-yl-3-{1-[3-(3-isopropyl-ureido)-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273297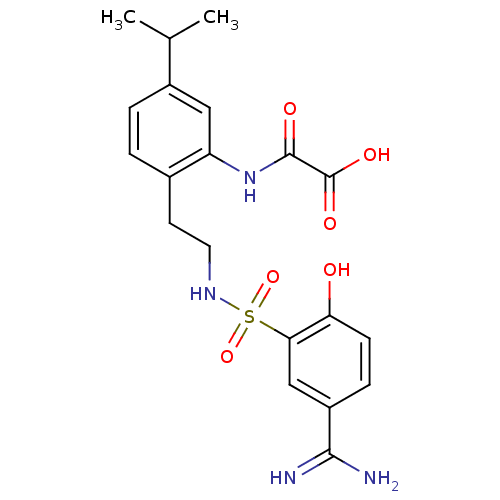 (CHEMBL459136 | N-{2-[2-(5-Carbamimidoyl-2-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078958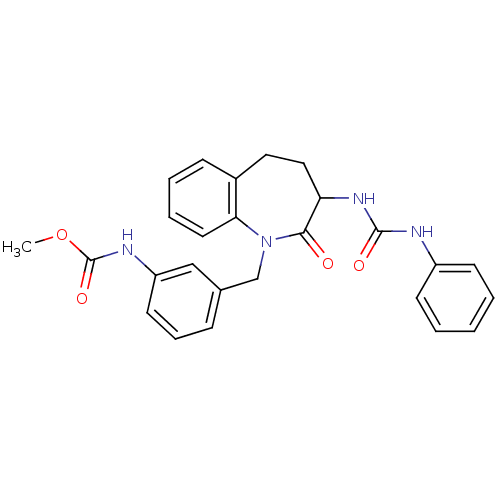 (CHEMBL433216 | {3-[2-Oxo-3-(3-phenyl-ureido)-2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078966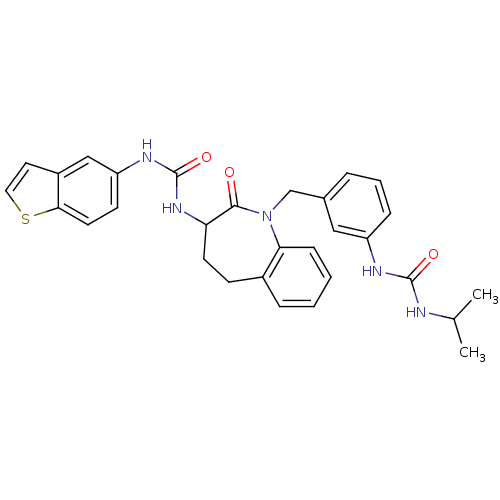 (1-Benzo[b]thiophen-5-yl-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273229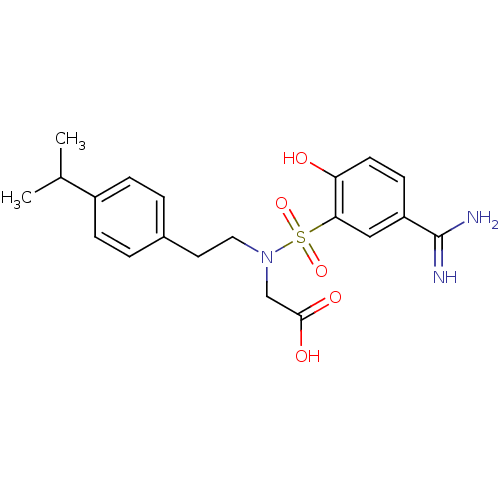 (CHEMBL455916 | {(5-Carbamimidoyl-2-hydroxy-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078974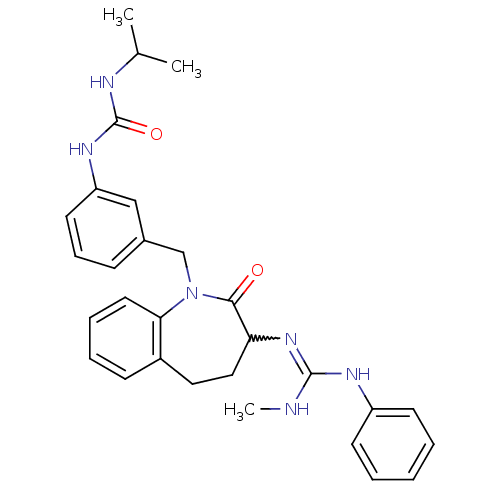 (1-Isopropyl-3-{3-[3-(N'-methyl-N''-phenyl-guanidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078994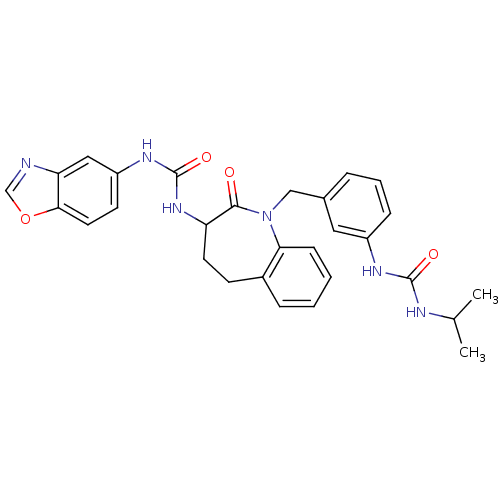 (1-Benzooxazol-5-yl-3-{1-[3-(3-isopropyl-ureido)-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078971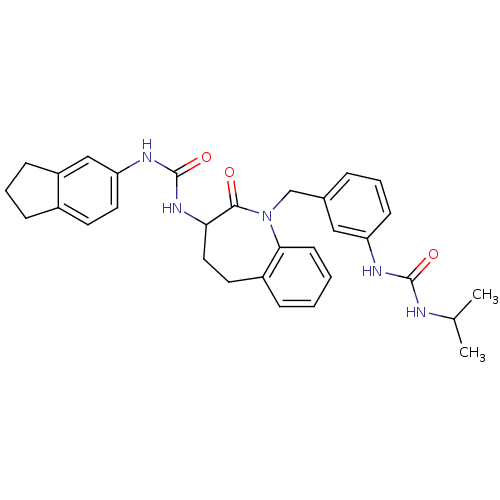 (1-Indan-5-yl-3-{1-[3-(3-isopropyl-ureido)-benzyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078981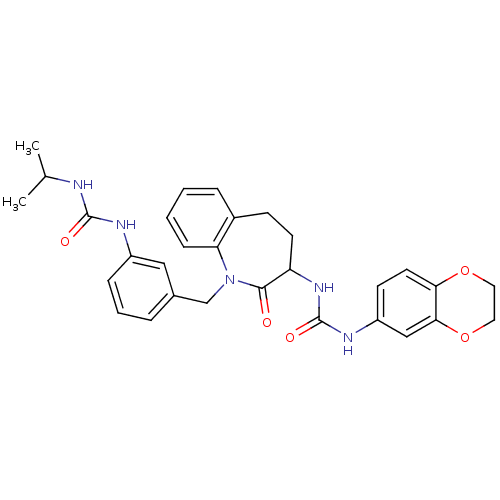 (1-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-3-{1-[3-(3-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078993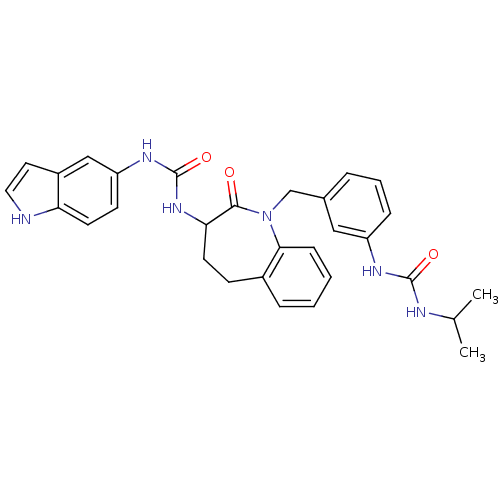 (1-(1H-Indol-5-yl)-3-{1-[3-(3-isopropyl-ureido)-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079017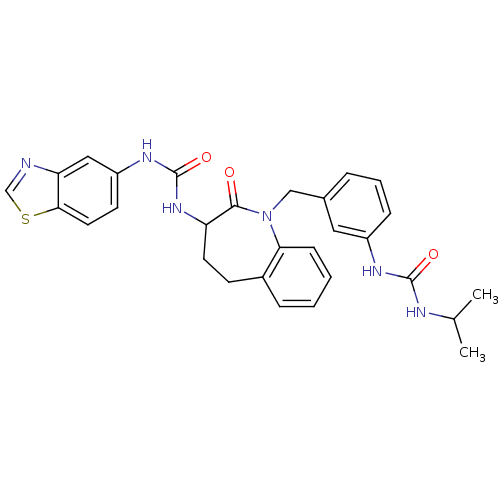 (1-Benzothiazol-5-yl-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078995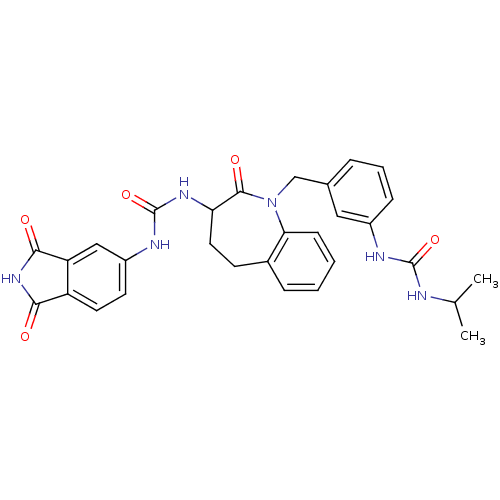 (1-(1,3-Dioxo-2,3-dihydro-1H-isoindol-5-yl)-3-{1-[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078959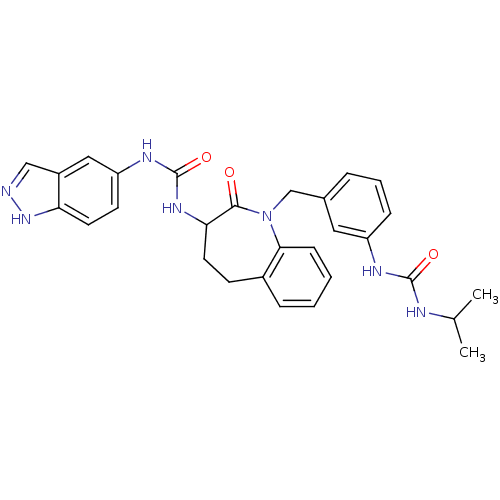 (1-(1H-Indazol-5-yl)-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273188 (3-[2-(4-tert-Butyl-phenyl)-ethylsulfamoyl]-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078975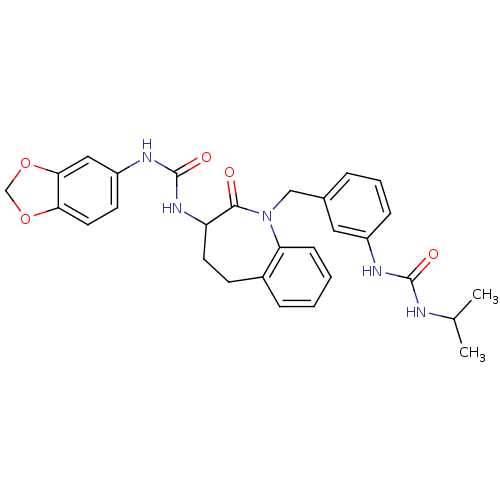 (1-Benzo[1,3]dioxol-5-yl-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273138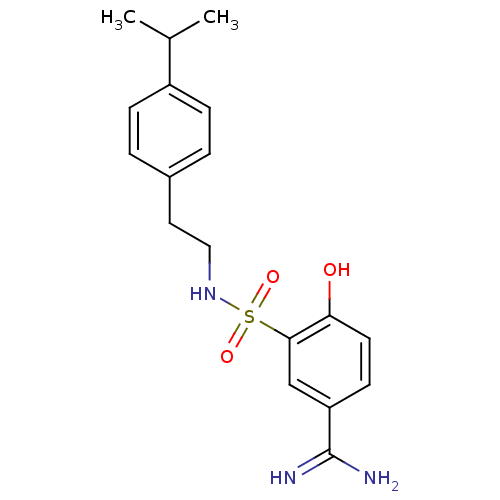 (4-Hydroxy-3-[2-(4-isopropyl-phenyl)-ethylsulfamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078985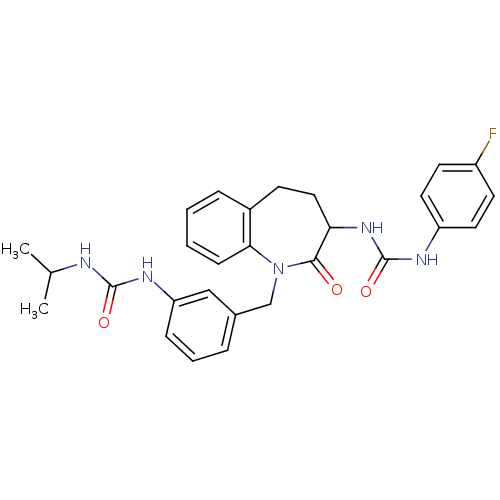 (1-(4-Fluoro-phenyl)-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078957 (1-(3-{3-[N'-(4-Hydroxy-phenyl)-N''-isopropyl-guani...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17283 ((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of factor 10a in human plasma | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078979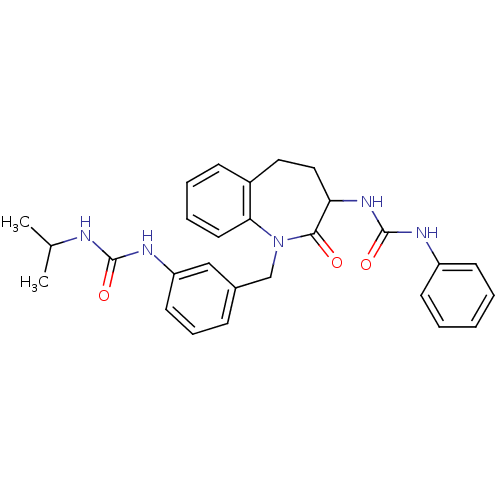 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273262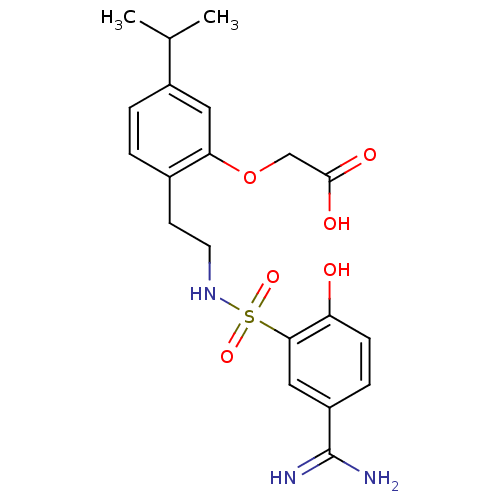 (CHEMBL458067 | {2-[2-(5-Carbamimidoyl-2-hydroxy-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079013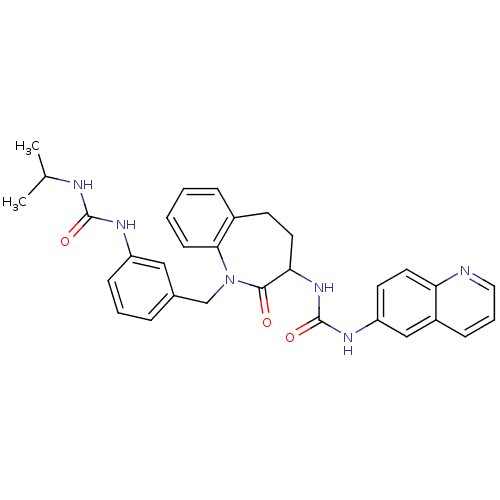 