

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231140 (CHEMBL77029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145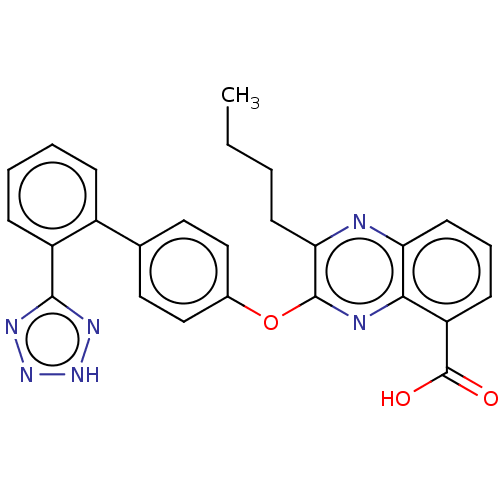 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231150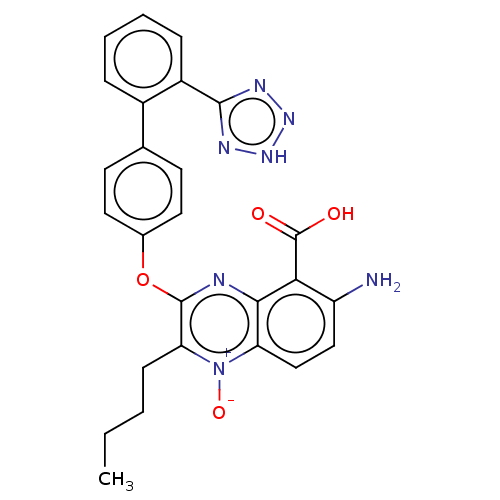 (CHEMBL77935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231139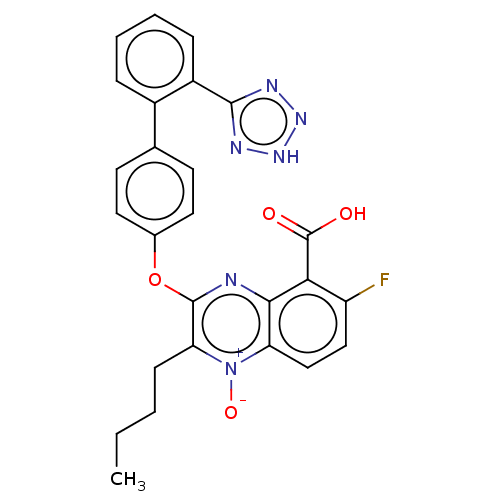 (CHEMBL77827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231149 (CHEMBL306278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231143 (CHEMBL75053) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231007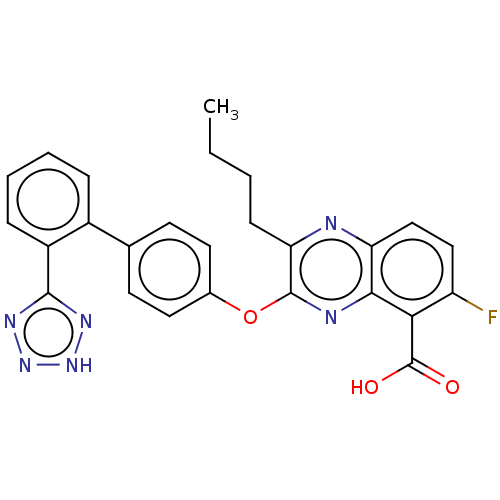 (CHEMBL74445) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231142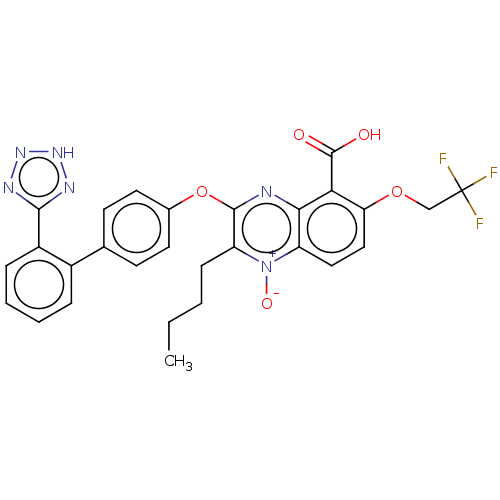 (CHEMBL312068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231148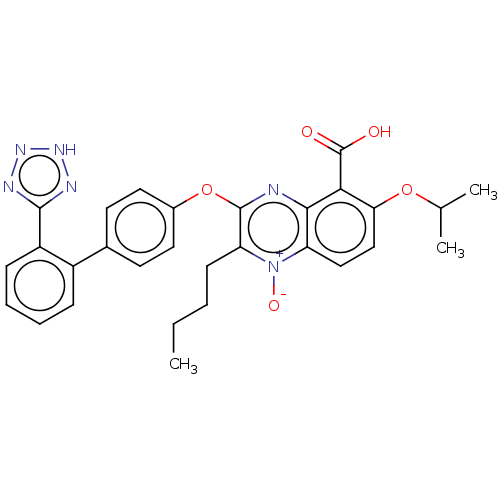 (CHEMBL77376) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231011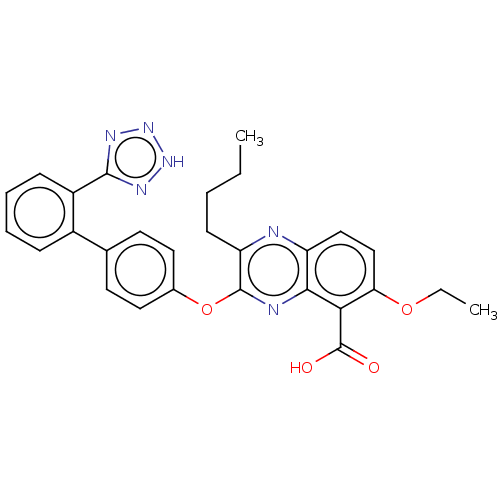 (CHEMBL309489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231009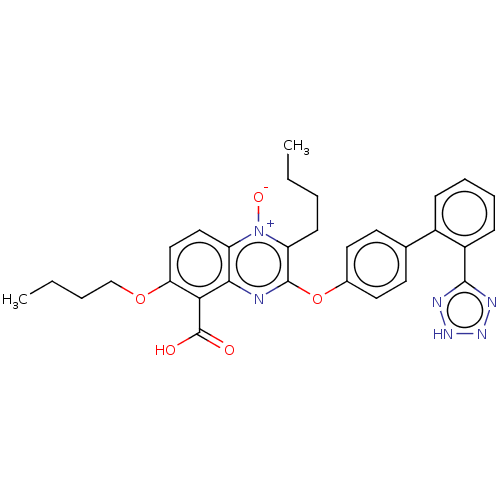 (CHEMBL76765) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231147 (CHEMBL433310) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231008 (CHEMBL75101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231008 (CHEMBL75101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231144 (CHEMBL76943) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231141 (CHEMBL77266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231146 (CHEMBL77185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231146 (CHEMBL77185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231008 (CHEMBL75101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.22% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231144 (CHEMBL76943) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.22% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231006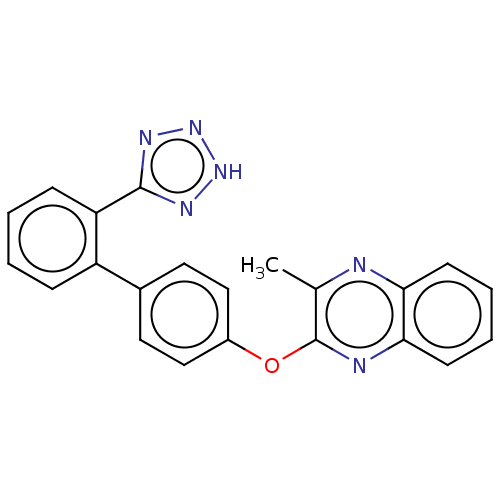 (CHEMBL307946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231006 (CHEMBL307946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231146 (CHEMBL77185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.22% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231006 (CHEMBL307946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.22% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50281226 (CHEMBL328736 | N-[(S)-1-[(2R,3S)-4-((R)-Butylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human renal renin at pH 7 | Bioorg Med Chem Lett 3: 2739-2744 (1993) Article DOI: 10.1016/S0960-894X(01)80755-1 BindingDB Entry DOI: 10.7270/Q2Q52PJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048982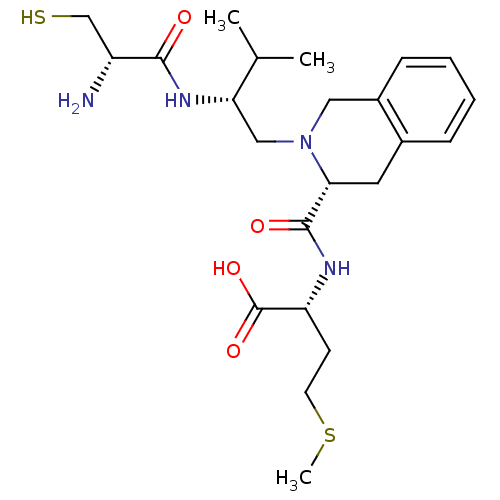 ((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048970 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048963 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048972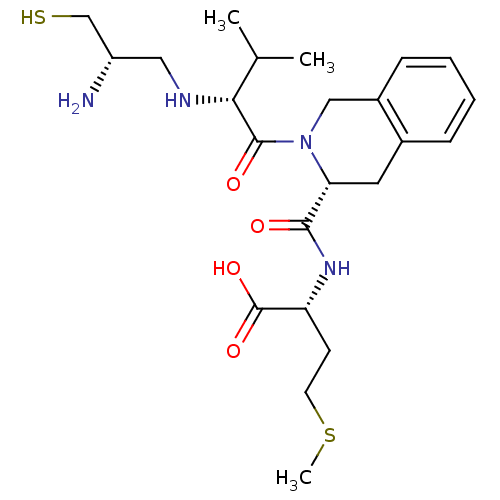 ((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048967 ((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048964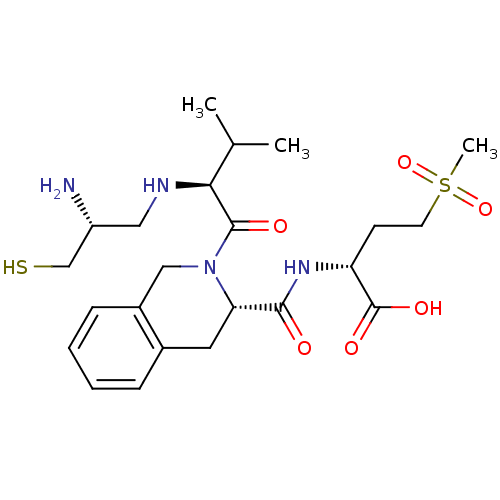 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048981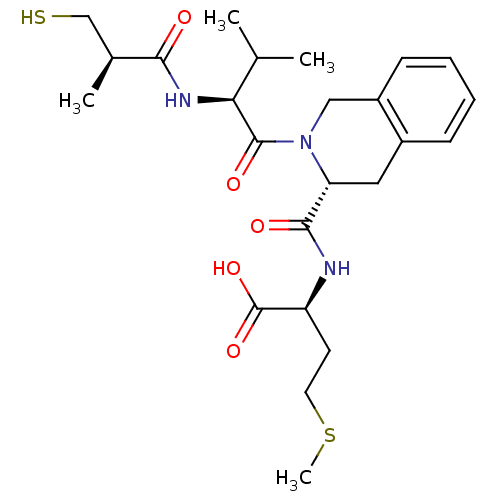 ((S)-2-({(R)-2-[(S)-2-((R)-3-Mercapto-2-methyl-prop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50048966 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Geranylgeranyl transferase type I | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048968 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50281231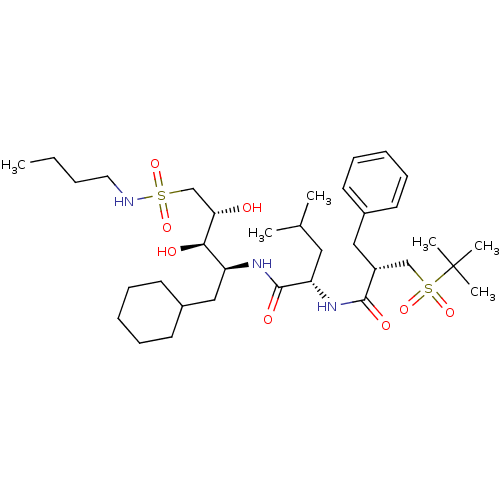 ((S)-4-Methyl-2-[(S)-2-(2-methyl-propane-2-sulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human renal renin at pH 7 | Bioorg Med Chem Lett 3: 2739-2744 (1993) Article DOI: 10.1016/S0960-894X(01)80755-1 BindingDB Entry DOI: 10.7270/Q2Q52PJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048969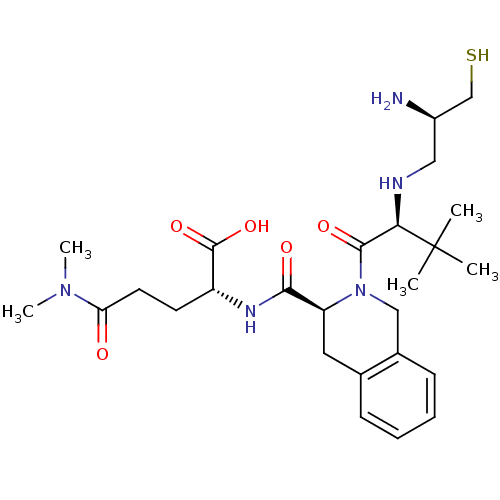 