

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163037 (US9061041, Compound C) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163034 (US9061041, 99) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 60 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163032 (US9061041, 26) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 70 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163036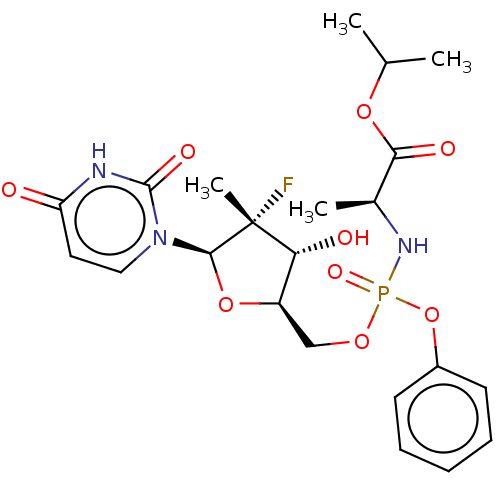 (US9061041, Compound B) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92463 (Imipenem) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163033 (US9061041, 24) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.70E+3 | -30.4 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM163035 (US9061041, 93) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.00E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9061041 (2015) BindingDB Entry DOI: 10.7270/Q2GM862K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50324669 (CHEMBL1221600 | bistramide A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chicago Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from human recombinant PKCdelta by competitive binding assay | Nat Chem Biol 1: 383-8 (2005) BindingDB Entry DOI: 