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078972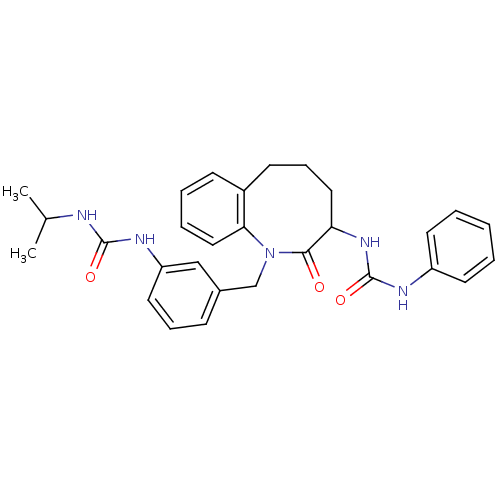 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273227 (4-Hydroxy-3-{[2-(4-isopropyl-phenyl)-ethyl]-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273265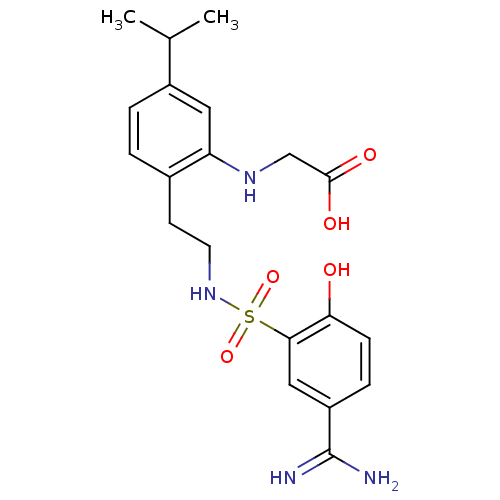 (CHEMBL457633 | {2-[2-(5-Carbamimidoyl-2-hydroxy-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078990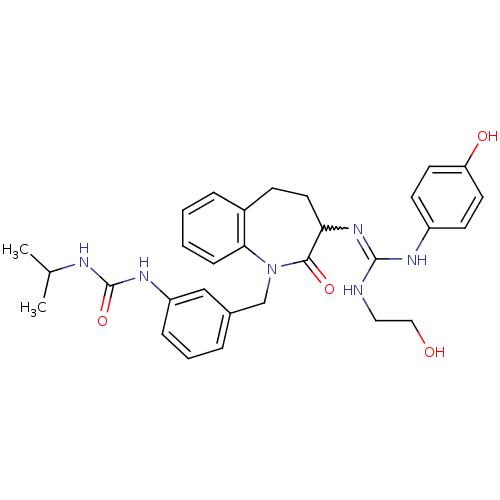 (1-(3-{3-[N'-(2-Hydroxy-ethyl)-N''-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078967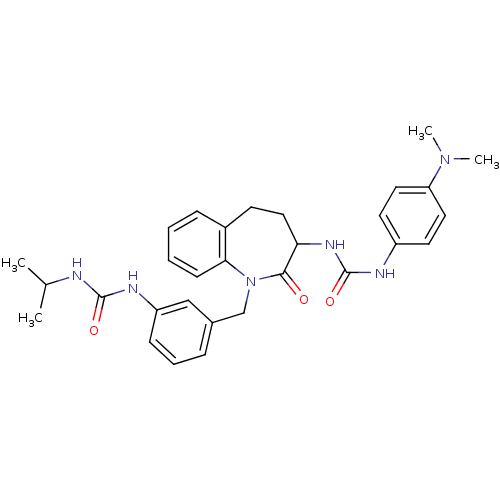 (1-(4-Dimethylamino-phenyl)-3-{1-[3-(3-isopropyl-ur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078998 (1-(4-Chloro-phenyl)-3-{1-[3-(3-isopropyl-ureido)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078976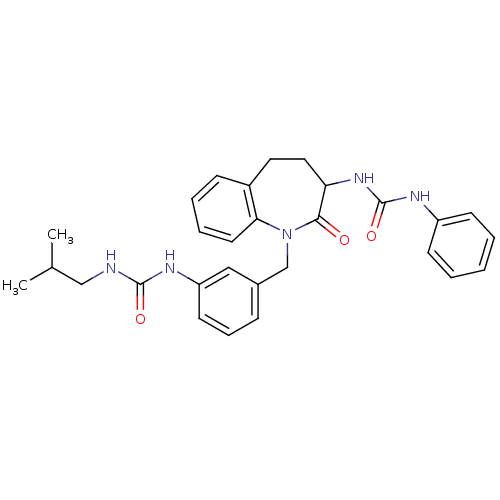 (1-{1-[3-(3-Isobutyl-ureido)-benzyl]-2-oxo-2,3,4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079002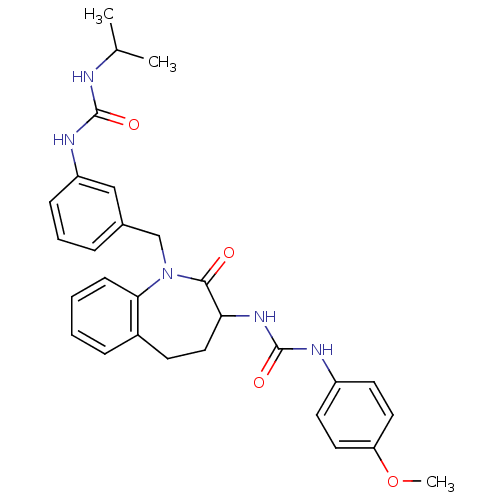 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078969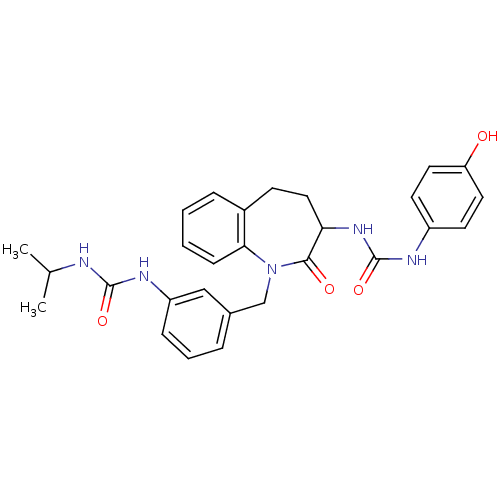 (1-(4-Hydroxy-phenyl)-3-{1-[3-(3-isopropyl-ureido)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079011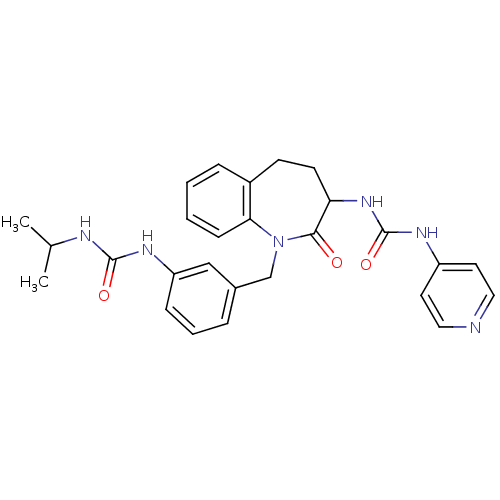 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078996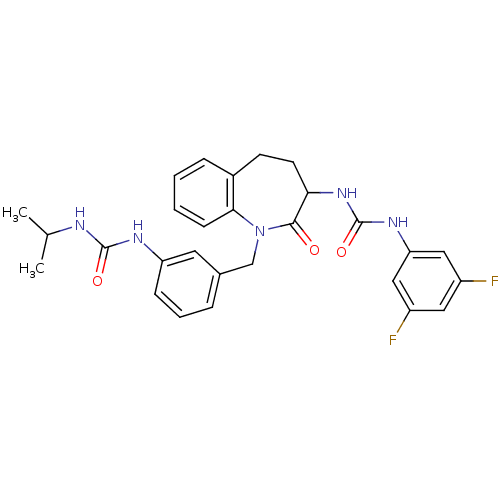 (1-(3,5-Difluoro-phenyl)-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078987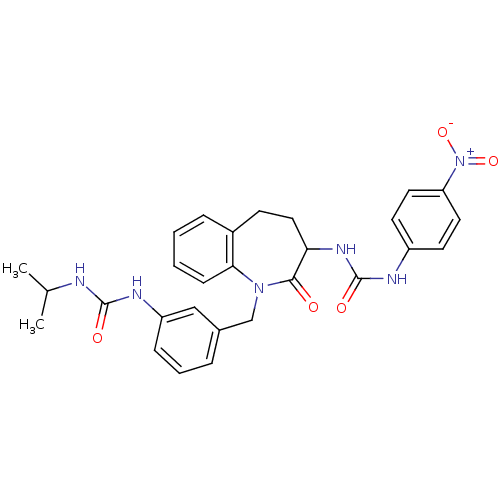 