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048974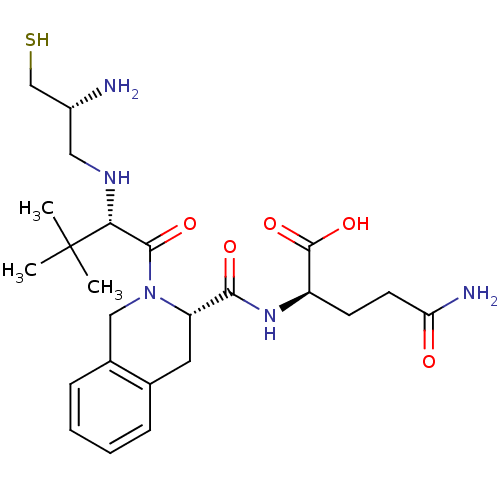 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048975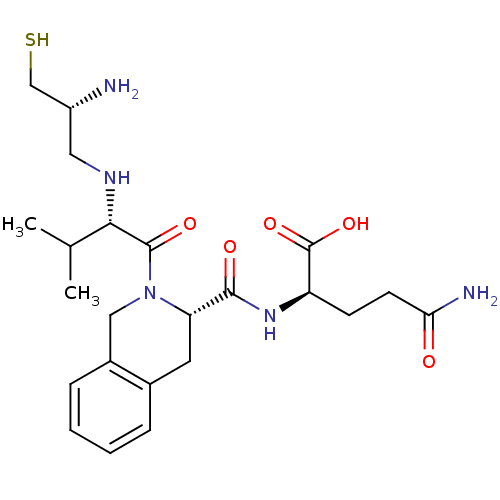 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50048963 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Geranylgeranyl transferase type I | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50281229 (1H-Indole-2-carboxylic acid [(S)-1-[4-((S,S,R)-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against human renal renin at pH 7 | Bioorg Med Chem Lett 3: 2739-2744 (1993) Article DOI: 10.1016/S0960-894X(01)80755-1 BindingDB Entry DOI: 10.7270/Q2Q52PJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048983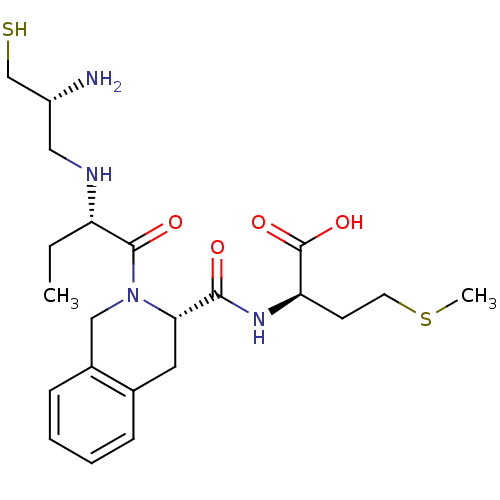 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50048970 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Geranylgeranyl transferase type I | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50048968 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Geranylgeranyl transferase type I | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048965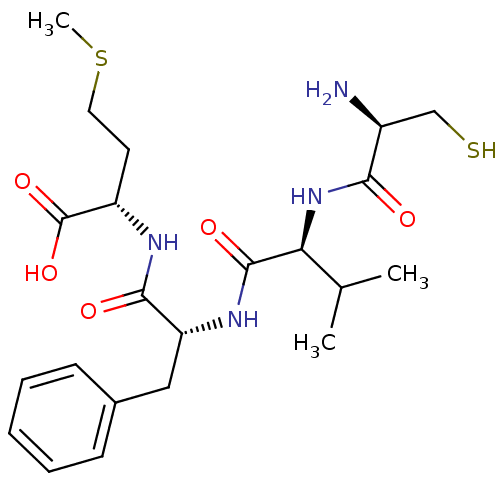 ((S)-2-{(R)-2-[(S)-2-((R)-2-Amino-3-mercapto-propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048973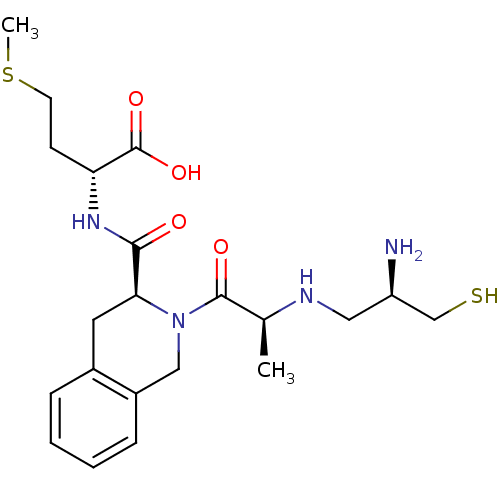 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |