

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202364 (US9238626, (-)-(Ib) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202363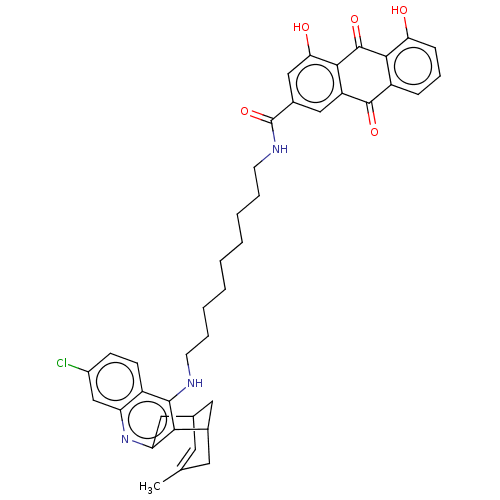 (US9238626, (+/-)-(Ib) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202362 (US9238626, (+/-)-(Ia) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202366 (US9238626, (+/-)-(Ic) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM202364 (US9238626, (-)-(Ib) HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379274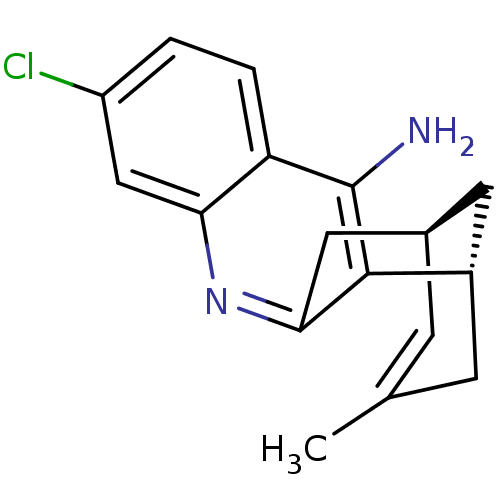 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM202367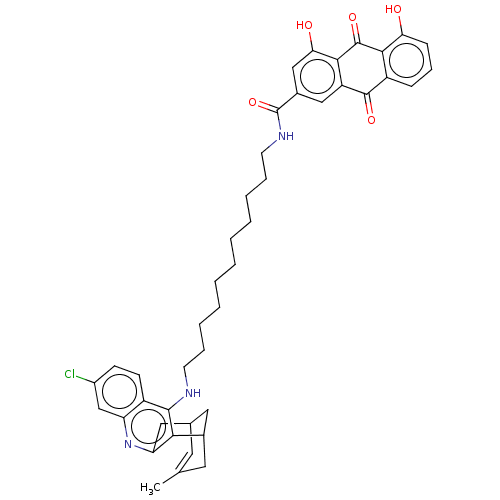 (US9238626, (+/-)-(Id) HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Cagliari Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells assessed as using transformed p-tyramine after 15 mins by flu... | Bioorg Med Chem Lett 22: 258-61 (2011) Article DOI: 10.1016/j.bmcl.2011.11.020 BindingDB Entry DOI: 10.7270/Q2TH8N4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202367 (US9238626, (+/-)-(Id) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202368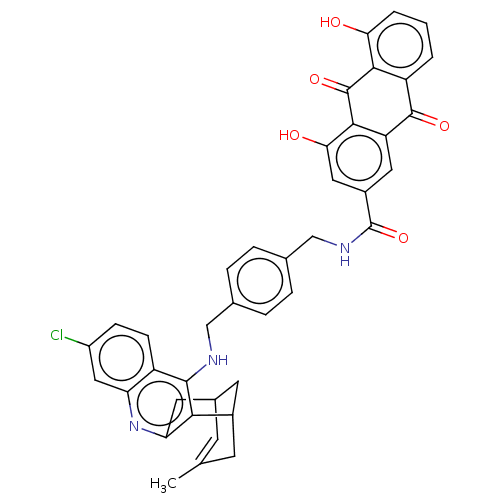 (US9238626, (+/-)-(Ie) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM202363 (US9238626, (+/-)-(Ib) HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32.6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM202366 (US9238626, (+/-)-(Ic) HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 46.7 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM202368 (US9238626, (+/-)-(Ie) HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202365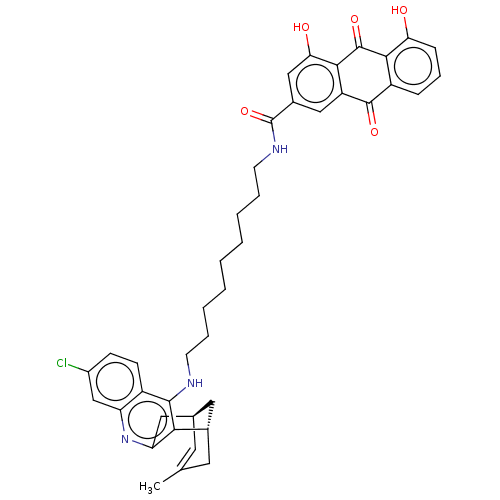 (US9238626, (+)-(Ib) HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202364 (US9238626, (-)-(Ib) HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM202362 (US9238626, (+/-)-(Ia) HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 88.6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM202365 (US9238626, (+)-(Ib) HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 98.9 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202363 (US9238626, (+/-)-(Ib) HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 181 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 222 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM202365 (US9238626, (+)-(Ib) HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 265 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM202362 (US9238626, (+/-)-(Ia) HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 