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50079010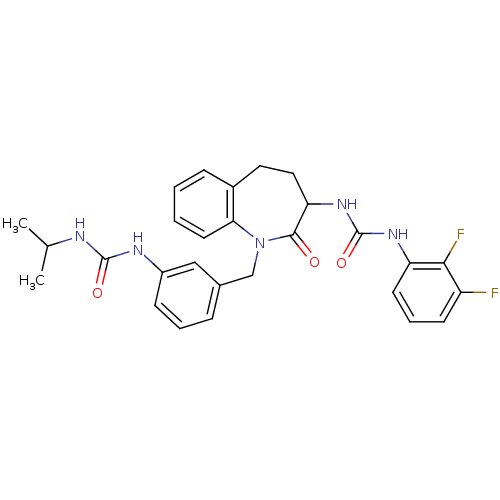 (1-(2,3-Difluoro-phenyl)-3-{1-[3-(3-isopropyl-ureid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50273187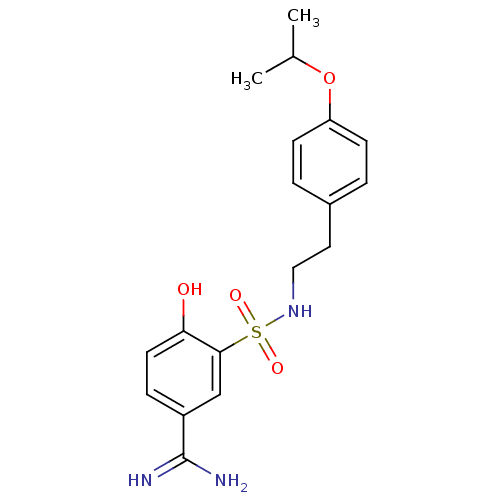 (4-Hydroxy-3-[2-(4-isopropoxy-phenyl)-ethylsulfamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human factor 10a by Dixon-plot method | Bioorg Med Chem Lett 18: 4682-7 (2008) Article DOI: 10.1016/j.bmcl.2008.07.009 BindingDB Entry DOI: 10.7270/Q208654H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078988 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078973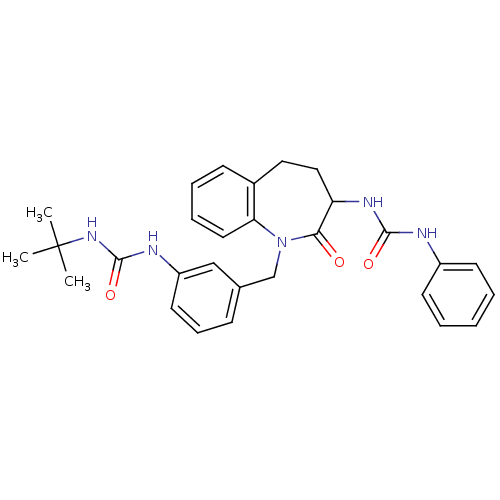 (1-{1-[3-(3-tert-Butyl-ureido)-benzyl]-2-oxo-2,3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078956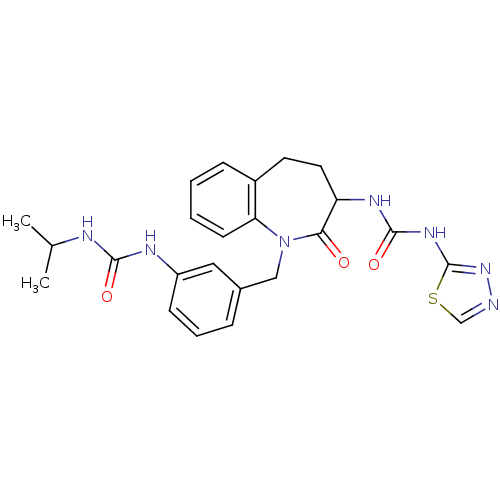 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078960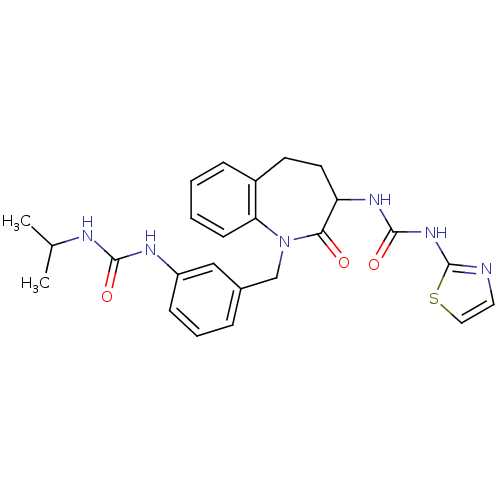 (1-{1-[3-(3-Isopropyl-ureido)-benzyl]-2-oxo-2,3,4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50078984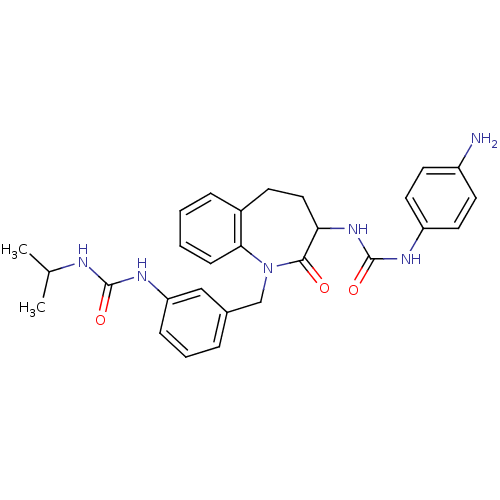 (1-(4-Amino-phenyl)-3-{1-[3-(3-isopropyl-ureido)-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards Neuropeptide Y receptor type 1 was determined as potency to displace [125I]-peptide YY binding to human neuroblasto... | J Med Chem 42: 2621-32 (1999) Article DOI: 10.1021/jm990044m BindingDB Entry DOI: 10.7270/Q2XK8DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 470 total ) | Next | Last >> |