

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108220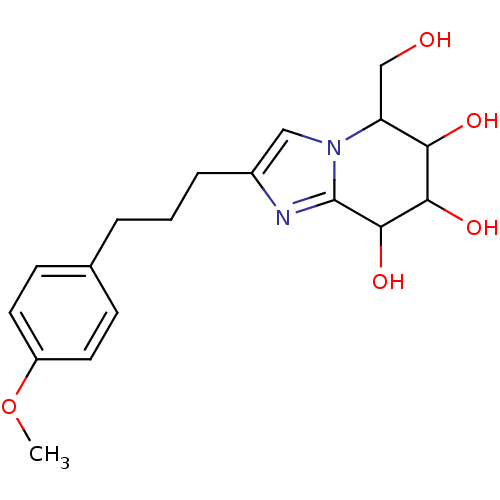 (5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108219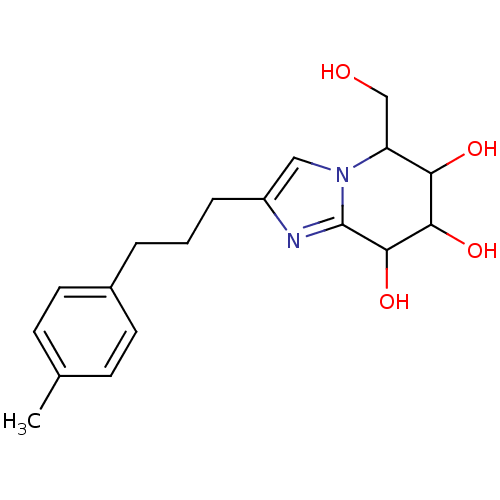 (5-(hydroxymethyl)-2-[3-(4-methylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108222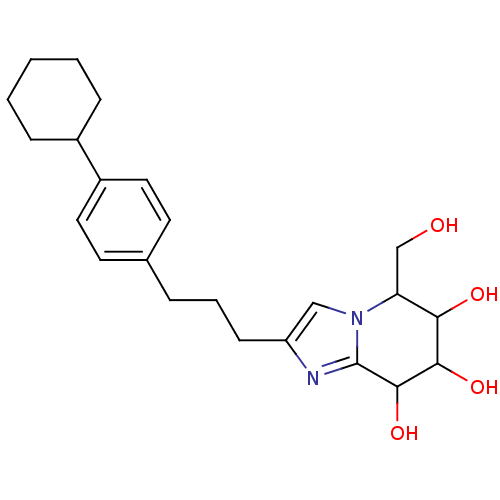 (2-[3-(4-cyclohexylphenyl)propyl]-5-(hydroxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108216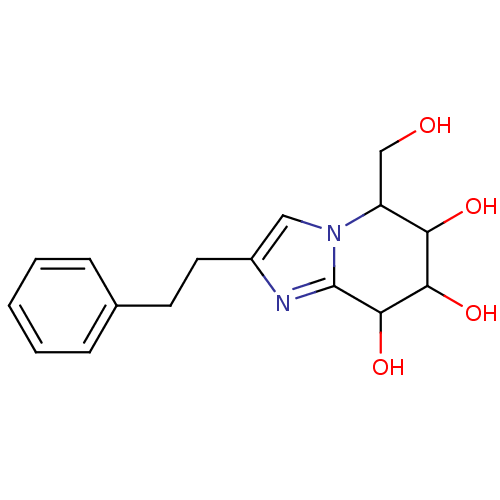 (5-(hydroxymethyl)-2-(2-phenylethyl)-5H,6H,7H,8H- i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.19 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108223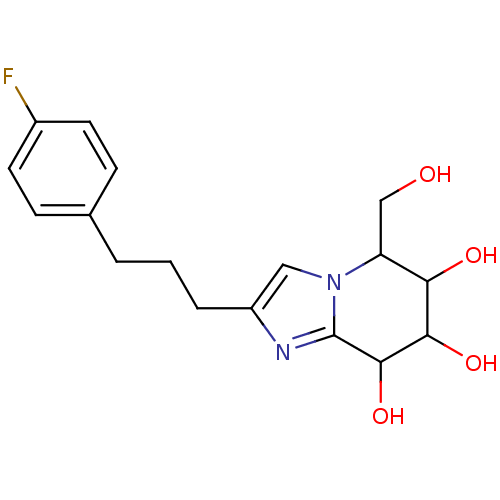 (2-[3-(4-fluorophenyl)propyl]-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.48 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108221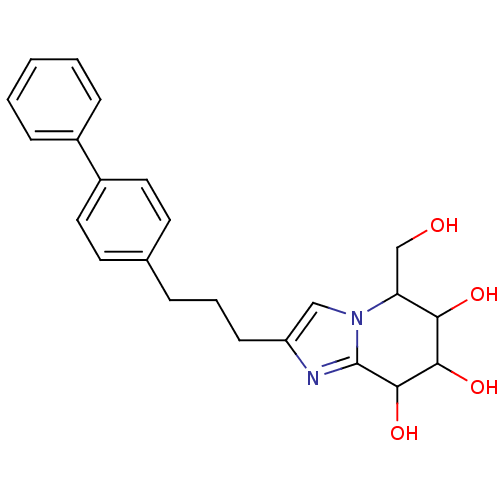 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.62 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108224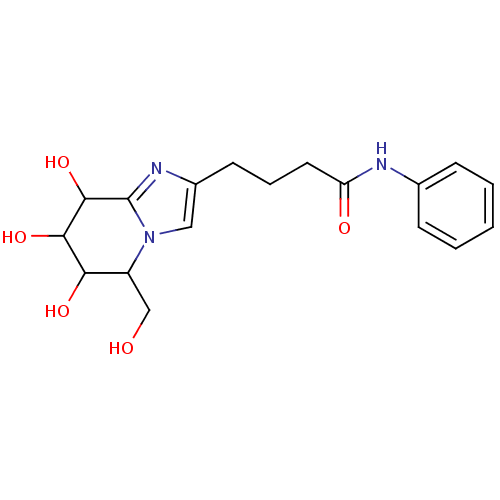 (N-phenyl-4-[6,7,8-trihydroxy-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.18 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108217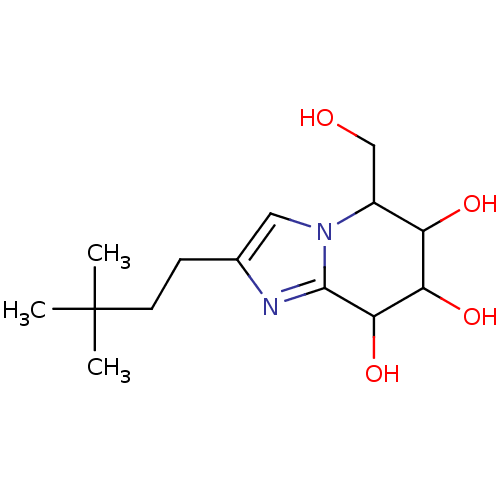 (2-(3,3-dimethylbutyl)-5-(hydroxymethyl)- 5H,6H,7H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.34 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108218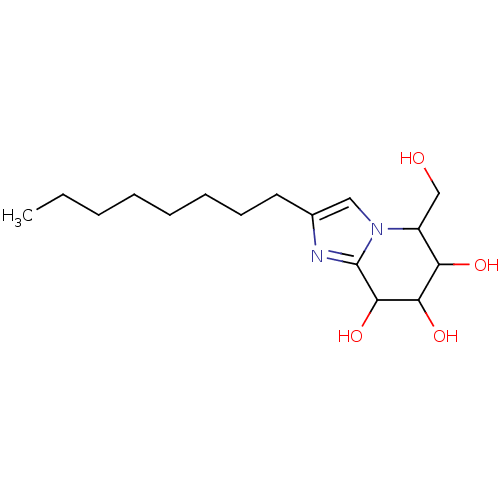 (5-(hydroxymethyl)-2-octyl-5H,6H,7H,8H-imidazo[1,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.38 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108225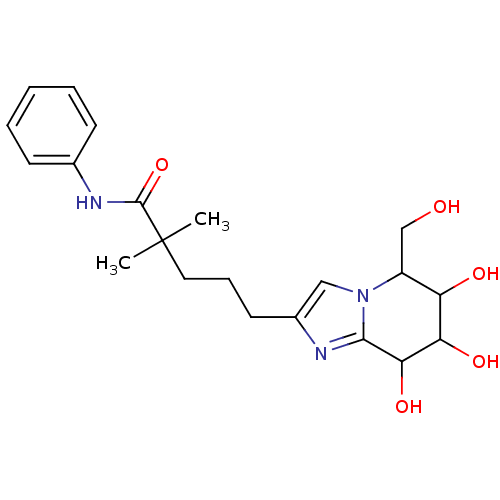 (2,2-dimethyl-N-phenyl-5-[6,7,8-trihydroxy-5- (hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18.9 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108215 (5-(hydroxymethyl)-5H,6H,7H,8H-imidazo[1,2- a]pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 33.1 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50394493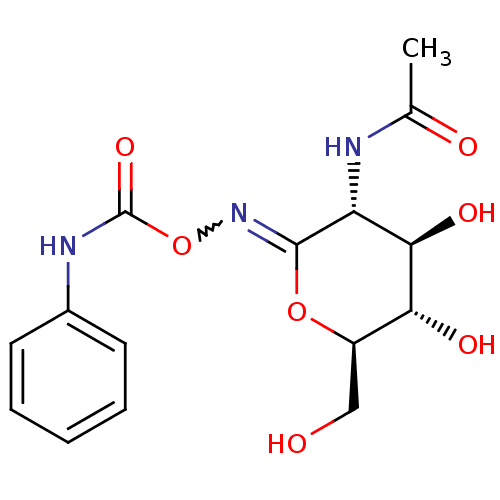 (CHEMBL2160124) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of human OGA using 4-MU-GlcNAc as substrate after 30 mins by Dixon plot analysis | Bioorg Med Chem Lett 22: 6854-7 (2012) Article DOI: 10.1016/j.bmcl.2012.09.042 BindingDB Entry DOI: 10.7270/Q2KP838N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase PKM (Homo sapiens (Human)) | BDBM50455474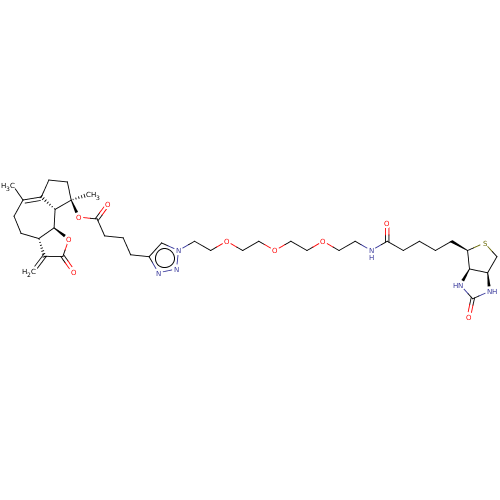 (CHEMBL4218770) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Irreversible binding affinity to recombinant human PKM2 expressed in Escherichia coli BL21 | J Med Chem 61: 4155-4164 (2018) Article DOI: 10.1021/acs.jmedchem.8b00241 BindingDB Entry DOI: 10.7270/Q2C82CWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50156357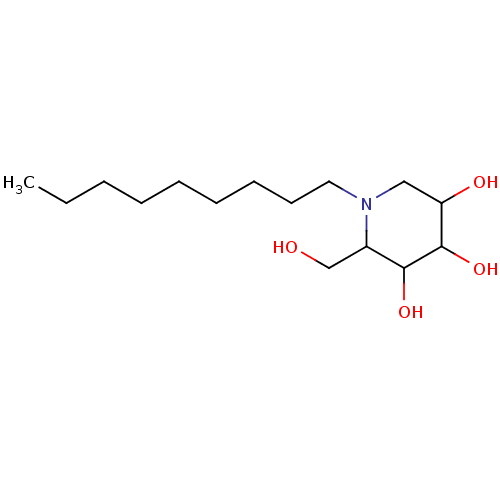 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 488 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50394492 (CHEMBL1765471) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Competitive inhibition of human OGA using 4-MU-GlcNAc as substrate after 10 mins by Lineweaver Burk plot analysis | Bioorg Med Chem Lett 22: 6854-7 (2012) Article DOI: 10.1016/j.bmcl.2012.09.042 BindingDB Entry DOI: 10.7270/Q2KP838N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM50394492 (CHEMBL1765471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of human Hex A using 4-MU-GlcNAc as substrate after 10 mins by Lineweaver Burk plot analysis | Bioorg Med Chem Lett 22: 6854-7 (2012) Article DOI: 10.1016/j.bmcl.2012.09.042 BindingDB Entry DOI: 10.7270/Q2KP838N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM108221 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | 10.6 | 37 |
Nankai University | Assay Description In a 96-well plates, 10µL of commercial enzyme solutions without (control) or with 20µL of inhibitor were incubated at 37°C for 5 min.... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50234138 (CHEMBL4084635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.978 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using S-2765 as substrate preincubated with AT-3 followed by factor 10a addition for 60 secs and subsequent... | Eur J Med Chem 126: 1039-1055 (2017) Article DOI: 10.1016/j.ejmech.2016.12.004 BindingDB Entry DOI: 10.7270/Q2Z321W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM108222 (2-[3-(4-cyclohexylphenyl)propyl]-5-(hydroxymethyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 10.6 | 37 |
Nankai University | Assay Description In a 96-well plates, 10µL of commercial enzyme solutions without (control) or with 20µL of inhibitor were incubated at 37°C for 5 min.... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50234135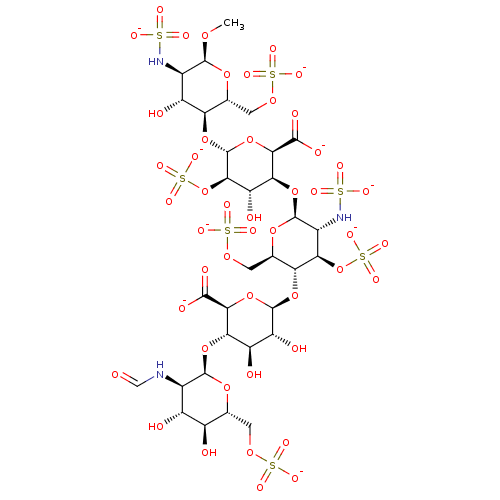 (CHEMBL4072190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using S-2765 as substrate preincubated with AT-3 followed by factor 10a addition for 60 secs and subsequent... | Eur J Med Chem 126: 1039-1055 (2017) Article DOI: 10.1016/j.ejmech.2016.12.004 BindingDB Entry DOI: 10.7270/Q2Z321W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50234131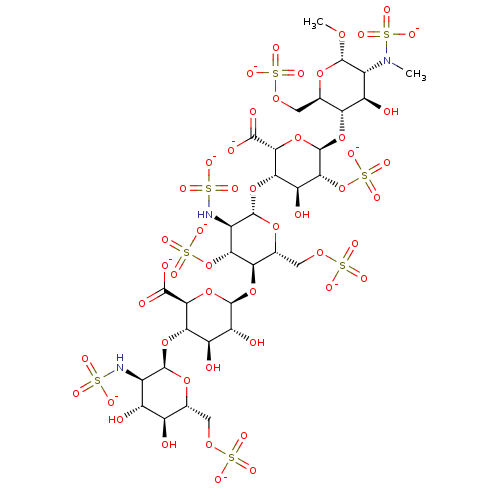 (CHEMBL4081127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using S-2765 as substrate preincubated with AT-3 followed by factor 10a addition for 60 secs and subsequent... | Eur J Med Chem 126: 1039-1055 (2017) Article DOI: 10.1016/j.ejmech.2016.12.004 BindingDB Entry DOI: 10.7270/Q2Z321W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108216 (5-(hydroxymethyl)-2-(2-phenylethyl)-5H,6H,7H,8H- i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108220 (5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50234136 (CHEMBL4082681) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using S-2765 as substrate preincubated with AT-3 followed by factor 10a addition for 60 secs and subsequent... | Eur J Med Chem 126: 1039-1055 (2017) Article DOI: 10.1016/j.ejmech.2016.12.004 BindingDB Entry DOI: 10.7270/Q2Z321W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108220 (5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.62 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108216 (5-(hydroxymethyl)-2-(2-phenylethyl)-5H,6H,7H,8H- i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.71 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prunasin hydrolase (Prunus dulcis (Almond)) | BDBM108222 (2-[3-(4-cyclohexylphenyl)propyl]-5-(hydroxymethyl)...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 10.6 | 37 |
Nankai University | Assay Description In a 96-well plates, 10µL of commercial enzyme solutions without (control) or with 20µL of inhibitor were incubated at 37°C for 5 min.... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50485177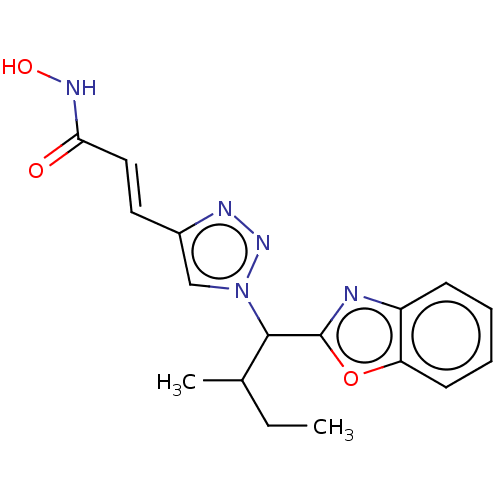 (CHEMBL2036478) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extracts using Ac-Arg-Gly-Lys(Ac)-AMC substrate by fluorimetry | J Med Chem 55: 3066-75 (2012) Article DOI: 10.1021/jm201496g BindingDB Entry DOI: 10.7270/Q2V40Z27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108217 (2-(3,3-dimethylbutyl)-5-(hydroxymethyl)- 5H,6H,7H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.26 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108219 (5-(hydroxymethyl)-2-[3-(4-methylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.73 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108223 (2-[3-(4-fluorophenyl)propyl]-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.75 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50485179 (CHEMBL2036476) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extracts using Ac-Arg-Gly-Lys(Ac)-AMC substrate by fluorimetry | J Med Chem 55: 3066-75 (2012) Article DOI: 10.1021/jm201496g BindingDB Entry DOI: 10.7270/Q2V40Z27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108218 (5-(hydroxymethyl)-2-octyl-5H,6H,7H,8H-imidazo[1,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108221 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.11 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108221 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.27 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108217 (2-(3,3-dimethylbutyl)-5-(hydroxymethyl)- 5H,6H,7H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.34 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108219 (5-(hydroxymethyl)-2-[3-(4-methylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.44 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM108221 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 10.6 | 37 |
Nankai University | Assay Description In a 96-well plates, 10µL of commercial enzyme solutions without (control) or with 20µL of inhibitor were incubated at 37°C for 5 min.... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108223 (2-[3-(4-fluorophenyl)propyl]-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.23 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108224 (N-phenyl-4-[6,7,8-trihydroxy-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.96 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50485172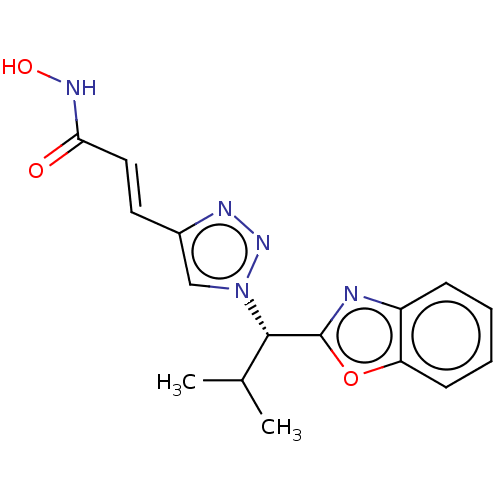 (CHEMBL2036482) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extracts using Ac-Arg-Gly-Lys(Ac)-AMC substrate by fluorimetry | J Med Chem 55: 3066-75 (2012) Article DOI: 10.1021/jm201496g BindingDB Entry DOI: 10.7270/Q2V40Z27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108224 (N-phenyl-4-[6,7,8-trihydroxy-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.67 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108222 (2-[3-(4-cyclohexylphenyl)propyl]-5-(hydroxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108218 (5-(hydroxymethyl)-2-octyl-5H,6H,7H,8H-imidazo[1,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.39 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108222 (2-[3-(4-cyclohexylphenyl)propyl]-5-(hydroxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.42 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50234139 (CHEMBL4100670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using S-2765 as substrate preincubated with AT-3 followed by factor 10a addition for 60 secs and subsequent... | Eur J Med Chem 126: 1039-1055 (2017) Article DOI: 10.1016/j.ejmech.2016.12.004 BindingDB Entry DOI: 10.7270/Q2Z321W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108225 (2,2-dimethyl-N-phenyl-5-[6,7,8-trihydroxy-5- (hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108225 (2,2-dimethyl-N-phenyl-5-[6,7,8-trihydroxy-5- (hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description The assays were performed at 37°C with 4-MU-β-ᴅ-Glu as the substrate in Mcllvaine buffer (sodium citrate (100mM) and sodium phosphate... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50485174 (CHEMBL2036479) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cell nuclear extracts using Ac-Arg-Gly-Lys(Ac)-AMC substrate by fluorimetry | J Med Chem 55: 3066-75 (2012) Article DOI: 10.1021/jm201496g BindingDB Entry DOI: 10.7270/Q2V40Z27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50234132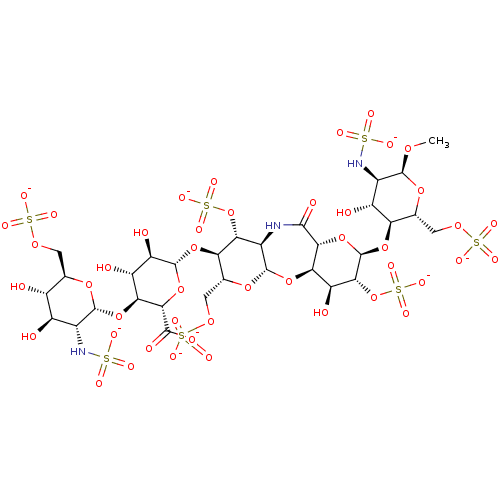 (CHEMBL4085202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University Curated by ChEMBL | Assay Description Inhibition of factor 10a (unknown origin) using S-2765 as substrate preincubated with AT-3 followed by factor 10a addition for 60 secs and subsequent... | Eur J Med Chem 126: 1039-1055 (2017) Article DOI: 10.1016/j.ejmech.2016.12.004 BindingDB Entry DOI: 10.7270/Q2Z321W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 125 total ) | Next | Last >> |