

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha2-beta2 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 using [3H]epibatidine | Bioorg Med Chem Lett 14: 1855-8 (2004) Article DOI: 10.1016/j.bmcl.2003.10.071 BindingDB Entry DOI: 10.7270/Q269753N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha3-beta2 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha3-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha3-beta4 using [3H]epibatidine | Bioorg Med Chem Lett 14: 1855-8 (2004) Article DOI: 10.1016/j.bmcl.2003.10.071 BindingDB Entry DOI: 10.7270/Q269753N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139926 (US8901087, 2) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to rat Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards nicotinic acetylcholine receptor alpha2-beta2 using [3H]epibatidine | Bioorg Med Chem Lett 14: 1855-8 (2004) Article DOI: 10.1016/j.bmcl.2003.10.071 BindingDB Entry DOI: 10.7270/Q269753N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha2-beta4 using [3H]epibatidine | Bioorg Med Chem Lett 14: 1855-8 (2004) Article DOI: 10.1016/j.bmcl.2003.10.071 BindingDB Entry DOI: 10.7270/Q269753N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha2-beta4 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50133235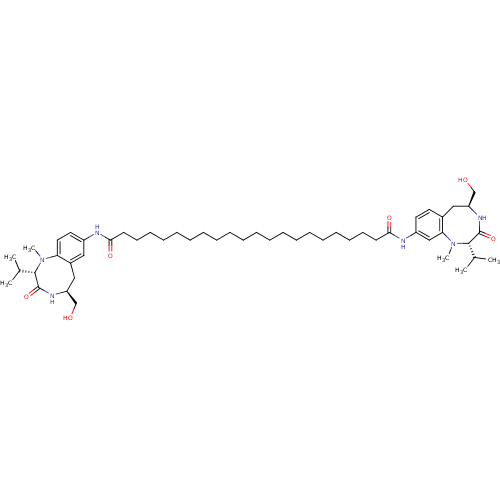 (CHEMBL132720 | Docosanedioic acid ((S)-5-hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for ability to displace [20e3] phorbol 12,13- dibutyrate binding from recombinant C1b domain of murine Protein kinase C delta in presence of [... | J Med Chem 46: 4196-204 (2003) Article DOI: 10.1021/jm0302041 BindingDB Entry DOI: 10.7270/Q2GF0SX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139933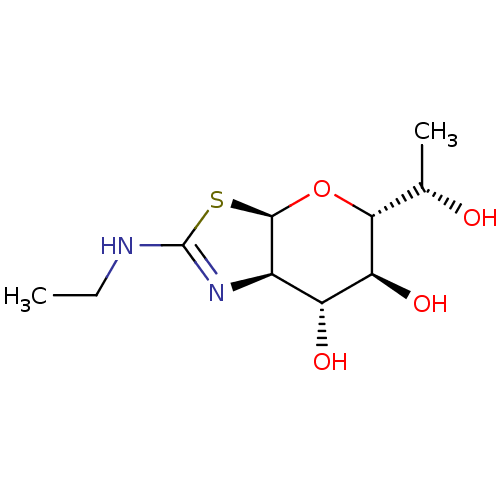 (US8901087, 27) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139959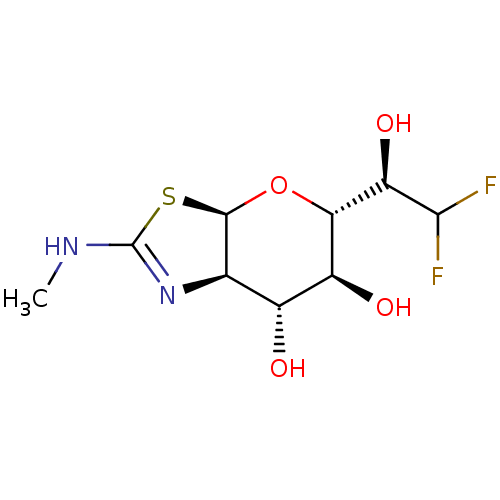 (US8901087, 191) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | -56.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50133238 (CHEMBL334853 | Hexadecanedioic acid ((S)-5-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for ability to displace [20e3] phorbol 12,13- dibutyrate binding from recombinant C1b domain of murine Protein kinase C delta in presence of [... | J Med Chem 46: 4196-204 (2003) Article DOI: 10.1021/jm0302041 BindingDB Entry DOI: 10.7270/Q2GF0SX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards nicotinic acetylcholine receptor alpha2-beta2 using [3H]epibatidine | Bioorg Med Chem Lett 14: 1855-8 (2004) Article DOI: 10.1016/j.bmcl.2003.10.071 BindingDB Entry DOI: 10.7270/Q269753N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha4-beta4 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139951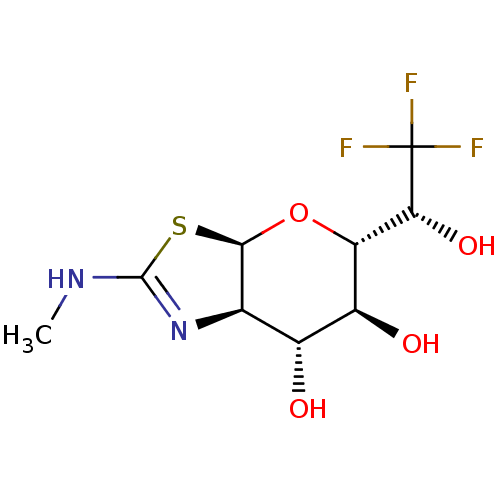 (US8901087, 95) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM314813 ((3aR,5S,6S,7R,7aR)-5-((R)-1-(4- benzylbenzyloxy)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALECTOS THERAPEUTICS, INC.; MERCK SHARP & DOHME CORP. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9611275 (2017) BindingDB Entry DOI: 10.7270/Q2833V4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139952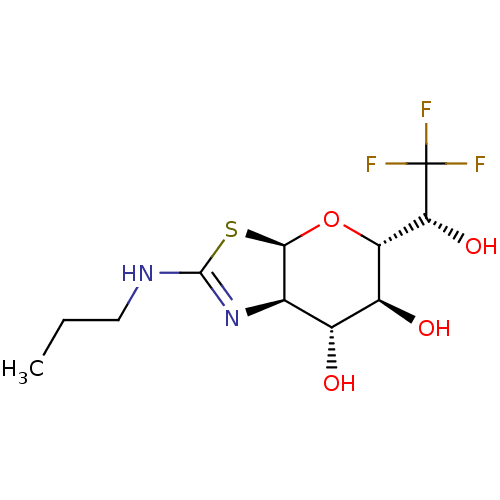 (US8901087, 97) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139925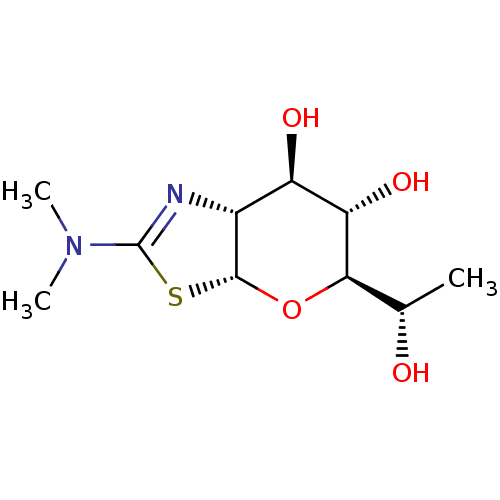 (US8901087, 1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139946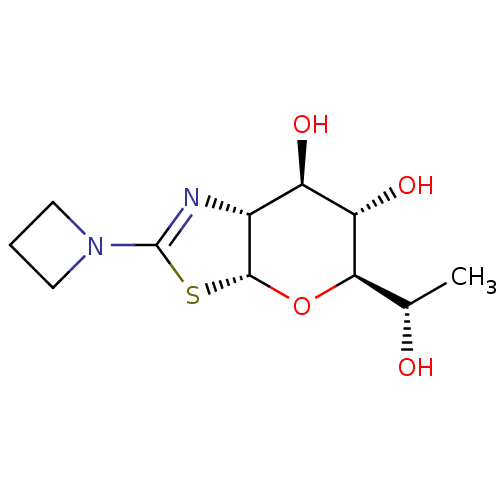 (US8901087, 76) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.180 | -55.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM314809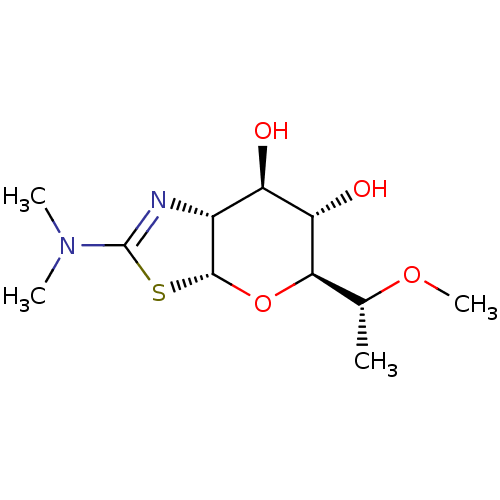 ((3aR,5S,6S,7R,7aR)-2-(dimethylamino)-5-((R)-1-meth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALECTOS THERAPEUTICS, INC.; MERCK SHARP & DOHME CORP. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9611275 (2017) BindingDB Entry DOI: 10.7270/Q2833V4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM46008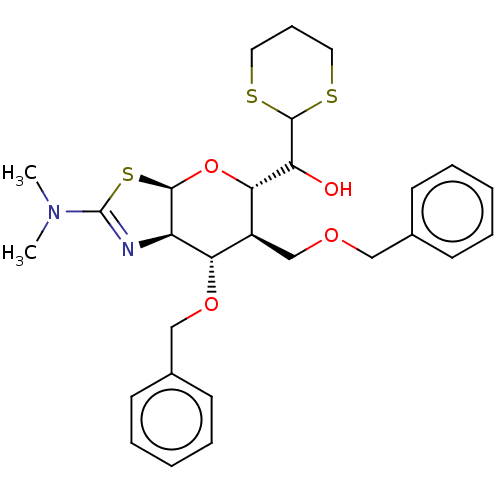 (US8901087, 96) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139941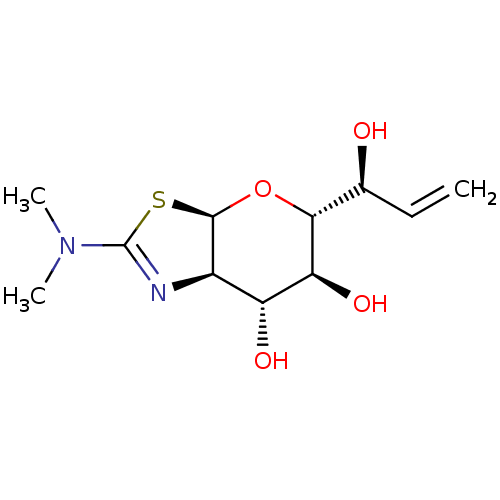 (US8901087, 63) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Homo sapiens (Human)) | BDBM50162983 (6-[5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-pyridin-3-yl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to Nicotinic acetylcholine receptor alpha3-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139930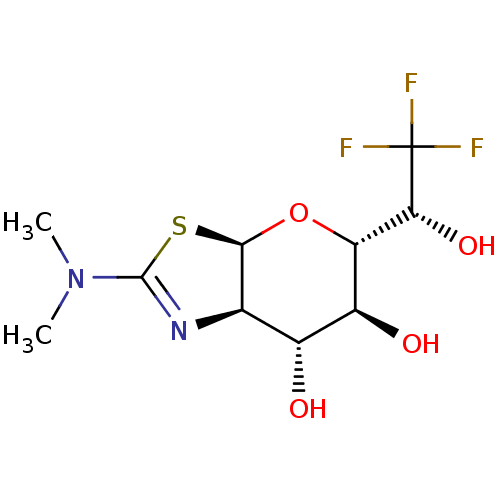 (US8901087, 11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM205418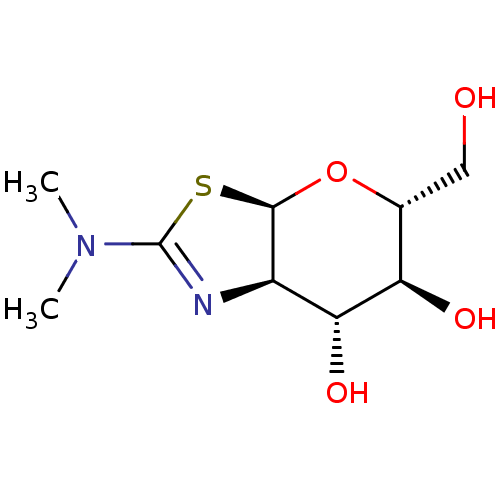 (US9243020, (3aR,5R,6S,7R,7aR)-2-(ethylamino)-5-(hy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics, Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9815861 (2017) BindingDB Entry DOI: 10.7270/Q2R78HJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50133236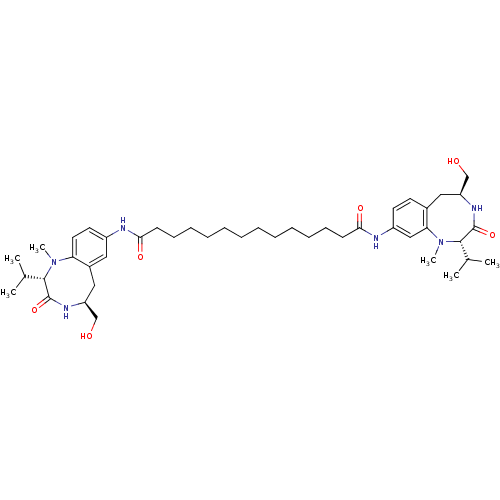 (CHEMBL337834 | Tetradecanedioic acid ((S)-5-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for ability to displace [20e3] phorbol 12,13- dibutyrate binding from recombinant C1b domain of murine Protein kinase C delta in presence of [... | J Med Chem 46: 4196-204 (2003) Article DOI: 10.1021/jm0302041 BindingDB Entry DOI: 10.7270/Q2GF0SX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139939 (US8901087, 57) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.330 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50133238 (CHEMBL334853 | Hexadecanedioic acid ((S)-5-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for ability to displace [20e3] phorbol 12,13- dibutyrate binding from recombinant murine Protein kinase C delta in presence of [Ca2+] | J Med Chem 46: 4196-204 (2003) Article DOI: 10.1021/jm0302041 BindingDB Entry DOI: 10.7270/Q2GF0SX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50513934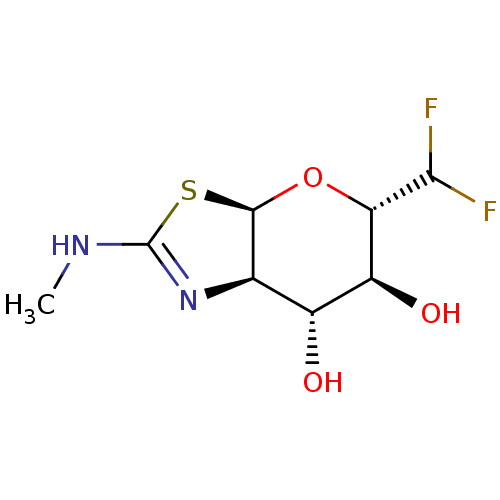 (CHEMBL4443587) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of recombinant human OGA | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50133235 (CHEMBL132720 | Docosanedioic acid ((S)-5-hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for ability to displace [20e3] phorbol 12,13- dibutyrate binding from recombinant murine Protein kinase C delta in presence of [Ca2+] | J Med Chem 46: 4196-204 (2003) Article DOI: 10.1021/jm0302041 BindingDB Entry DOI: 10.7270/Q2GF0SX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139931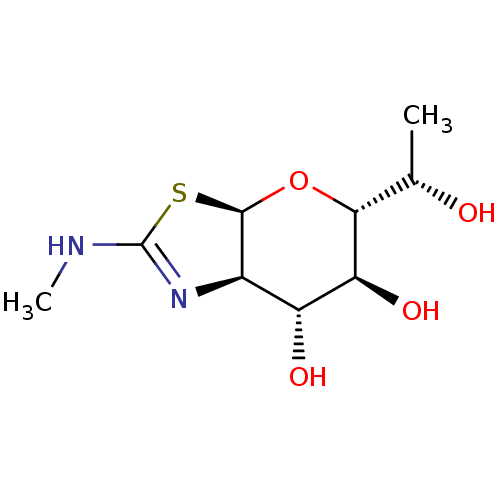 (US8901087, 13) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50323697 ((3AR,5R,6S,7R,7AR)-2-(ETHYLAMINO)-5-(HYDROXYMETHYL...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics, Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9815861 (2017) BindingDB Entry DOI: 10.7270/Q2R78HJ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50323697 ((3AR,5R,6S,7R,7AR)-2-(ETHYLAMINO)-5-(HYDROXYMETHYL...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of recombinant human OGA | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139961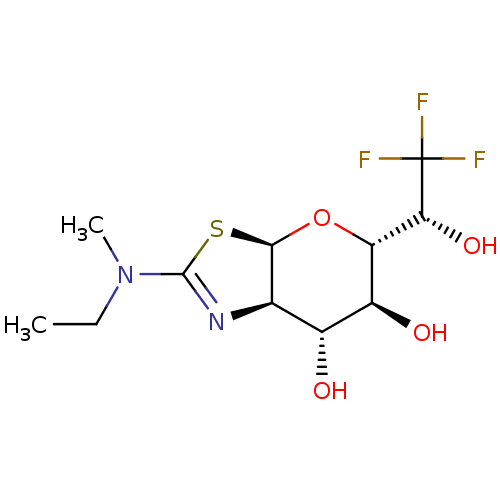 (US8901087, 211) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.440 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM314781 ((3aR,5S,6S,7R,7aR)-2- (dimethylamino)-5-((R)-2,2,2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALECTOS THERAPEUTICS, INC.; MERCK SHARP & DOHME CORP. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9611275 (2017) BindingDB Entry DOI: 10.7270/Q2833V4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139942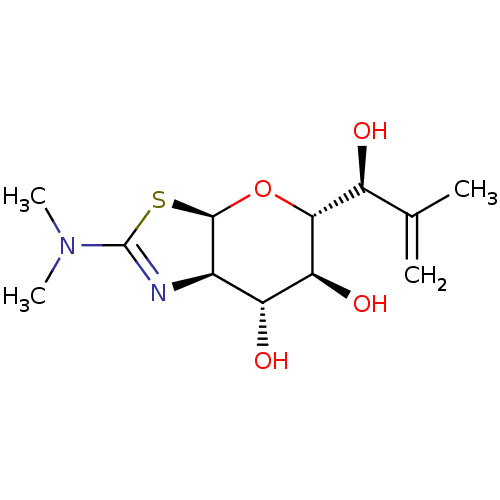 (US8901087, 65) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.450 | -53.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50133249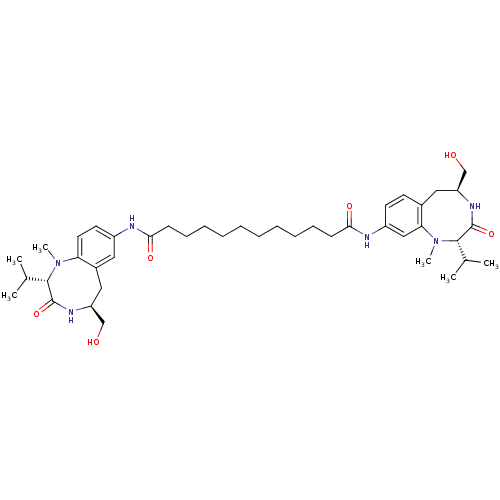 (CHEMBL133853 | Dodecanedioic acid [(S)-5-hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for ability to displace [20e3] phorbol 12,13- dibutyrate binding from recombinant C1b domain of murine Protein kinase C delta in presence of [... | J Med Chem 46: 4196-204 (2003) Article DOI: 10.1021/jm0302041 BindingDB Entry DOI: 10.7270/Q2GF0SX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM205423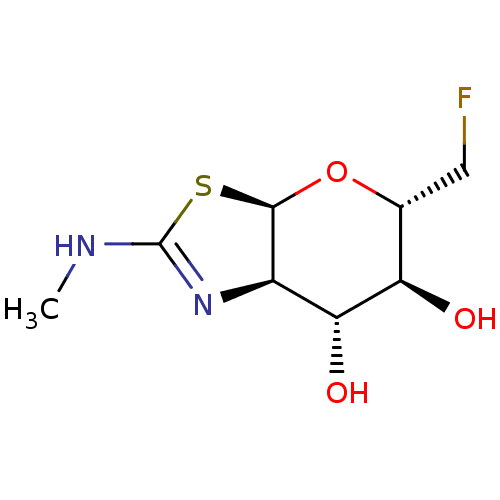 (US9243020, 17 | US9815861, Example 17) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics, Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9815861 (2017) BindingDB Entry DOI: 10.7270/Q2R78HJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139948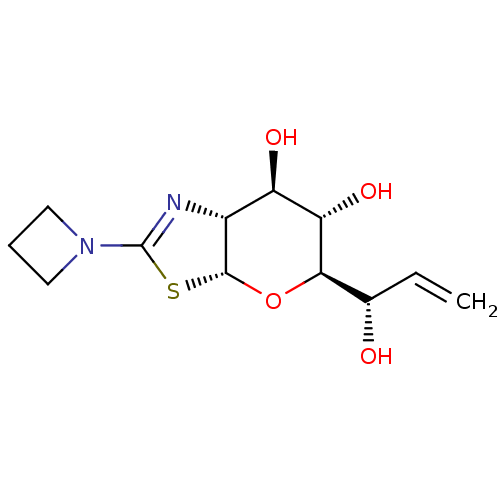 (US8901087, 80) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50097613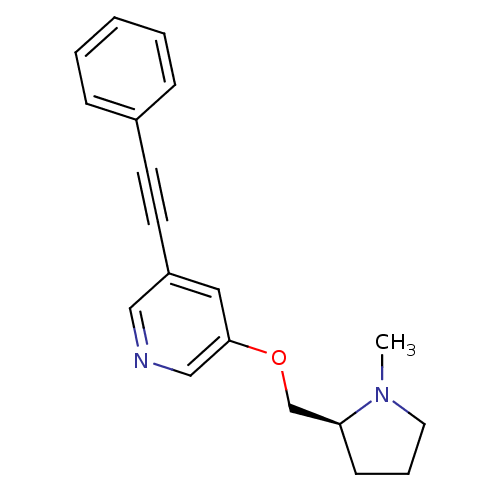 (3-((S)-1-Methyl-pyrrolidin-2-ylmethoxy)-5-phenylet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to rat Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139950 (US8901087, 91) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.530 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM50513931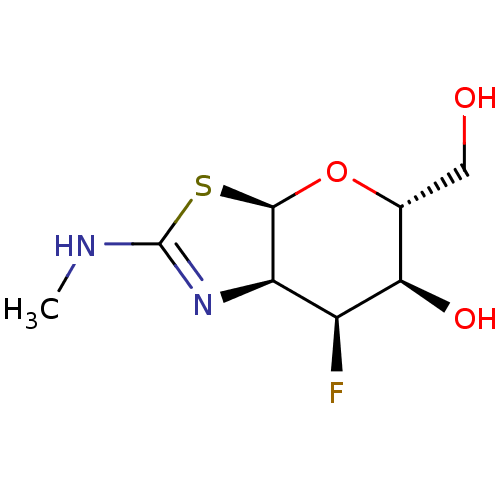 (CHEMBL4444446) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of recombinant human OGA | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM205423 (US9243020, 17 | US9815861, Example 17) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of recombinant human OGA | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50133236 (CHEMBL337834 | Tetradecanedioic acid ((S)-5-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C epsilon | J Med Chem 46: 4196-204 (2003) Article DOI: 10.1021/jm0302041 BindingDB Entry DOI: 10.7270/Q2GF0SX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta4 using [3H]epibatidine | Bioorg Med Chem Lett 14: 1855-8 (2004) Article DOI: 10.1016/j.bmcl.2003.10.071 BindingDB Entry DOI: 10.7270/Q269753N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Tested for binding affinity against Nicotinic acetylcholine receptor alpha3-beta4 | J Med Chem 46: 921-4 (2003) Article DOI: 10.1021/jm025613w BindingDB Entry DOI: 10.7270/Q2P271VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of [3H]epibatidine binding to rat Nicotinic acetylcholine receptor alpha3-beta4 | J Med Chem 48: 1721-4 (2005) Article DOI: 10.1021/jm0492406 BindingDB Entry DOI: 10.7270/Q2BZ65JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3541 total ) | Next | Last >> |