

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132351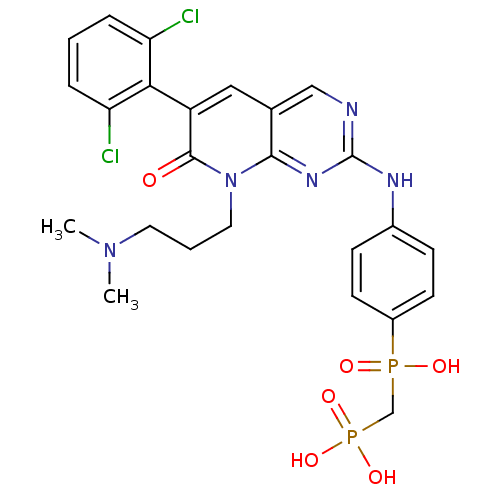 (({4-[6-(2,6-Dichloro-phenyl)-8-(3-dimethylamino-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50314074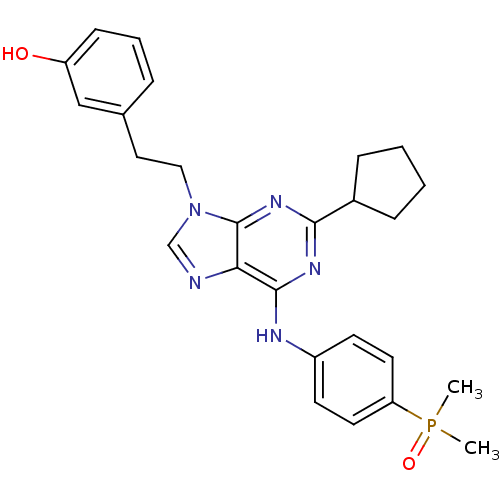 (2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals | Assay Description Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... | Chem Biol Drug Des 67: 46-57 (2006) Article DOI: 10.1111/j.1747-0285.2005.00316.x BindingDB Entry DOI: 10.7270/Q2M61HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132348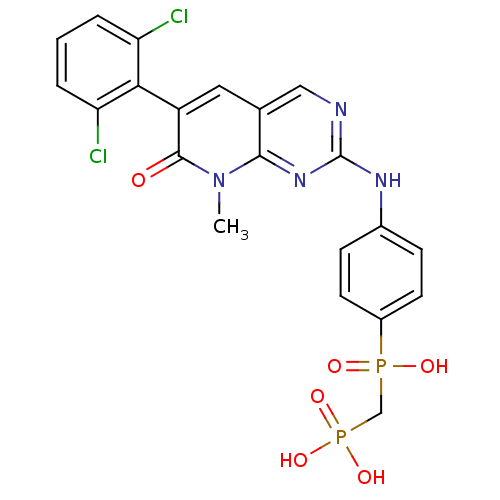 (({4-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM27216 ((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2 (CDK2) | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132322 (({2-[(2-{4-[4-Amino-5-(3-hydroxy-phenyl)-pyrrolo[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3071 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 38 | 2-ani...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221656 (CHEMBL3706663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM81618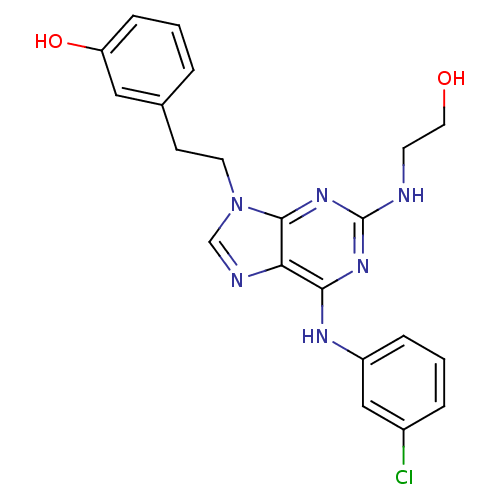 (2,6,9-Trisubstitute purine, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals | Assay Description Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... | Chem Biol Drug Des 67: 46-57 (2006) Article DOI: 10.1111/j.1747-0285.2005.00316.x BindingDB Entry DOI: 10.7270/Q2M61HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM81619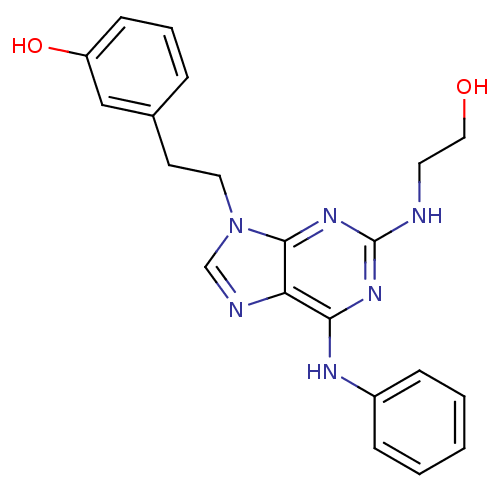 (2,6,9-Trisubstitute purine, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals | Assay Description Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... | Chem Biol Drug Des 67: 46-57 (2006) Article DOI: 10.1111/j.1747-0285.2005.00316.x BindingDB Entry DOI: 10.7270/Q2M61HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM81620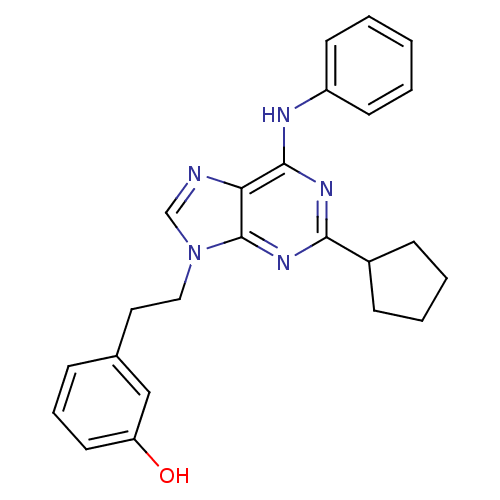 (2,6,9-Trisubstitute purine, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals | Assay Description Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... | Chem Biol Drug Des 67: 46-57 (2006) Article DOI: 10.1111/j.1747-0285.2005.00316.x BindingDB Entry DOI: 10.7270/Q2M61HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132339 (3-[4-Amino-7-(4-{2-[(2-hydroxy-ethyl)-methyl-amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132324 (3-{4-Amino-7-[4-(2-hydroxy-ethyl)-phenyl]-7H-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50451556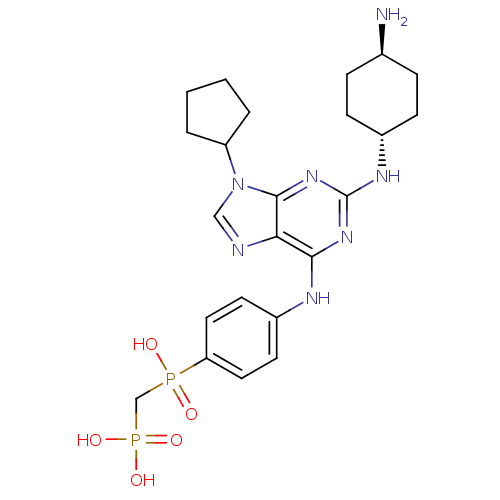 (CHEMBL3084838) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assay | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132334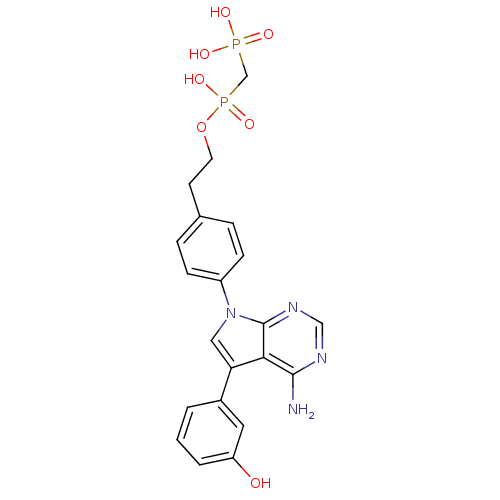 (CHEMBL104215 | [(2-{4-[4-Amino-5-(3-hydroxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM81623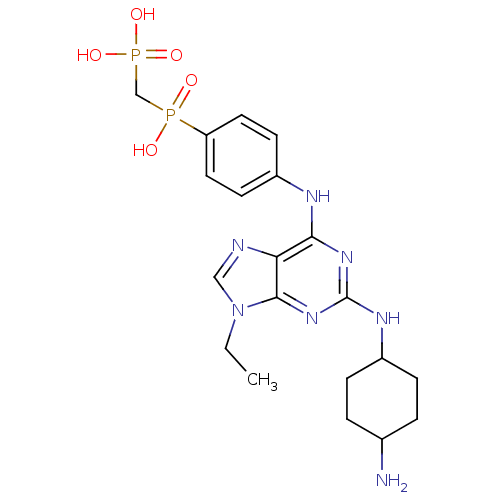 (2,6,9-Trisubstitute purine, 9 (AP23451)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals | Assay Description Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... | Chem Biol Drug Des 67: 46-57 (2006) Article DOI: 10.1111/j.1747-0285.2005.00316.x BindingDB Entry DOI: 10.7270/Q2M61HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM50318870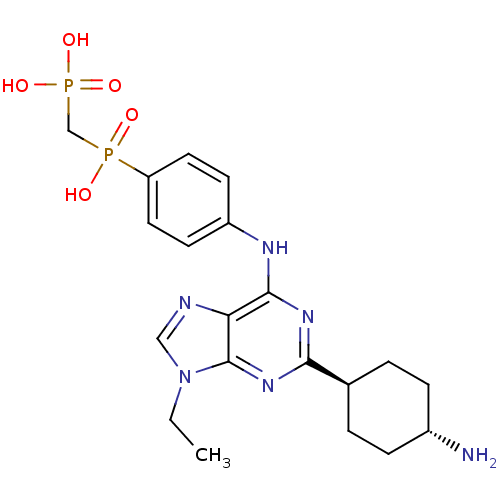 (((4-(2-(cis-4-aminocyclohexyl)-9-ethyl-9H-purin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM27216 ((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals | Assay Description Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... | Chem Biol Drug Des 67: 46-57 (2006) Article DOI: 10.1111/j.1747-0285.2005.00316.x BindingDB Entry DOI: 10.7270/Q2M61HRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81725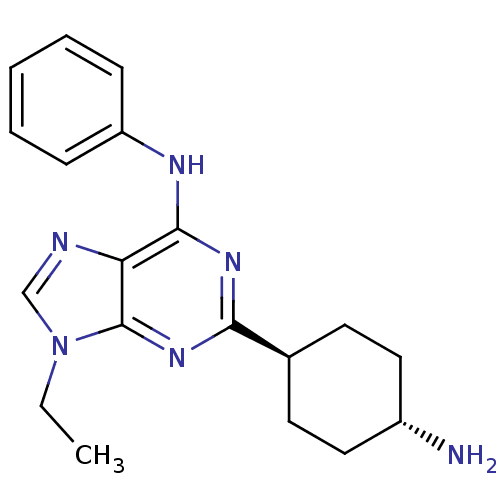 (2,6,9-Trisubstituted Purine, AP23517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132352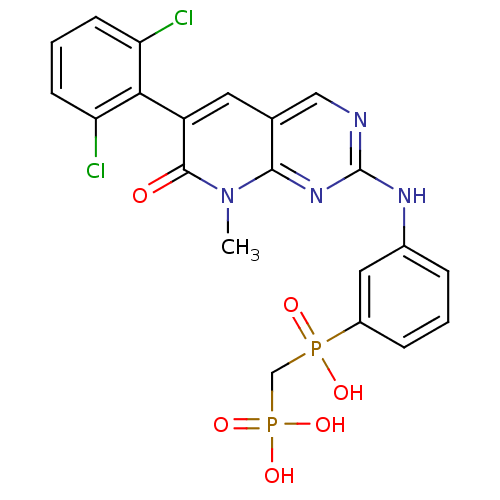 (({3-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81723 (2,6,9-Trisubstituted Purine, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132330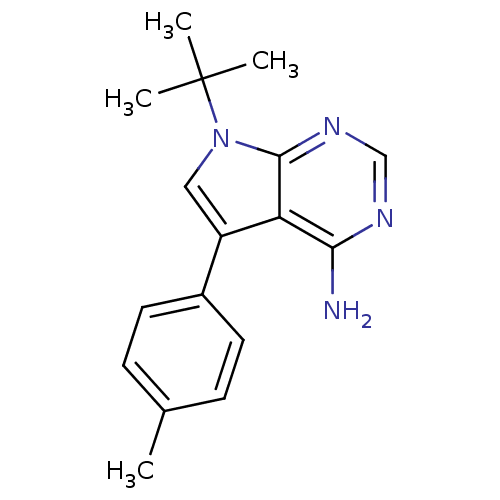 (7-tert-Butyl-5-p-tolyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50451553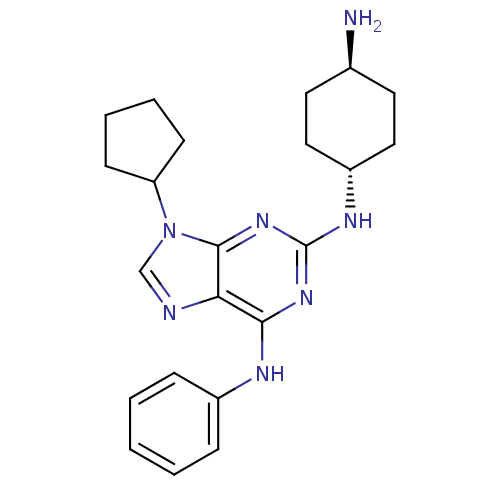 (CHEMBL3084839) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assay | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50088903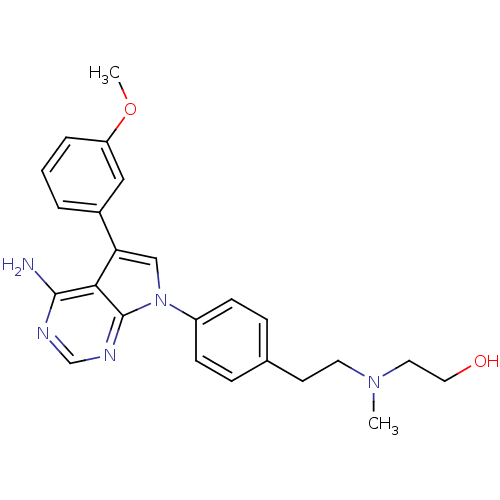 (2-((4-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2,3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190583 (US9181219, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190579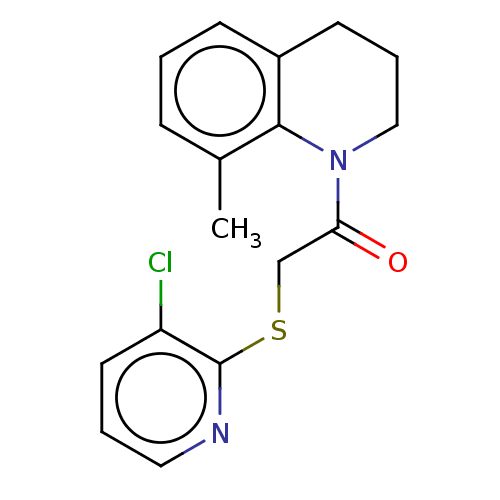 (US9181219, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132353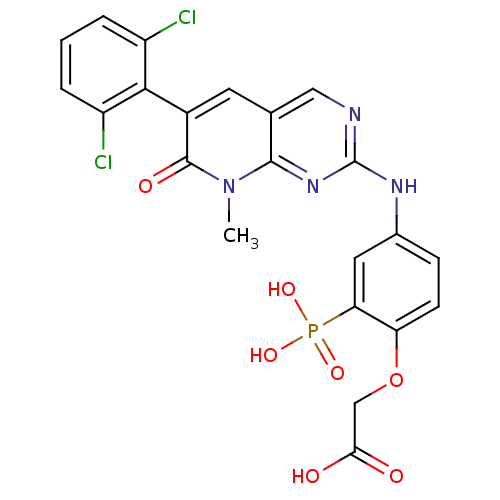 (CHEMBL102801 | {4-[6-(2,6-Dichloro-phenyl)-8-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 3071-4 (2003) BindingDB Entry DOI: 10.7270/Q21J995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190585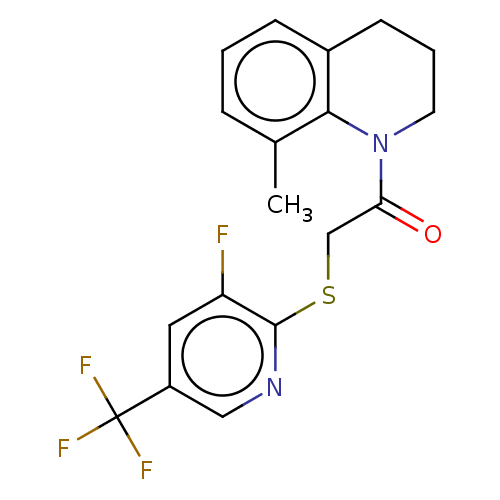 (US9181219, 2-{[3-fluoro-5-(trifluoromethyl)pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190588 (US9181219, 2-[(3,5-dichloropyridin-2-yl)sulfanyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190593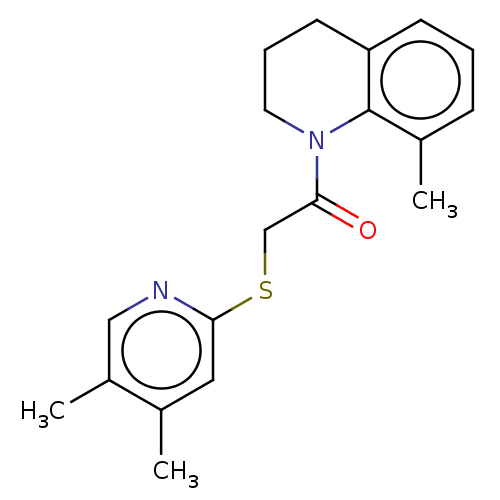 (US9181219, 2-[(4,5-dimethylpyridin-2-yl)sulfanyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190593 (US9181219, 2-[(4,5-dimethylpyridin-2-yl)sulfanyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190576 (US9181219, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190585 (US9181219, 2-{[3-fluoro-5-(trifluoromethyl)pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190588 (US9181219, 2-[(3,5-dichloropyridin-2-yl)sulfanyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM81722 (2,6,9-Trisubstituted Purine, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM81622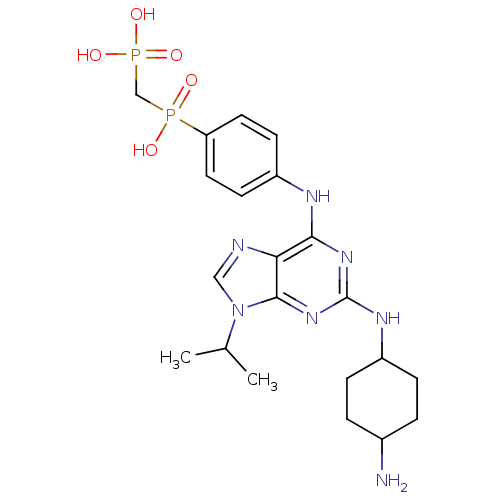 (2,6,9-Trisubstitute purine, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 239 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals | Assay Description Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... | Chem Biol Drug Des 67: 46-57 (2006) Article DOI: 10.1111/j.1747-0285.2005.00316.x BindingDB Entry DOI: 10.7270/Q2M61HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM27216 ((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.4 | 4 |
ARIAD Pharmaceuticals | Assay Description Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR... | Chem Biol Drug Des 67: 46-57 (2006) Article DOI: 10.1111/j.1747-0285.2005.00316.x BindingDB Entry DOI: 10.7270/Q2M61HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132335 (CHEMBL104857 | [(3-{4-[4-Amino-5-(3-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Mus musculus (Mouse)) | BDBM50318870 (((4-(2-(cis-4-aminocyclohexyl)-9-ethyl-9H-purin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. | Assay Description 2,6,9-Trisubstituted purines were evaluated for their src kinase inhibitory activities using an ELISA assay. | Chem Biol Drug Des 71: 97-105 (2008) Article DOI: 10.1111/j.1747-0285.2007.00615.x BindingDB Entry DOI: 10.7270/Q2FF3QTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132333 (({2-[(2-{4-[4-Amino-5-(3-methoxy-phenyl)-pyrrolo[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221655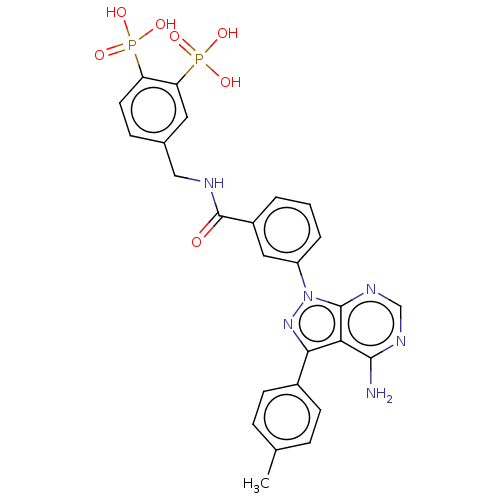 (CHEMBL3706661) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097348 (CHEMBL348743 | [(4-{2-Acetylamino-2-[1-(3-carbamoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of binding to Src SH2 domain | J Med Chem 44: 660-3 (2001) BindingDB Entry DOI: 10.7270/Q2DJ5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132328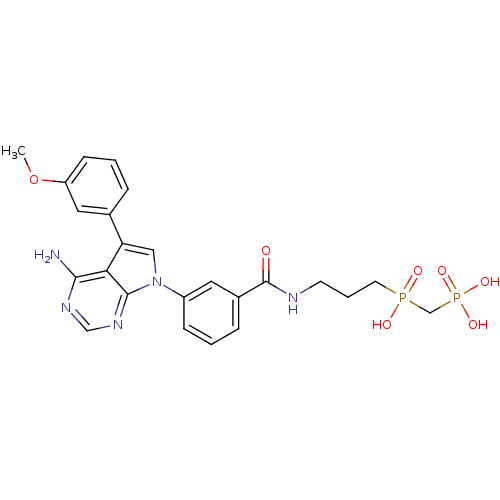 (CHEMBL321116 | [(3-{3-[4-Amino-5-(3-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221654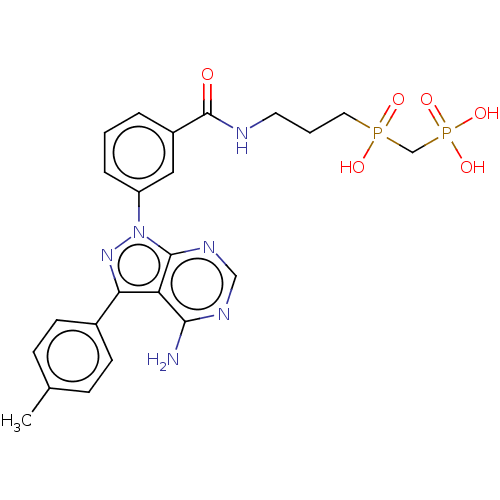 (CHEMBL3706662) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190591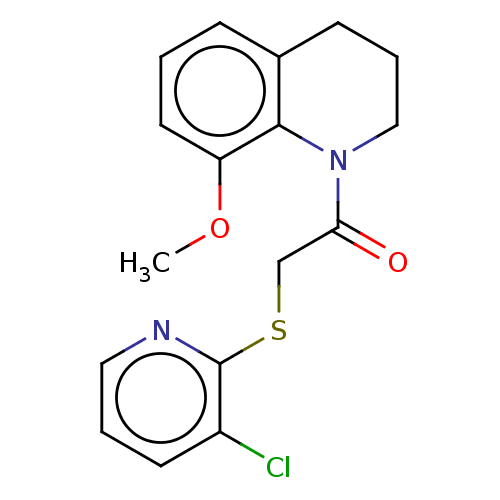 (US9181219, 2-[(3-chloropyridin-2-yl)sulfanyl]-1-(8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190578 (US9181219, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190578 (US9181219, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190591 (US9181219, 2-[(3-chloropyridin-2-yl)sulfanyl]-1-(8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190590 (US9181219, 2-{[2-(8-methyl-1,2,3,4-tetrahydroquino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190586 (US9181219, 2-[(5-bromopyridin-2-yl)sulfanyl]-1-(8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description Phase 1: TRPV3 cells were induced 20-48 hours, removed from growth plates, and replated at low density (to attain good single-cell physical separatio... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 3 (Homo sapiens (Human)) | BDBM190586 (US9181219, 2-[(5-bromopyridin-2-yl)sulfanyl]-1-(8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
HYDRA BIOSCIENCES, INC. US Patent | Assay Description To determine whether compounds were selective for TRPV3 inhibition over inhibition of other ion channel types, the human ERG (hERG), NaV1.2, and TRPV... | US Patent US9181219 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 124 total ) | Next | Last >> |