373 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM202368 (US9238626, (+/-)-(Ie) HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM202364 (US9238626, (-)-(Ib) HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 513 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM202363 (US9238626, (+/-)-(Ib) HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM32021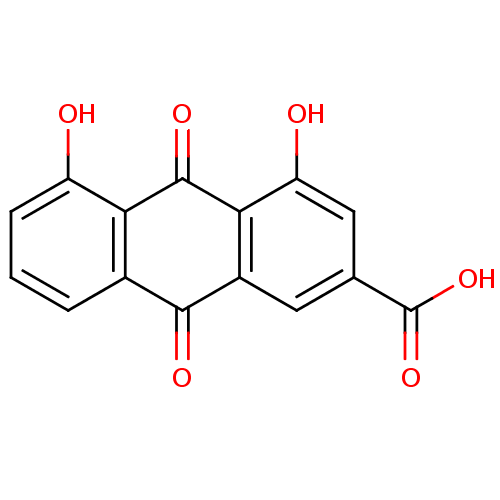 (4,5-bis(oxidanyl)-9,10-bis(oxidanylidene)anthracen...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 637 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM202367 (US9238626, (+/-)-(Id) HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 645 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202362 (US9238626, (+/-)-(Ia) HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM202366 (US9238626, (+/-)-(Ic) HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202366 (US9238626, (+/-)-(Ic) HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202367 (US9238626, (+/-)-(Id) HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202368 (US9238626, (+/-)-(Ie) HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix protein 2 [1-96] (Influenza A virus) | BDBM50343543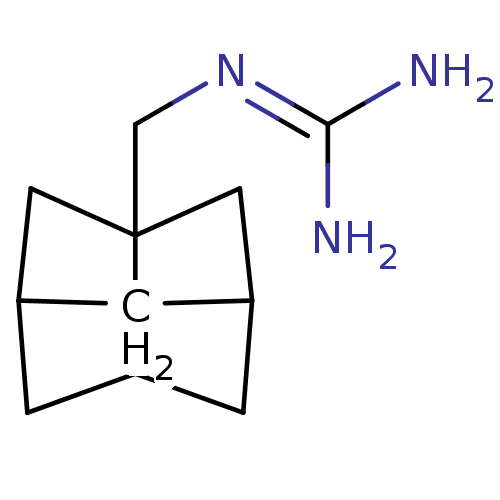 (CHEMBL499125 | N-(Hexahydro-2,5-methano-pentalen-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Udorn/72) wild type matrix protein 2 expressed in xenopus oocytes after 2 mins by two-electrode voltage clamp assa... | J Med Chem 54: 2646-57 (2011) Article DOI: 10.1021/jm101334y BindingDB Entry DOI: 10.7270/Q2H41RR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50360811 (CHEMBL1934677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Cagliari Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells assessed as using transformed p-tyramine after 15 mins by flu... | Bioorg Med Chem Lett 22: 258-61 (2011) Article DOI: 10.1016/j.bmcl.2011.11.020 BindingDB Entry DOI: 10.7270/Q2TH8N4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202365 (US9238626, (+)-(Ib) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description β-Secretase (BACE-1, Sigma) inhibition studies were performed by employing a peptide mimicking APP sequence as substrate (methoxycoumarin-Ser-... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM29136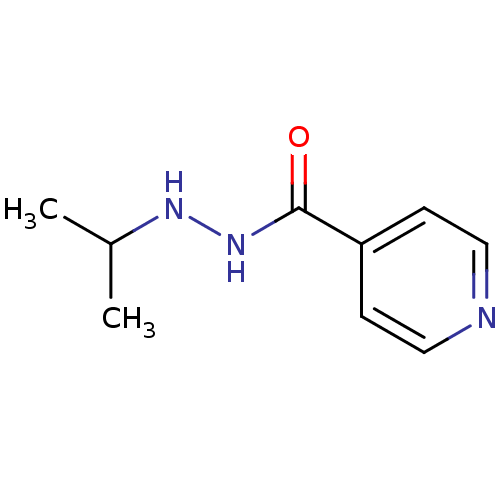 (CHEMBL92401 | Euphozid | Iprazid | Iproniazid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Cagliari Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA expressed in baculovirus-infected BTI insect cells assessed as conversion of p-tyramine into p-hydroxyphenyl-ace... | Bioorg Med Chem Lett 22: 258-61 (2011) Article DOI: 10.1016/j.bmcl.2011.11.020 BindingDB Entry DOI: 10.7270/Q2TH8N4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix protein 2 [1-96] (Influenza A virus) | BDBM50343541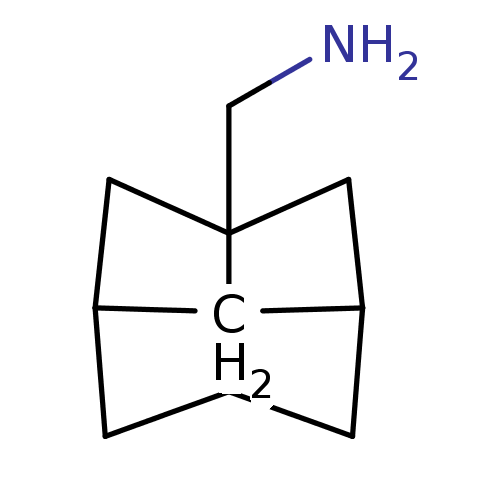 (C-(Hexahydro-2,5-methano-pentalen-3a-yl)-methylami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Udorn/72) wild type matrix protein 2 expressed in xenopus oocytes after 2 mins by two-electrode voltage clamp assa... | J Med Chem 54: 2646-57 (2011) Article DOI: 10.1021/jm101334y BindingDB Entry DOI: 10.7270/Q2H41RR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix protein 2 [1-96] (Influenza A virus) | BDBM50343546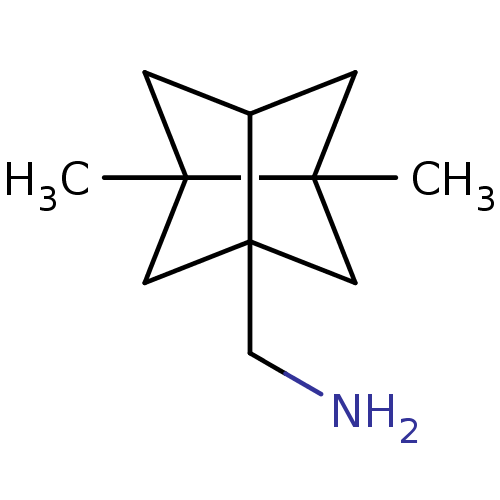 (C-(2,5-Dimethyl-hexahydro-2,5-cyclo-pentalen-3a-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Udorn/72) wild type matrix protein 2 expressed in xenopus oocytes after 2 mins by two-electrode voltage clamp assa... | J Med Chem 54: 2646-57 (2011) Article DOI: 10.1021/jm101334y BindingDB Entry DOI: 10.7270/Q2H41RR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM29136 (CHEMBL92401 | Euphozid | Iprazid | Iproniazid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Cagliari Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells assessed as using transformed p-tyramine after 15 mins by flu... | Bioorg Med Chem Lett 22: 258-61 (2011) Article DOI: 10.1016/j.bmcl.2011.11.020 BindingDB Entry DOI: 10.7270/Q2TH8N4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50360809 (CHEMBL1934675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Cagliari Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells assessed as using transformed p-tyramine after 15 mins by flu... | Bioorg Med Chem Lett 22: 258-61 (2011) Article DOI: 10.1016/j.bmcl.2011.11.020 BindingDB Entry DOI: 10.7270/Q2TH8N4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM87351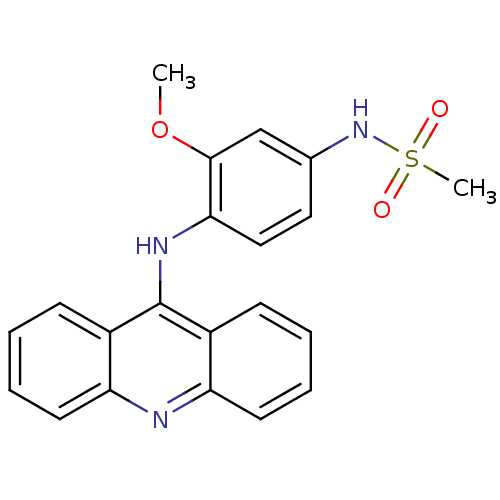 (Amsacrine hydrochloride | CHEMBL43 | MLS002153376 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2-mediated Crithidia fasciculata kDNA decatenation using ethidium bromide staining by agarose gel electrophores... | Bioorg Med Chem 17: 3266-77 (2009) Article DOI: 10.1016/j.bmc.2009.03.052 BindingDB Entry DOI: 10.7270/Q21N811C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM32021 (4,5-bis(oxidanyl)-9,10-bis(oxidanylidene)anthracen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |