 Found 319 hits with Last Name = 'visnick' and Initial = 'm'
Found 319 hits with Last Name = 'visnick' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25077
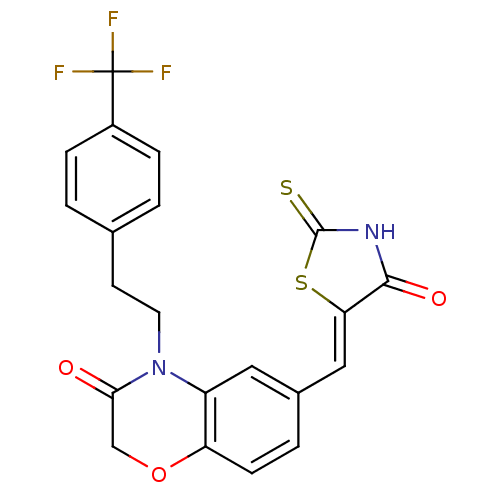
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES FC(F)(F)c1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H15F3N2O3S2/c22-21(23,24)14-4-1-12(2-5-14)7-8-26-15-9-13(3-6-16(15)29-11-18(26)27)10-17-19(28)25-20(30)31-17/h1-6,9-10H,7-8,11H2,(H,25,28,30)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25073
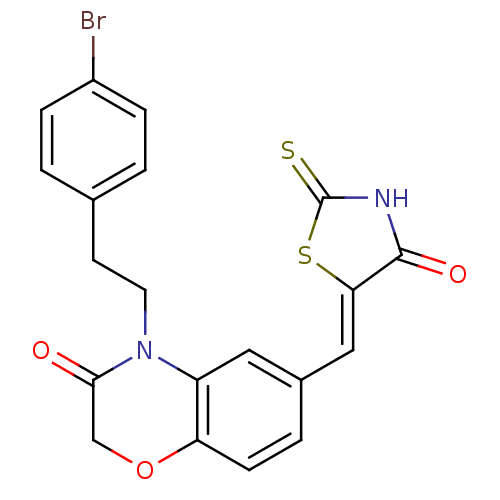
(4-[2-(4-bromophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Brc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H15BrN2O3S2/c21-14-4-1-12(2-5-14)7-8-23-15-9-13(3-6-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-6,9-10H,7-8,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25061
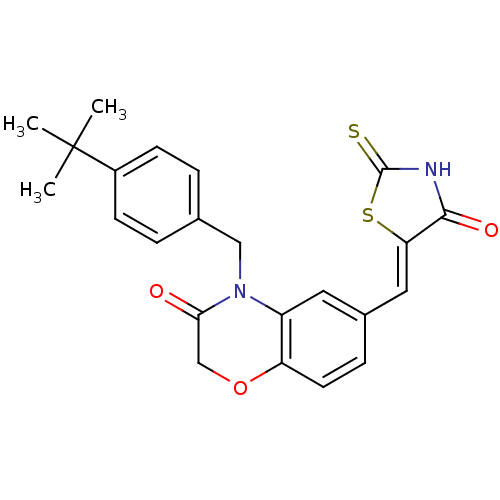
(4-[(4-tert-butylphenyl)methyl]-6-{[(5Z)-4-oxo-2-su...)Show SMILES CC(C)(C)c1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C23H22N2O3S2/c1-23(2,3)16-7-4-14(5-8-16)12-25-17-10-15(6-9-18(17)28-13-20(25)26)11-19-21(27)24-22(29)30-19/h4-11H,12-13H2,1-3H3,(H,24,27,29)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25066
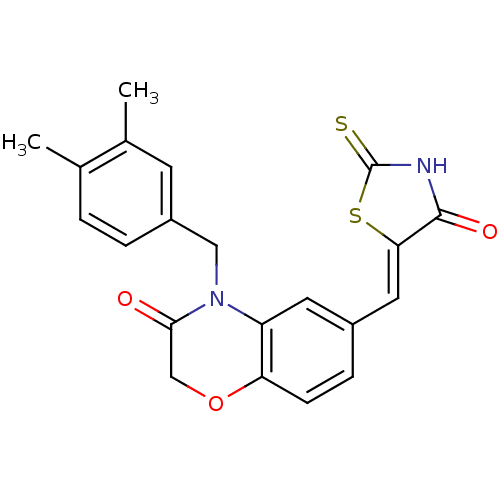
(4-[(3,4-dimethylphenyl)methyl]-6-{[(5Z)-4-oxo-2-su...)Show SMILES Cc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1C Show InChI InChI=1S/C21H18N2O3S2/c1-12-3-4-15(7-13(12)2)10-23-16-8-14(5-6-17(16)26-11-19(23)24)9-18-20(25)22-21(27)28-18/h3-9H,10-11H2,1-2H3,(H,22,25,27)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.59 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25068
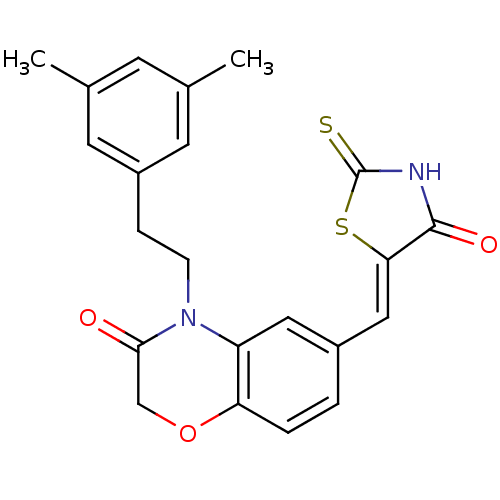
(4-[2-(3,5-dimethylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Cc1cc(C)cc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C22H20N2O3S2/c1-13-7-14(2)9-16(8-13)5-6-24-17-10-15(3-4-18(17)27-12-20(24)25)11-19-21(26)23-22(28)29-19/h3-4,7-11H,5-6,12H2,1-2H3,(H,23,26,28)/b19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25080
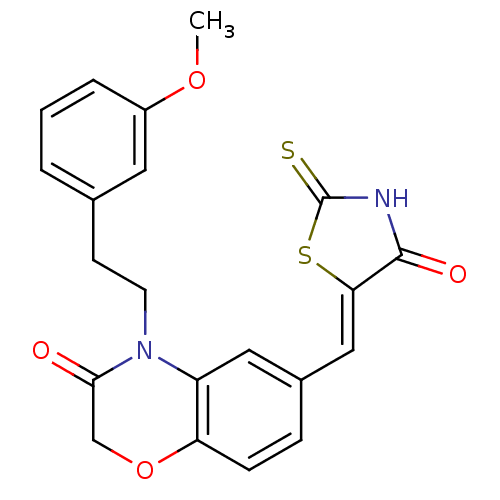