10.7270/Q2GM87HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50140672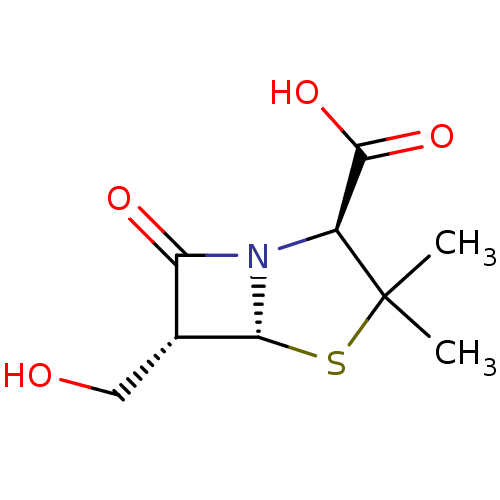 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92461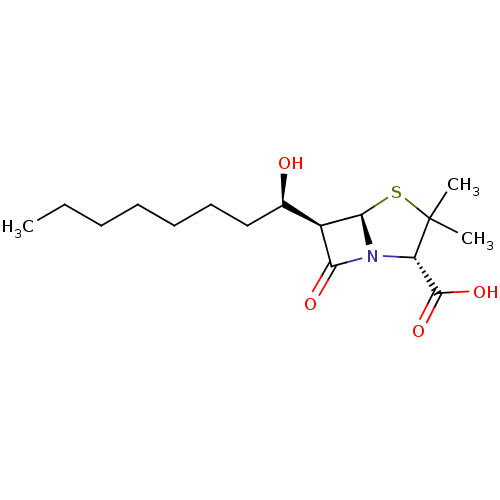 (Beta-lactam compound, 4) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92464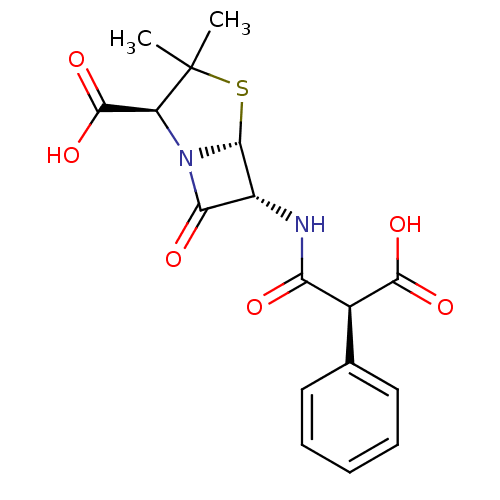 (Carbenicillin) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50140671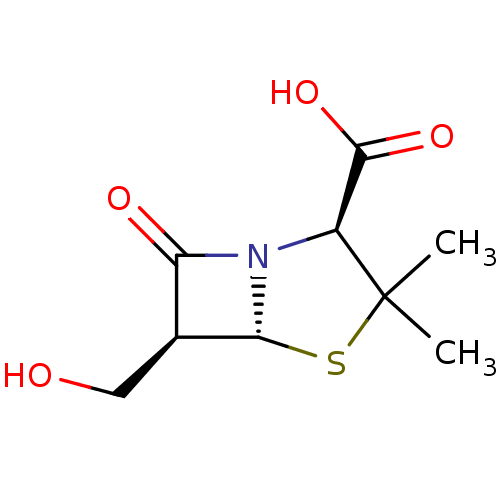 ((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92462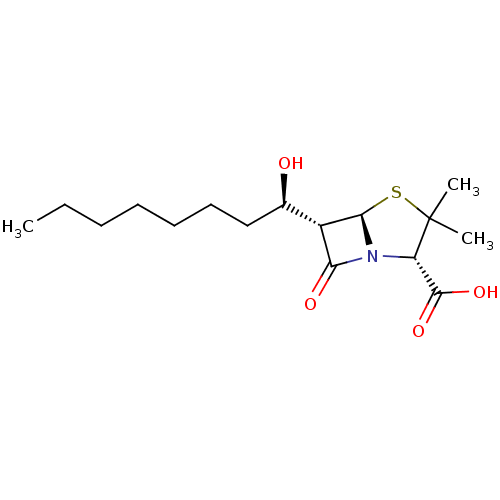 (Beta-lactam compound, 3) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437915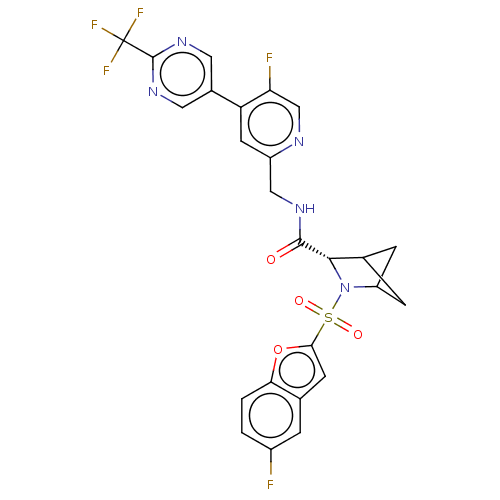 ((2S)-3-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[5-fl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437925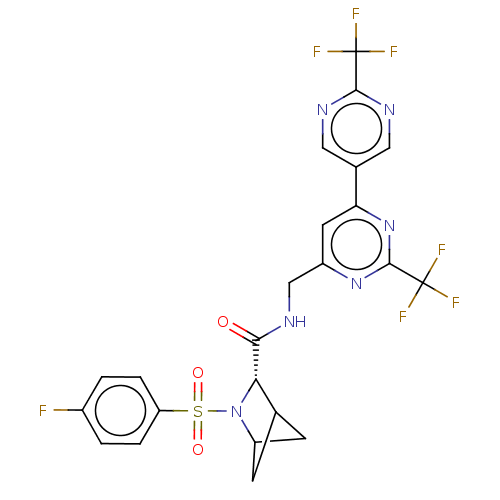 ((2S)-3-(4- fluorophenyl)sulfonyl-N-[[2- (trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437848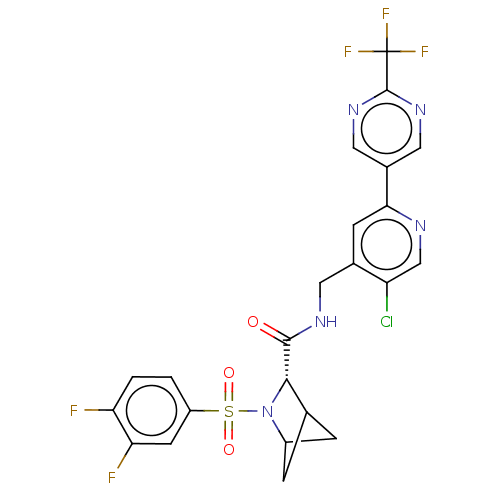 ((2S)-N-[[5-chloro-2-[2- (trifluoromethyl)pyrimidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437932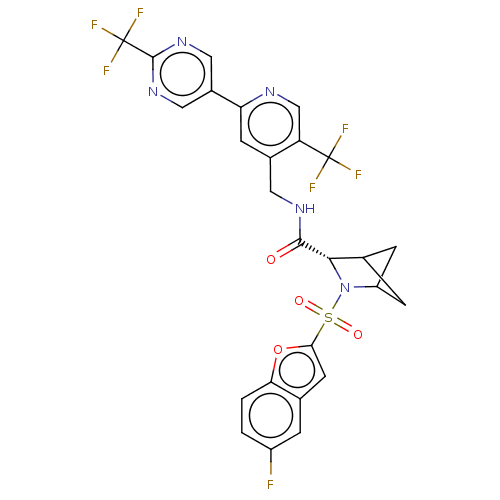 ((2S)-3-(5-fluorobenzofuran-2- yl)sulfonyl-N-[[5- (...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437842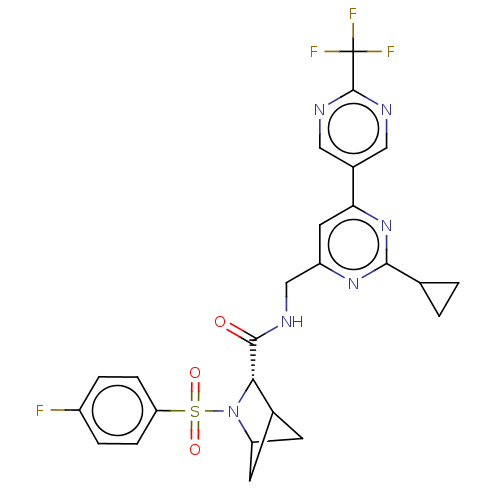 ((2S)-N-[[2-cyclopropyl-6-[2- (trifluoromethyl)pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437894 ((2S)-3-(4- fluorophenyl)sulfonyl-N-[[5- (trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437854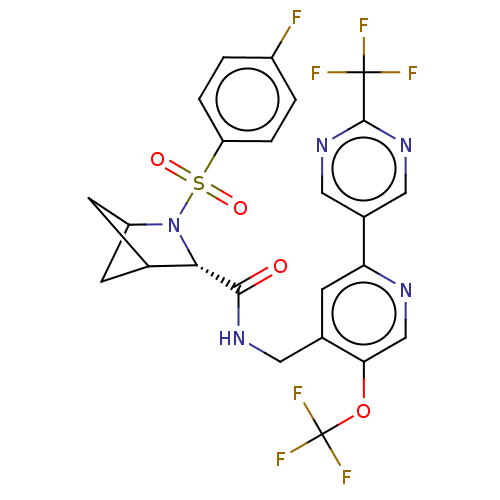 ((2S)-3-(4- fluorophenyl)sulfonyl-N-[[5- (trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM535104 (Preparation of (1S,2S,5R)óN-(2-fluoro-5-(2-(triflu...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437955 ((2S)-N-[[2-cyclopropyl-6-[2- (trifluoromethyl)pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437851 ((2S)-N-[[5-bromo-2-[2- (trifluoromethyl)pyrimidin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM535107 (Preparation of (2S,5S)-1-(4-fluorophenylsulfonyl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM535018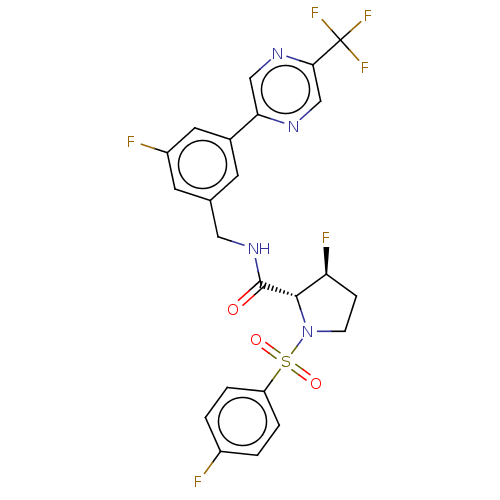 (Preparation of (2R,3S)-3-fluoro-N-([3-fluoro-5-[5-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM535079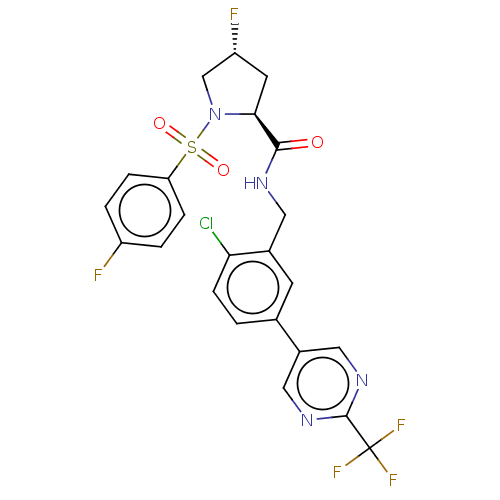 (Preparation of (2S,4R)óN-[[2-chloro-5-[2-(trifluor...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437976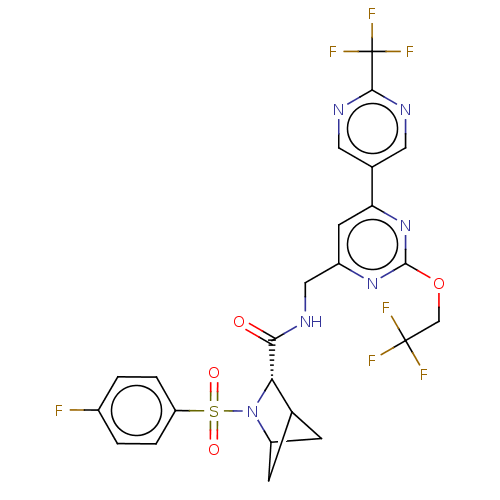 ((2S)-3-(4- fluorophenyl)sulfonyl-N-[[2- (2,2,2-tri...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437802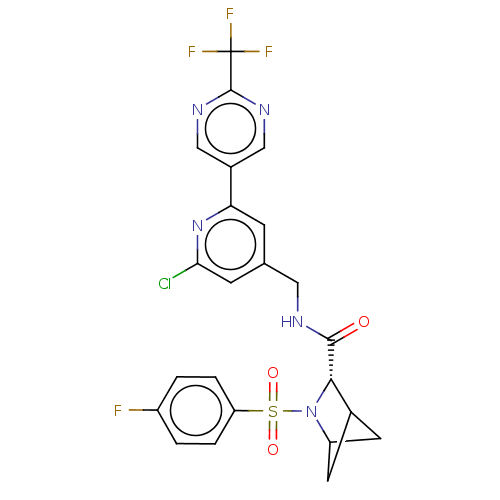 ((2S)-N-[[2-chloro-6-[2- (trifluoromethyl)pyrimidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437920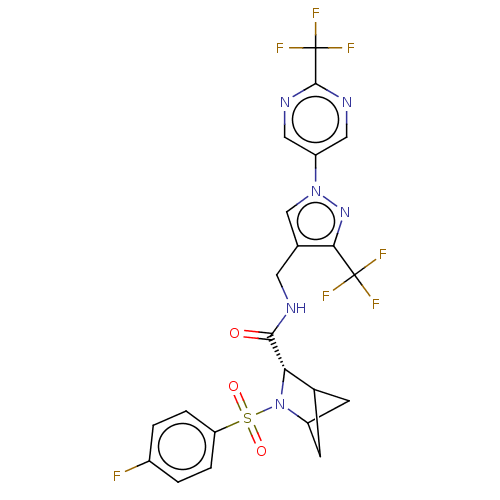 ((2S)-3-(4- fluorophenyl)sulfonyl-N-[[3- (trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.84 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM111376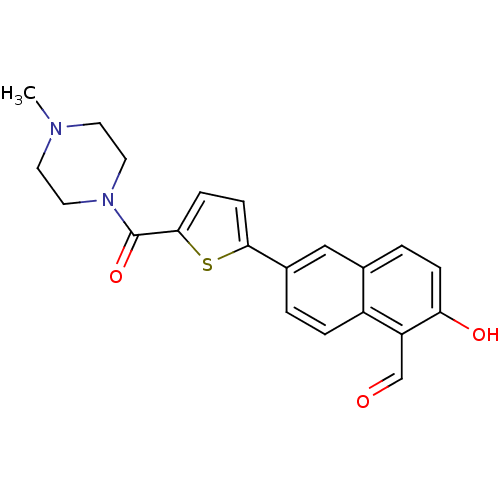 (US8614253, 32-12 | US8614253, 33-3 | US9241942, 33...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IRE1 in human RPMI 8226 cells assessed as reduction in XBP1 splicing incubated for 3 hrs by RT-PCR method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01177 BindingDB Entry DOI: 10.7270/Q2DB85MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM535098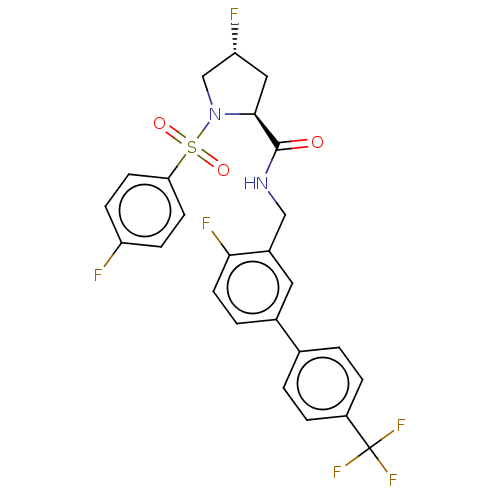 (Preparation of (2S,4R)-4-fluoro-N-((4-fluoro-4R...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263547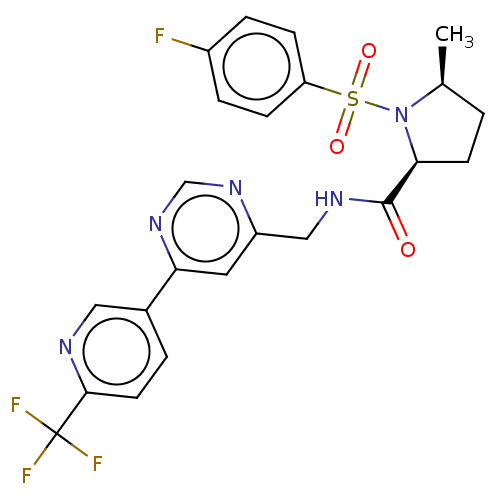 (CHEMBL4077957 | US11236046, Example 41) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263509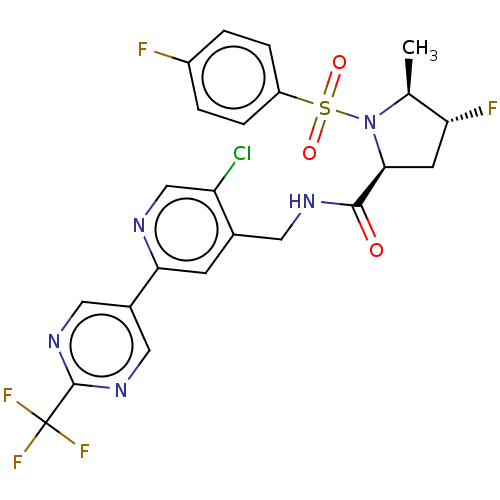 (CHEMBL4103545) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA , Beijing 100176 , P. R. China. Curated by ChEMBL | Assay Description Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by... | J Med Chem 61: 3641-3659 (2018) Article DOI: 10.1021/acs.jmedchem.8b00117 BindingDB Entry DOI: 10.7270/Q2VX0JZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50263528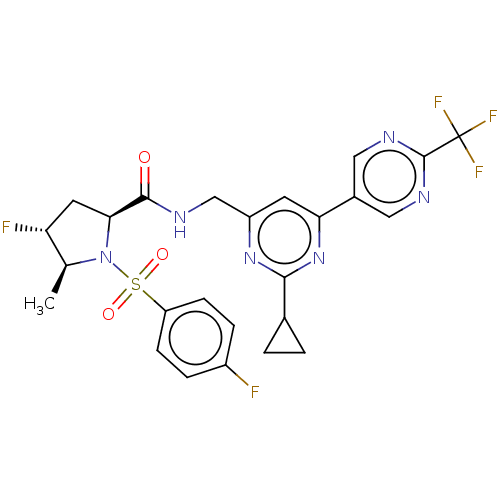 (CHEMBL4079886) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co. Ltd. , 6 Taihe Road, BDA , Beijing 100176 , P. R. China. Curated by ChEMBL | Assay Description Inhibition of human TRPA1 expressed in HEK293 cells assessed as inhibition of cinnamaldehyde-induced Ca2+ influx preincubated for 20 mins followed by... | J Med Chem 61: 3641-3659 (2018) Article DOI: 10.1021/acs.jmedchem.8b00117 BindingDB Entry DOI: 10.7270/Q2VX0JZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM534977 ((2S,4R)-4-fluoro-1-(4- fluorophenyl)sulfonyl-N-[[2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM462050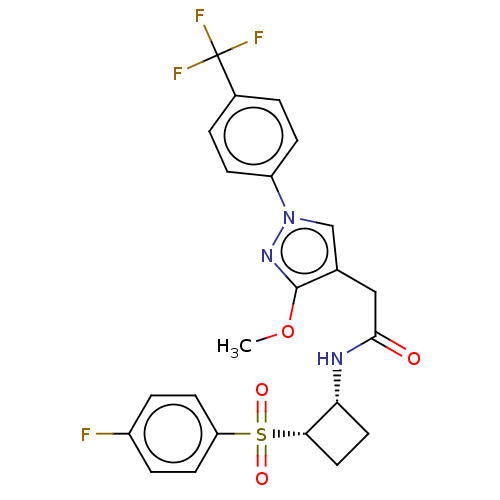 (N-((1R,2S)-2-((4- fluorophenyl)sulfonyl)cyclobutyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 values (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expr... | US Patent US10766878 (2020) BindingDB Entry DOI: 10.7270/Q2GM8BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM462050 (N-((1R,2S)-2-((4- fluorophenyl)sulfonyl)cyclobutyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 values (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expr... | US Patent US10766878 (2020) BindingDB Entry DOI: 10.7270/Q2GM8BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM535108 (Preparation of (2S,4R)-4-fluoro-N-(2-fluoro-5-(2-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437862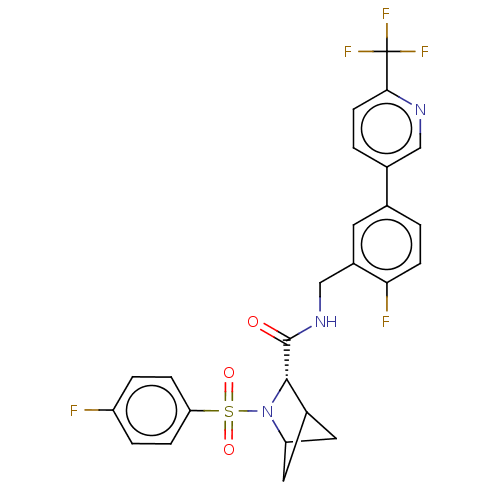 ((2S)-3-(4- fluorophenyl)sulfonyl-N-[[2- fluoro-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437845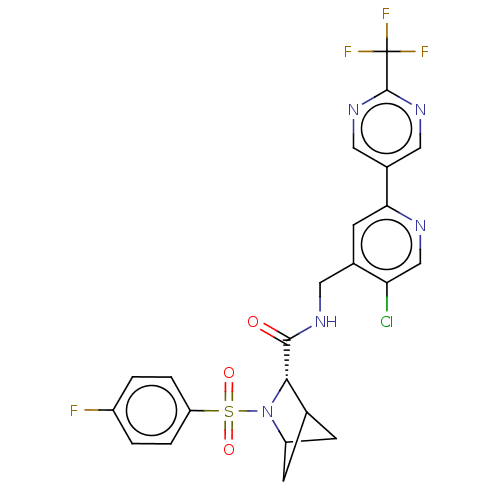 ((2S)-N-[[5-chloro-4-[2- (trifluoromethyl)pyrimidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437886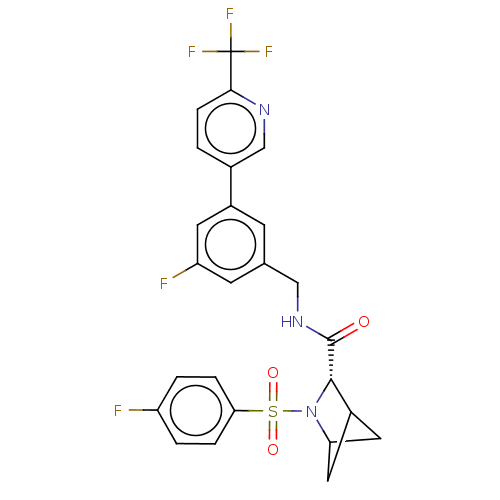 ((2S)-3-(4- fluorophenyl)sulfonyl-N-[[3- fluoro-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM534992 (Preparation of (2R,3S)-3-fluoro-1-(4-fluorophenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM534988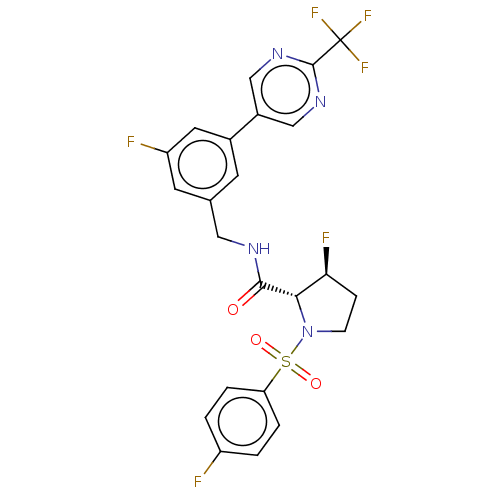 (Preparation of (2R,3S)-3-fluoro-1-(4-fluorophenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM534998 (Preparation of (2S,4R)óN-([5-cyano-2-[4-(trifluoro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM534932 (Preparation of (2S,4R)-4-fluoro-1-(4-fluorophenyls...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM535031 (Preparation of (2R,3S)-3-fluoro-1-(4-fluorophenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)-Rattus norvegicus) | BDBM437935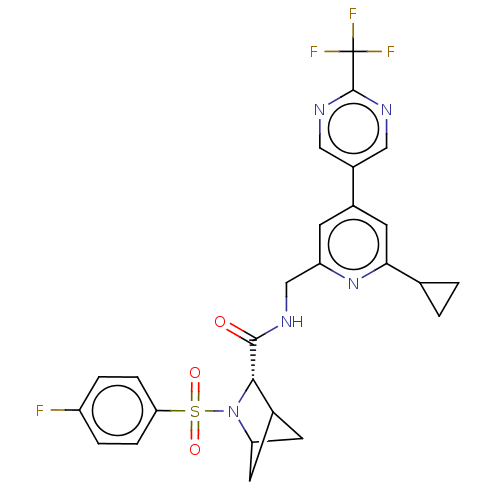 ((2S)-N-[[6-cyclopropyl-4-[2- (trifluoromethyl)pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing ... | US Patent US10597383 (2020) BindingDB Entry DOI: 10.7270/Q2348PF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM462039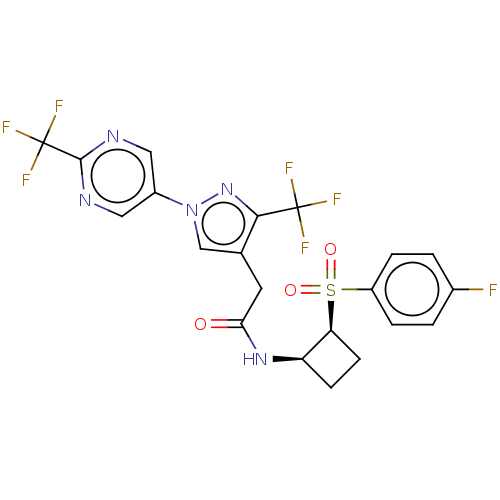 (N-((1R,2S)-2-(4- fluorophenylsulfonyl)cyclobutyl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 values (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expr... | US Patent US10766878 (2020) BindingDB Entry DOI: 10.7270/Q2GM8BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM462039 (N-((1R,2S)-2-(4- fluorophenylsulfonyl)cyclobutyl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description IC50 values (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expr... | US Patent US10766878 (2020) BindingDB Entry DOI: 10.7270/Q2GM8BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM534927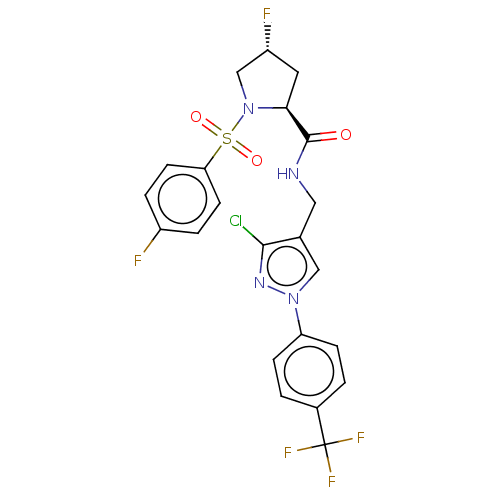 (Preparation of (2S,4R)óN-([3-chloro-1-[4-(trifluor...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50s (effective concentration) of compounds on the human and rat TRPA1 channels were determined using a FLIPR Tetra instrument. CHO cells expressing... | Citation and Details BindingDB Entry DOI: 10.7270/Q25T3PPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 421 total ) | Next | Last >> |