(4-[2-(3-methoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulf...)Show SMILES COc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C21H18N2O4S2/c1-26-15-4-2-3-13(9-15)7-8-23-16-10-14(5-6-17(16)27-12-19(23)24)11-18-20(25)22-21(28)29-18/h2-6,9-11H,7-8,12H2,1H3,(H,22,25,28)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.66 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25069
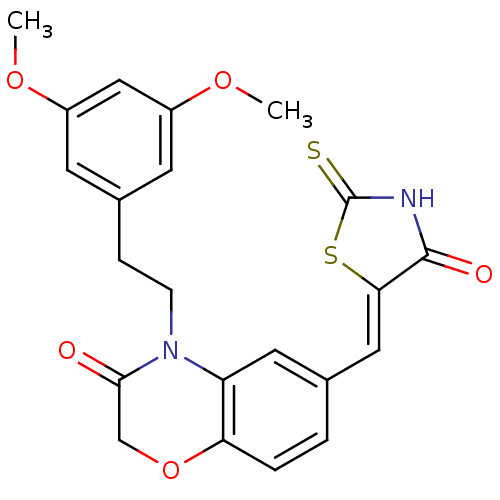
(4-[2-(3,5-dimethoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-...)Show SMILES COc1cc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc(OC)c1 Show InChI InChI=1S/C22H20N2O5S2/c1-27-15-7-14(8-16(11-15)28-2)5-6-24-17-9-13(3-4-18(17)29-12-20(24)25)10-19-21(26)23-22(30)31-19/h3-4,7-11H,5-6,12H2,1-2H3,(H,23,26,30)/b19-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.79 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25074
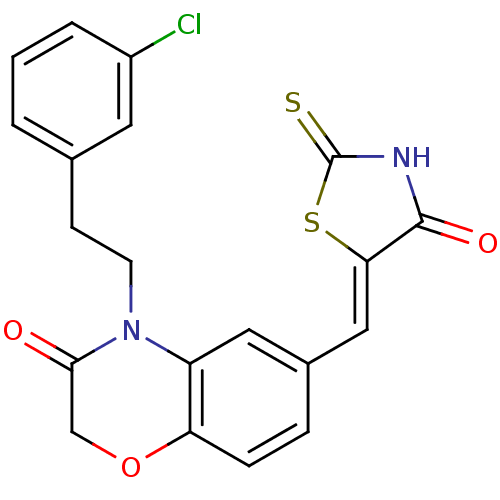
(4-[2-(3-chlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Clc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C20H15ClN2O3S2/c21-14-3-1-2-12(8-14)6-7-23-15-9-13(4-5-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-5,8-10H,6-7,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.27 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25078
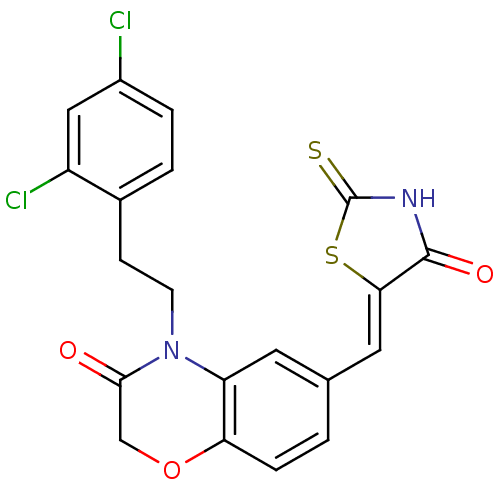
(4-[2-(2,4-dichlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c(Cl)c1 Show InChI InChI=1S/C20H14Cl2N2O3S2/c21-13-3-2-12(14(22)9-13)5-6-24-15-7-11(1-4-16(15)27-10-18(24)25)8-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.28 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25075
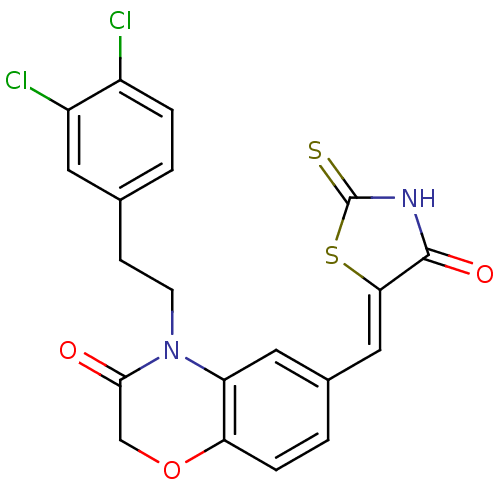
(4-[2-(3,4-dichlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1Cl Show InChI InChI=1S/C20H14Cl2N2O3S2/c21-13-3-1-11(7-14(13)22)5-6-24-15-8-12(2-4-16(15)27-10-18(24)25)9-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.36 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25070
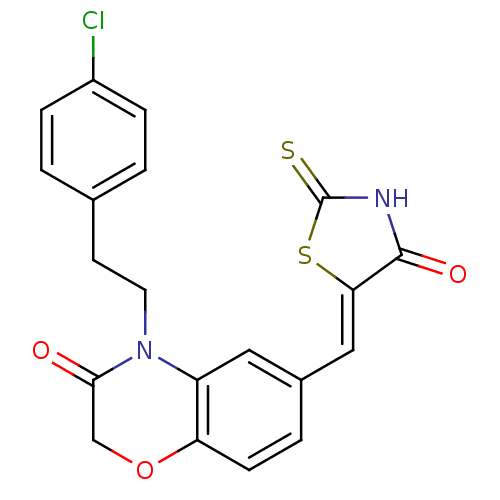
(4-[2-(4-chlorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Clc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H15ClN2O3S2/c21-14-4-1-12(2-5-14)7-8-23-15-9-13(3-6-16(15)26-11-18(23)24)10-17-19(25)22-20(27)28-17/h1-6,9-10H,7-8,11H2,(H,22,25,27)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.76 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25081
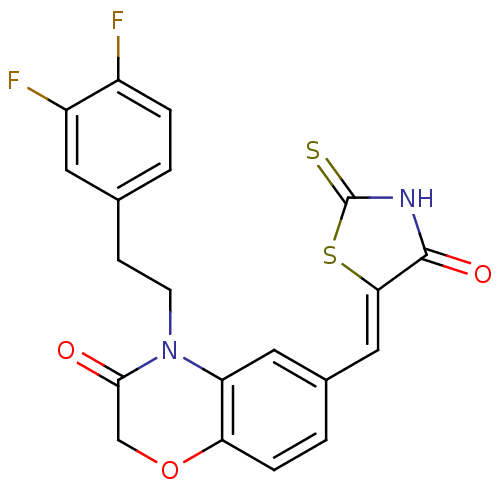
(4-[2-(3,4-difluorophenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES Fc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1F Show InChI InChI=1S/C20H14F2N2O3S2/c21-13-3-1-11(7-14(13)22)5-6-24-15-8-12(2-4-16(15)27-10-18(24)25)9-17-19(26)23-20(28)29-17/h1-4,7-9H,5-6,10H2,(H,23,26,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.84 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25079
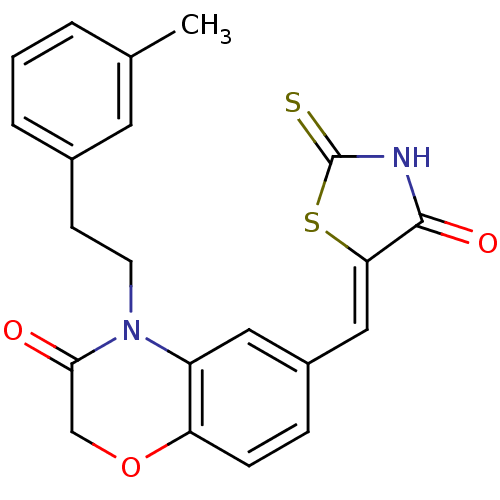
(4-[2-(3-methylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Cc1cccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)c1 Show InChI InChI=1S/C21H18N2O3S2/c1-13-3-2-4-14(9-13)7-8-23-16-10-15(5-6-17(16)26-12-19(23)24)11-18-20(25)22-21(27)28-18/h2-6,9-11H,7-8,12H2,1H3,(H,22,25,27)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.96 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25071
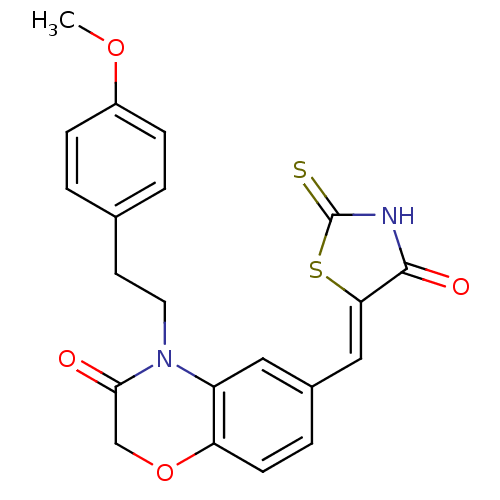
(4-[2-(4-methoxyphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulf...)Show SMILES COc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H18N2O4S2/c1-26-15-5-2-13(3-6-15)8-9-23-16-10-14(4-7-17(16)27-12-19(23)24)11-18-20(25)22-21(28)29-18/h2-7,10-11H,8-9,12H2,1H3,(H,22,25,28)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.57 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25062
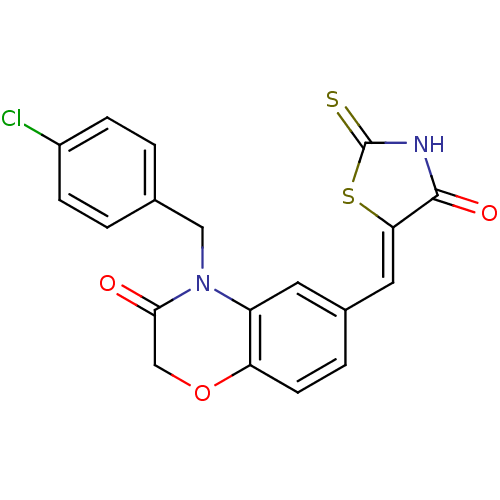
(4-[(4-chlorophenyl)methyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Clc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C19H13ClN2O3S2/c20-13-4-1-11(2-5-13)9-22-14-7-12(3-6-15(14)25-10-17(22)23)8-16-18(24)21-19(26)27-16/h1-8H,9-10H2,(H,21,24,26)/b16-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.16 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25072
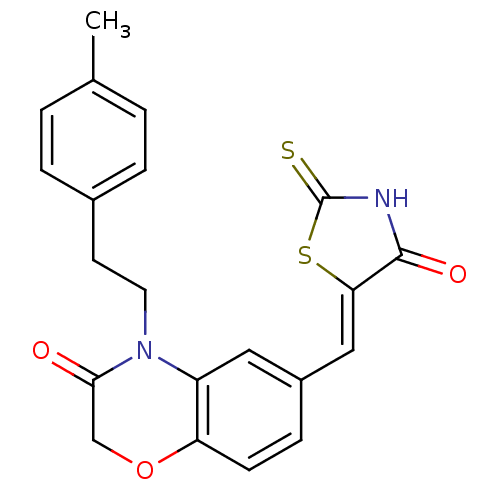
(4-[2-(4-methylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-sulfa...)Show SMILES Cc1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C21H18N2O3S2/c1-13-2-4-14(5-3-13)8-9-23-16-10-15(6-7-17(16)26-12-19(23)24)11-18-20(25)22-21(27)28-18/h2-7,10-11H,8-9,12H2,1H3,(H,22,25,27)/b18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.83 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM511498

(US11059792, Compound T12)Show SMILES C[C@H]1[C@@H]2CCc3c(nc(CCO)n3-c3ccccc3)[C@@]2(C)C=C(C#N)C1=O |r,t:24| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QN69XS |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544866

(CHEMBL4643904 | US11292781, Compound T146)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2CCC)-c1ccnc(c1)C1CC1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C31H29FN4O/c1-3-6-21-24-12-11-23-27(22-7-4-5-8-25(22)32)35-30(19-13-14-34-26(15-19)18-9-10-18)36-29(23)31(24,2)16-20(17-33)28(21)37/h4-5,7-8,13-16,18,21,24H,3,6,9-12H2,1-2H3/t21-,24-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544867

(CHEMBL4642696)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2CCC)-c1ccnc(c1)C(F)(F)F)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C29H24F4N4O/c1-3-6-18-21-10-9-20-24(19-7-4-5-8-22(19)30)36-27(16-11-12-35-23(13-16)29(31,32)33)37-26(20)28(21,2)14-17(15-34)25(18)38/h4-5,7-8,11-14,18,21H,3,6,9-10H2,1-2H3/t18-,21-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM511511
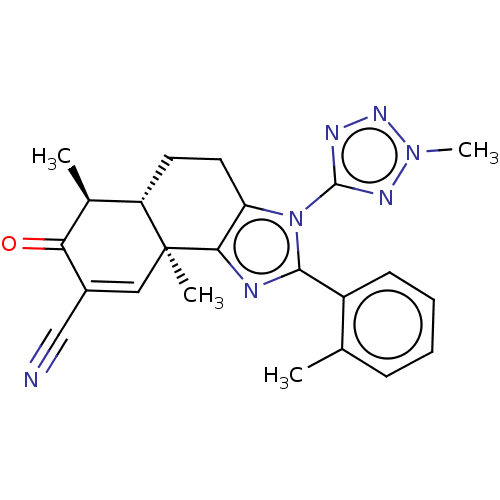
(US11059792, Compound T25)Show SMILES C[C@H]1[C@@H]2CCc3c(nc(-c4ccccc4C)n3-c3nnn(C)n3)[C@@]2(C)C=C(C#N)C1=O |r,t:29| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QN69XS |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544868

(CHEMBL4644765)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2CCC)-c1ccnc(CF)c1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C29H26F2N4O/c1-3-6-20-23-10-9-22-25(21-7-4-5-8-24(21)31)34-28(17-11-12-33-19(13-17)15-30)35-27(22)29(23,2)14-18(16-32)26(20)36/h4-5,7-8,11-14,20,23H,3,6,9-10,15H2,1-2H3/t20-,23-,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM15234
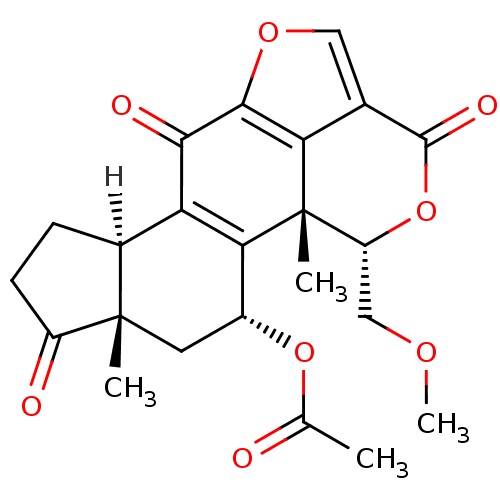
((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM511492
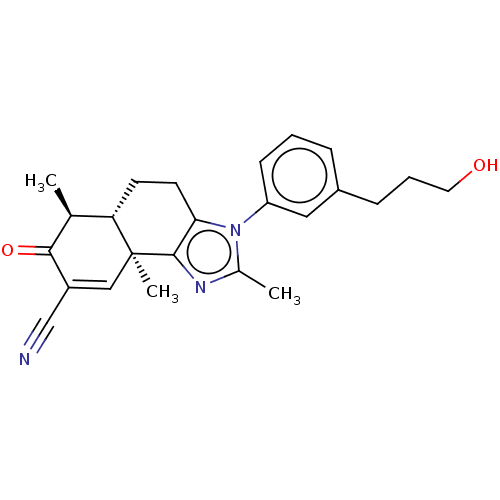
(US11059792, Compound T6)Show SMILES C[C@H]1[C@@H]2CCc3c(nc(C)n3-c3cccc(CCCO)c3)[C@@]2(C)C=C(C#N)C1=O |r,t:26| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 19.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QN69XS |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544865
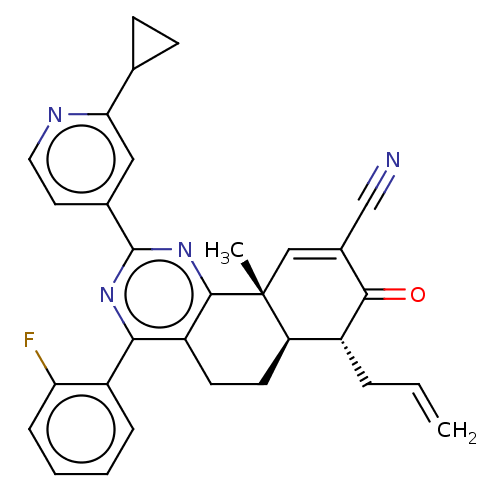
(CHEMBL4638971 | US11292781, Compound T145)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2CC=C)-c1ccnc(c1)C1CC1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C31H27FN4O/c1-3-6-21-24-12-11-23-27(22-7-4-5-8-25(22)32)35-30(19-13-14-34-26(15-19)18-9-10-18)36-29(23)31(24,2)16-20(17-33)28(21)37/h3-5,7-8,13-16,18,21,24H,1,6,9-12H2,2H3/t21-,24-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM511495

(US11059792, Compound T9)Show SMILES C[C@H]1[C@@H]2CCc3c(nc(-c4cnn(C)c4)n3-c3ccccc3)[C@@]2(C)C=C(C#N)C1=O |r,t:28| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 22.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QN69XS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25059
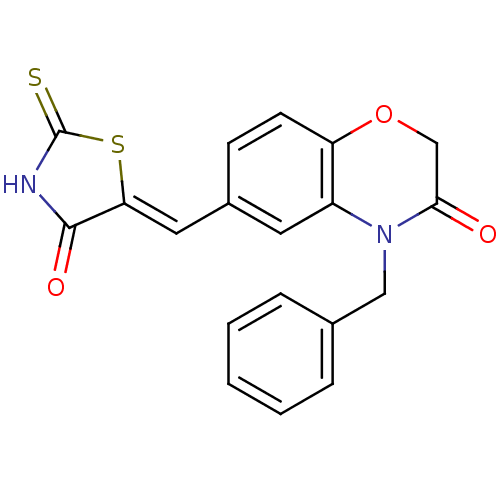
(4-benzyl-6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazo...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(Cc3ccccc3)c2c1 Show InChI InChI=1S/C19H14N2O3S2/c22-17-11-24-15-7-6-13(9-16-18(23)20-19(25)26-16)8-14(15)21(17)10-12-4-2-1-3-5-12/h1-9H,10-11H2,(H,20,23,25)/b16-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25067
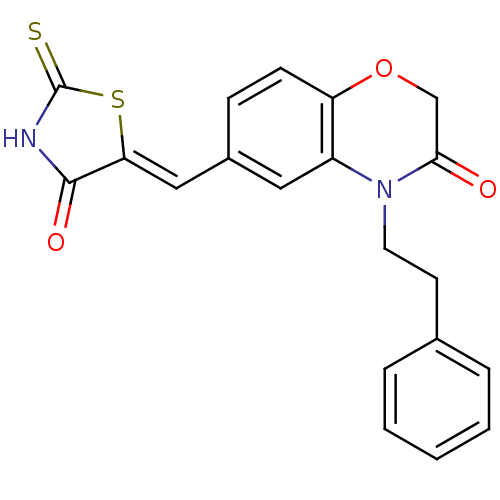
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(CCc3ccccc3)c2c1 Show InChI InChI=1S/C20H16N2O3S2/c23-18-12-25-16-7-6-14(11-17-19(24)21-20(26)27-17)10-15(16)22(18)9-8-13-4-2-1-3-5-13/h1-7,10-11H,8-9,12H2,(H,21,24,26)/b17-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544864

(CHEMBL4646395 | US11292781, Compound T169)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(CCC)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(c1)C1CC1)-c1ccccc1F |r,t:16| Show InChI InChI=1S/C31H29FN4O/c1-3-13-31-16-21(17-33)28(37)18(2)24(31)11-10-23-27(22-6-4-5-7-25(22)32)35-30(36-29(23)31)20-12-14-34-26(15-20)19-8-9-19/h4-7,12,14-16,18-19,24H,3,8-11,13H2,1-2H3/t18-,24-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25064
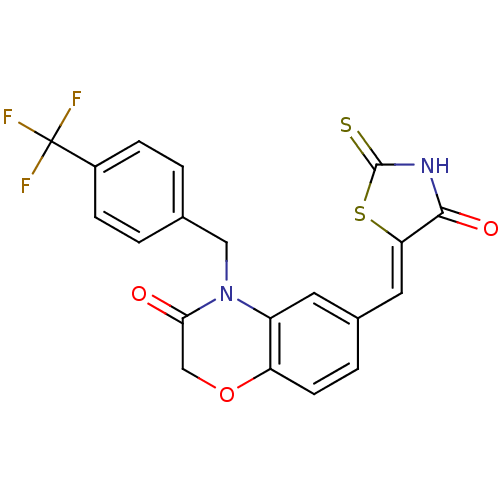
(6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-y...)Show SMILES FC(F)(F)c1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H13F3N2O3S2/c21-20(22,23)13-4-1-11(2-5-13)9-25-14-7-12(3-6-15(14)28-10-17(25)26)8-16-18(27)24-19(29)30-16/h1-8H,9-10H2,(H,24,27,29)/b16-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27.5 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM511512

(US11059792, Compound T26)Show SMILES C[C@H]1[C@@H]2CCc3c(nc(C#N)n3-c3ccccc3)[C@@]2(C)C=C(C#N)C1=O |r,t:23| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 27.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QN69XS |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544866

(CHEMBL4643904 | US11292781, Compound T146)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2CCC)-c1ccnc(c1)C1CC1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C31H29FN4O/c1-3-6-21-24-12-11-23-27(22-7-4-5-8-25(22)32)35-30(19-13-14-34-26(15-19)18-9-10-18)36-29(23)31(24,2)16-20(17-33)28(21)37/h4-5,7-8,13-16,18,21,24H,3,6,9-12H2,1-2H3/t21-,24-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at full-length human RORgammat C285S mutant co-transfected with ROR response element by luciferase based reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544861

(CHEMBL4634962 | US11292781, Compound T50)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(C)c1)-c1ccc(F)cc1 |r,t:14| Show InChI InChI=1S/C27H23FN4O/c1-15-12-18(10-11-30-15)26-31-23(17-4-6-20(28)7-5-17)21-8-9-22-16(2)24(33)19(14-29)13-27(22,3)25(21)32-26/h4-7,10-13,16,22H,8-9H2,1-3H3/t16-,22-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as inhibition of T cell to Th17 differentiation by flow cytometry |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM511494

(US11059792, Compound T8)Show SMILES C[C@H]1[C@@H]2CCc3c(nc(-c4ccccc4)n3-c3nnn(C)n3)[C@@]2(C)C=C(C#N)C1=O |r,t:28| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 28.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QN69XS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25060
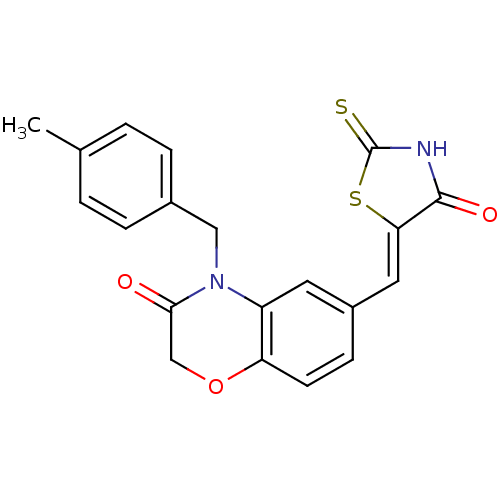
(4-[(4-methylphenyl)methyl]-6-{[(5Z)-4-oxo-2-sulfan...)Show SMILES Cc1ccc(CN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C20H16N2O3S2/c1-12-2-4-13(5-3-12)10-22-15-8-14(6-7-16(15)25-11-18(22)23)9-17-19(24)21-20(26)27-17/h2-9H,10-11H2,1H3,(H,21,24,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544866

(CHEMBL4643904 | US11292781, Compound T146)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2CCC)-c1ccnc(c1)C1CC1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C31H29FN4O/c1-3-6-21-24-12-11-23-27(22-7-4-5-8-25(22)32)35-30(19-13-14-34-26(15-19)18-9-10-18)36-29(23)31(24,2)16-20(17-33)28(21)37/h4-5,7-8,13-16,18,21,24H,3,6,9-12H2,1-2H3/t21-,24-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human full-length RORgammaT expressed in Jurkat cells co-transfected with 5 copies of ROR response element after 18 hrs b... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM511509

(US11059792, Compound T23)Show SMILES C[C@H]1[C@@H]2CCc3c(nc(Br)n3-c3ccccc3)[C@@]2(C)C=C(C#N)C1=O |r,t:22| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 31.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QN69XS |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM546677
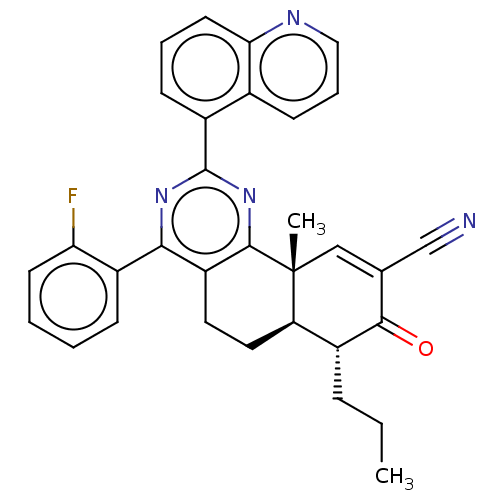
(US11292781, Compound T159)Show SMILES CCC[C@@H]1[C@H]2CCc3c(nc(nc3[C@]2(C)C=C(C#N)C1=O)-c1cccc2ncccc12)-c1ccccc1F |r,t:17| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The RORγ assay system was purchased from Indigo Biosciences. This nuclear receptor assay utilizes a human cell line that has been engineered to ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24F1TXR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25076
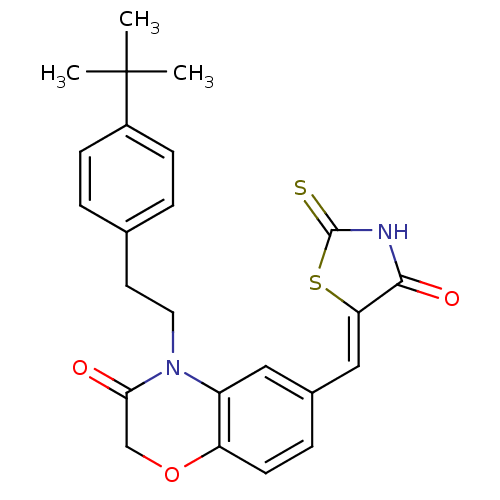
(4-[2-(4-tert-butylphenyl)ethyl]-6-{[(5Z)-4-oxo-2-s...)Show SMILES CC(C)(C)c1ccc(CCN2C(=O)COc3ccc(\C=C4/SC(=S)NC4=O)cc23)cc1 Show InChI InChI=1S/C24H24N2O3S2/c1-24(2,3)17-7-4-15(5-8-17)10-11-26-18-12-16(6-9-19(18)29-14-21(26)27)13-20-22(28)25-23(30)31-20/h4-9,12-13H,10-11,14H2,1-3H3,(H,25,28,30)/b20-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33.2 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544861

(CHEMBL4634962 | US11292781, Compound T50)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(C)c1)-c1ccc(F)cc1 |r,t:14| Show InChI InChI=1S/C27H23FN4O/c1-15-12-18(10-11-30-15)26-31-23(17-4-6-20(28)7-5-17)21-8-9-22-16(2)24(33)19(14-29)13-27(22,3)25(21)32-26/h4-7,10-13,16,22H,8-9H2,1-3H3/t16-,22-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at full-length human RORgammat C345S mutant co-transfected with ROR response element by luciferase based reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544862

(CHEMBL4645611 | US11292781, Compound T83)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(CF)c1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C27H22F2N4O/c1-15-21-8-7-20-23(19-5-3-4-6-22(19)29)32-26(16-9-10-31-18(11-16)13-28)33-25(20)27(21,2)12-17(14-30)24(15)34/h3-6,9-12,15,21H,7-8,13H2,1-2H3/t15-,21-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544874
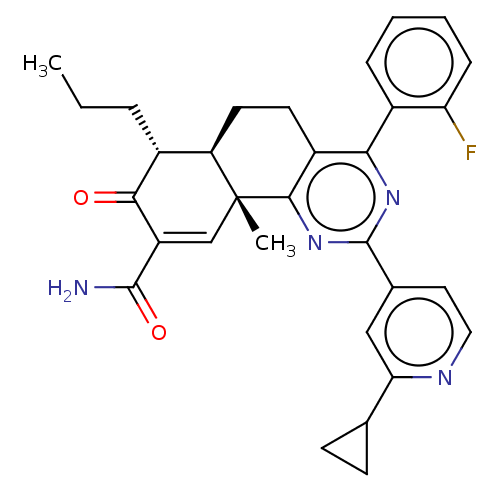
(CHEMBL4642370)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C(N)=O)C(=O)[C@@H]2CCC)-c1ccnc(c1)C1CC1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C31H31FN4O2/c1-3-6-19-23-12-11-21-26(20-7-4-5-8-24(20)32)35-30(18-13-14-34-25(15-18)17-9-10-17)36-28(21)31(23,2)16-22(27(19)37)29(33)38/h4-5,7-8,13-17,19,23H,3,6,9-12H2,1-2H3,(H2,33,38)/t19-,23-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM546675
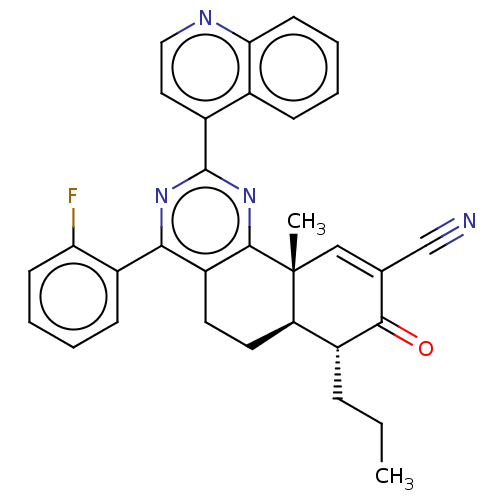
(US11292781, Compound T158)Show SMILES CCC[C@@H]1[C@H]2CCc3c(nc(nc3[C@]2(C)C=C(C#N)C1=O)-c1ccnc2ccccc12)-c1ccccc1F |r,t:17| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The RORγ assay system was purchased from Indigo Biosciences. This nuclear receptor assay utilizes a human cell line that has been engineered to ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24F1TXR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5
(Homo sapiens (Human)) | BDBM25063
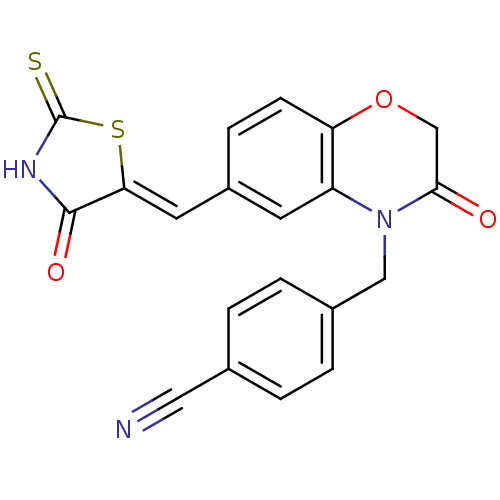
(4-[(3-oxo-6-{[(5Z)-4-oxo-2-sulfanylidene-1,3-thiaz...)Show SMILES O=C1NC(=S)S\C1=C/c1ccc2OCC(=O)N(Cc3ccc(cc3)C#N)c2c1 Show InChI InChI=1S/C20H13N3O3S2/c21-9-12-1-3-13(4-2-12)10-23-15-7-14(5-6-16(15)26-11-18(23)24)8-17-19(25)22-20(27)28-17/h1-8H,10-11H2,(H,22,25,27)/b17-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36.1 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer
| Assay Description
PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa... |
Bioorg Med Chem Lett 17: 756-60 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.080
BindingDB Entry DOI: 10.7270/Q2NS0S6N |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544861

(CHEMBL4634962 | US11292781, Compound T50)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(C)c1)-c1ccc(F)cc1 |r,t:14| Show InChI InChI=1S/C27H23FN4O/c1-15-12-18(10-11-30-15)26-31-23(17-4-6-20(28)7-5-17)21-8-9-22-16(2)24(33)19(14-29)13-27(22,3)25(21)32-26/h4-7,10-13,16,22H,8-9H2,1-3H3/t16-,22-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at full-length human RORgammat C320S mutant co-transfected with ROR response element by luciferase based reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544866

(CHEMBL4643904 | US11292781, Compound T146)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2CCC)-c1ccnc(c1)C1CC1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C31H29FN4O/c1-3-6-21-24-12-11-23-27(22-7-4-5-8-25(22)32)35-30(19-13-14-34-26(15-19)18-9-10-18)36-29(23)31(24,2)16-20(17-33)28(21)37/h4-5,7-8,13-16,18,21,24H,3,6,9-12H2,1-2H3/t21-,24-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at full-length human RORgammat C345S mutant co-transfected with ROR response element by luciferase based reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544861

(CHEMBL4634962 | US11292781, Compound T50)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(C)c1)-c1ccc(F)cc1 |r,t:14| Show InChI InChI=1S/C27H23FN4O/c1-15-12-18(10-11-30-15)26-31-23(17-4-6-20(28)7-5-17)21-8-9-22-16(2)24(33)19(14-29)13-27(22,3)25(21)32-26/h4-7,10-13,16,22H,8-9H2,1-3H3/t16-,22-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at full-length human RORgammat C455S mutant co-transfected with ROR response element by luciferase based reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544871

(CHEMBL4647788 | US11292781, Compound T182)Show SMILES [H][C@]12CCCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(C)c1)-c1ccccc1 |r,t:15| Show InChI InChI=1S/C28H26N4O/c1-17-14-20(12-13-30-17)27-31-24(19-8-5-4-6-9-19)22-10-7-11-23-18(2)25(33)21(16-29)15-28(23,3)26(22)32-27/h4-6,8-9,12-15,18,23H,7,10-11H2,1-3H3/t18-,23-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The RORγ assay system was purchased from Indigo Biosciences. This nuclear receptor assay utilizes a human cell line that has been engineered to ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24F1TXR |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544861

(CHEMBL4634962 | US11292781, Compound T50)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(C)c1)-c1ccc(F)cc1 |r,t:14| Show InChI InChI=1S/C27H23FN4O/c1-15-12-18(10-11-30-15)26-31-23(17-4-6-20(28)7-5-17)21-8-9-22-16(2)24(33)19(14-29)13-27(22,3)25(21)32-26/h4-7,10-13,16,22H,8-9H2,1-3H3/t16-,22-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at wild type full-length human RORgammat co-transfected with ROR response element by luciferase based reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544866

(CHEMBL4643904 | US11292781, Compound T146)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2CCC)-c1ccnc(c1)C1CC1)-c1ccccc1F |r,t:14| Show InChI InChI=1S/C31H29FN4O/c1-3-6-21-24-12-11-23-27(22-7-4-5-8-25(22)32)35-30(19-13-14-34-26(15-19)18-9-10-18)36-29(23)31(24,2)16-20(17-33)28(21)37/h4-5,7-8,13-16,18,21,24H,3,6,9-12H2,1-2H3/t21-,24-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at wild type full-length human RORgammat co-transfected with ROR response element by luciferase based reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50544861

(CHEMBL4634962 | US11292781, Compound T50)Show SMILES [H][C@]12CCc3c(nc(nc3[C@]1(C)C=C(C#N)C(=O)[C@@H]2C)-c1ccnc(C)c1)-c1ccc(F)cc1 |r,t:14| Show InChI InChI=1S/C27H23FN4O/c1-15-12-18(10-11-30-15)26-31-23(17-4-6-20(28)7-5-17)21-8-9-22-16(2)24(33)19(14-29)13-27(22,3)25(21)32-26/h4-7,10-13,16,22H,8-9H2,1-3H3/t16-,22-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Reata Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inverse agonist activity at full-length human RORgammat C285S mutant co-transfected with ROR response element by luciferase based reporter gene assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126967
BindingDB Entry DOI: 10.7270/Q23X8B6Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data