

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435486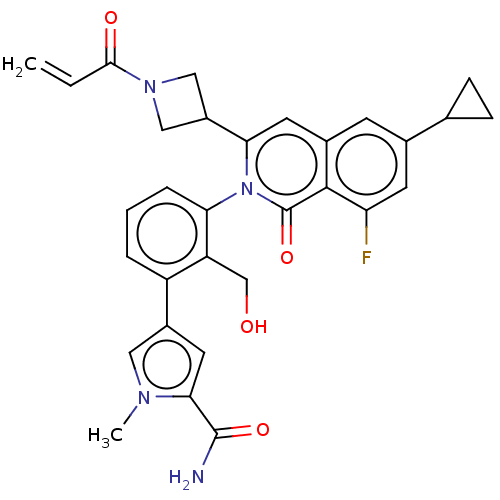 (US10570118, Example 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435467 (US10570118, Example 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435452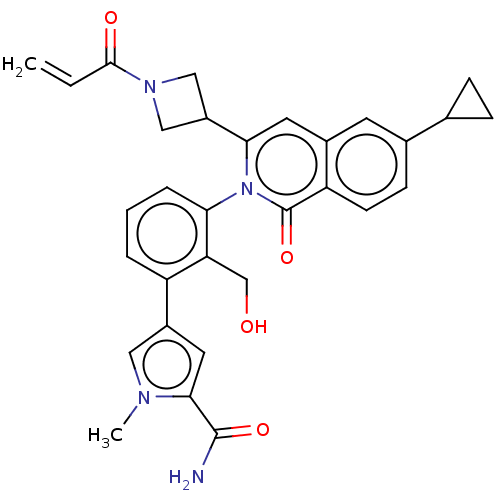 (US10570118, Example 42 | US10570118, Example 43 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435452 (US10570118, Example 42 | US10570118, Example 43 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435446 (US10570118, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435487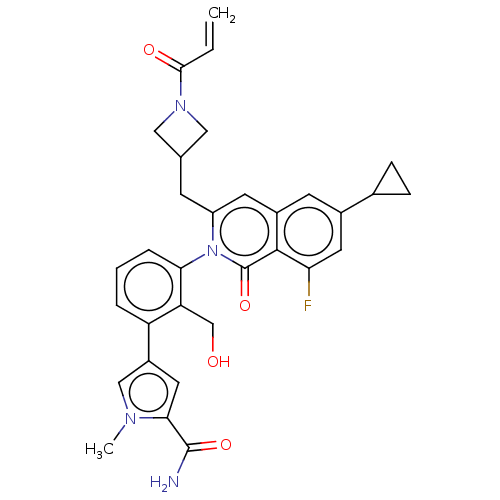 (US10570118, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435463 (US10570118, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435456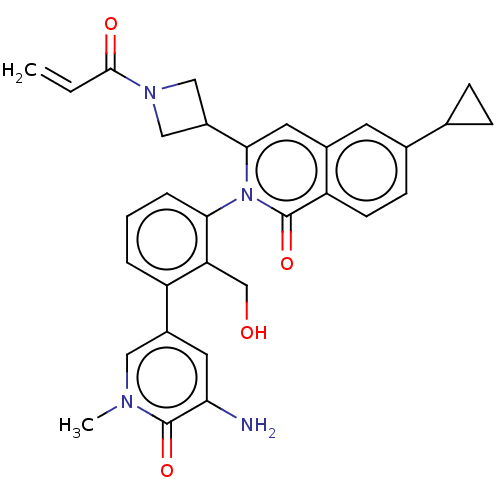 (US10570118, Example 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435448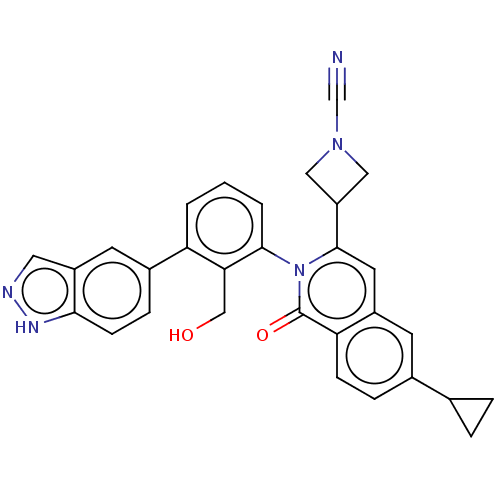 (US10570118, Example 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435458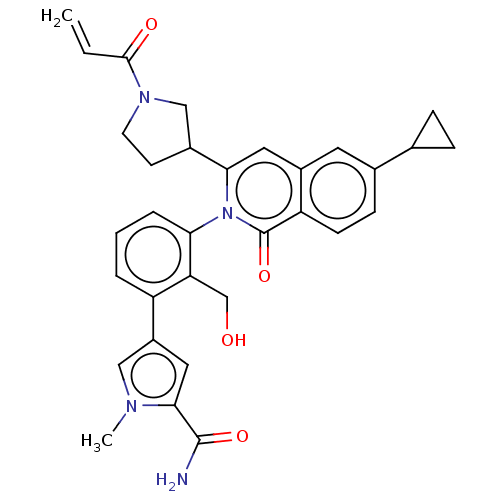 (US10570118, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435454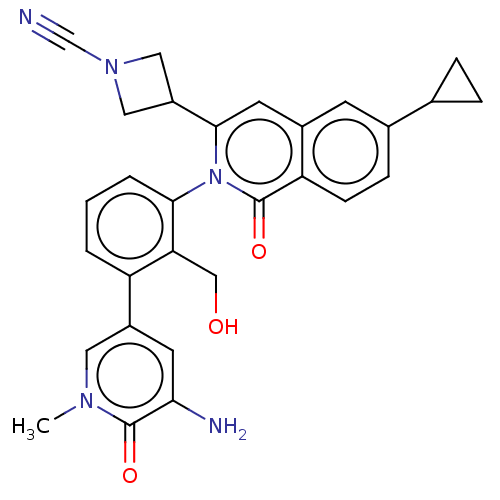 (US10570118, Example 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435455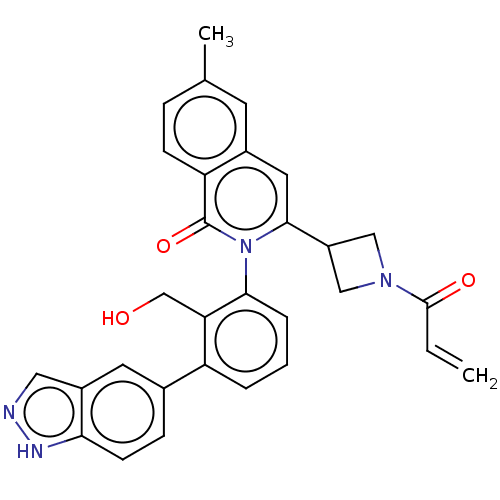 (US10570118, Example 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435457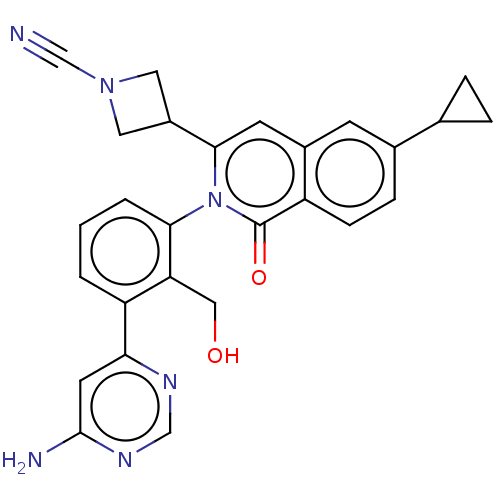 (US10570118, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435451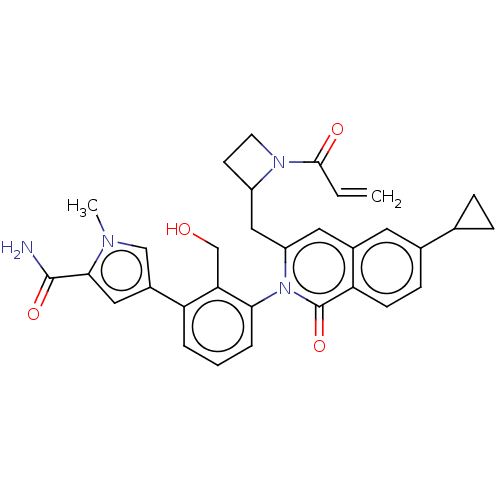 (US10570118, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361851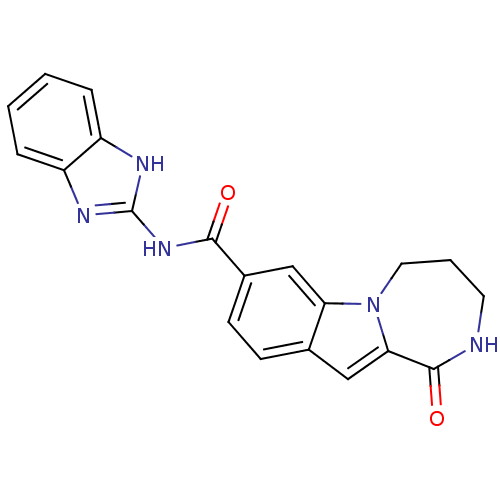 (CHEMBL1938803 | US9150577, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435447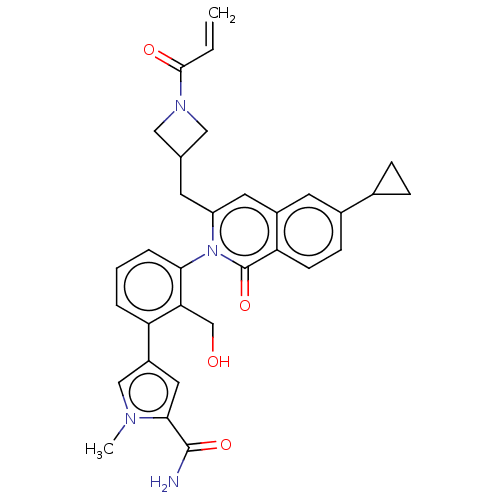 (US10570118, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435460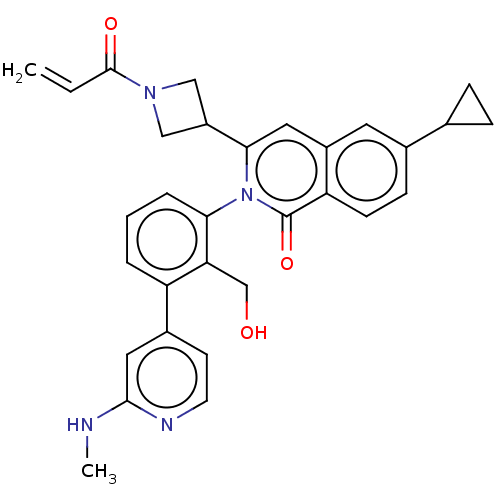 (US10570118, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435449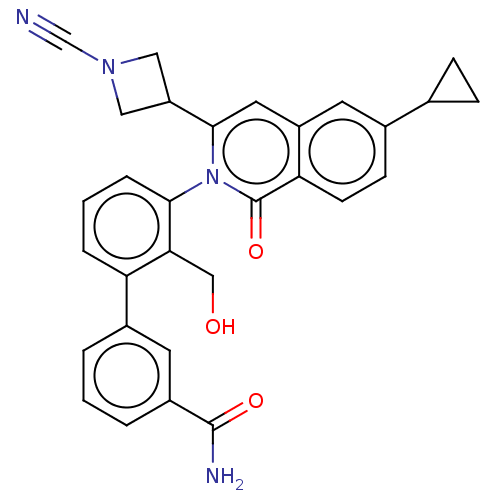 (US10570118, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435450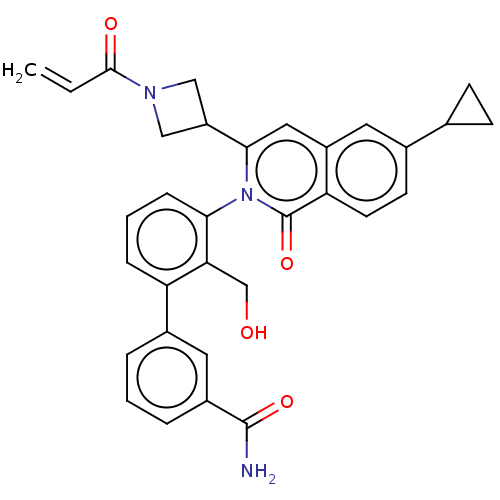 (US10570118, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435466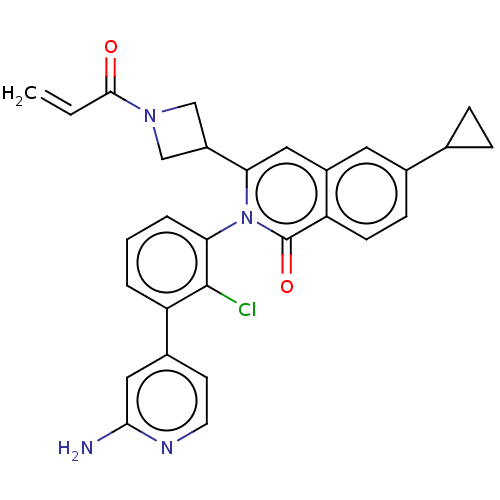 (US10570118, Example 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50360292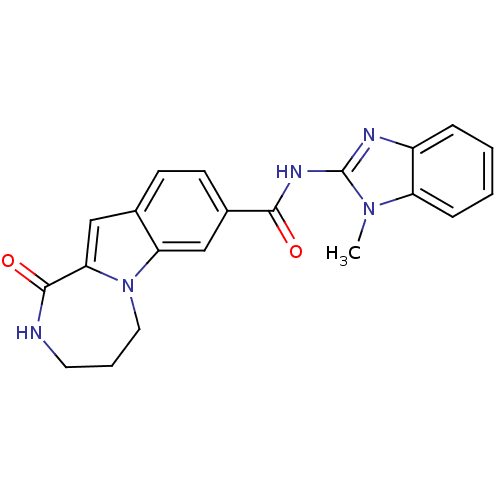 (CHEMBL1933279 | US9150577, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50360288 (CHEMBL1933148 | US9150577, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435453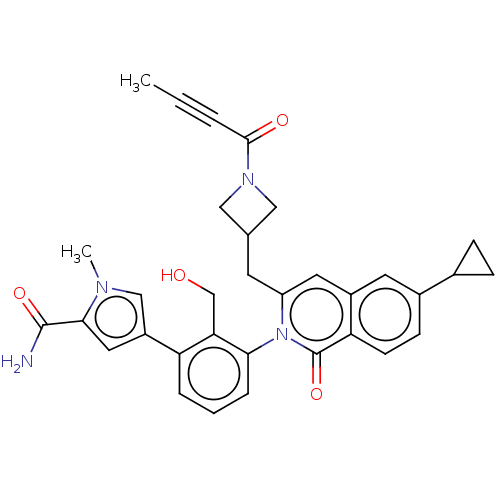 (US10570118, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50360282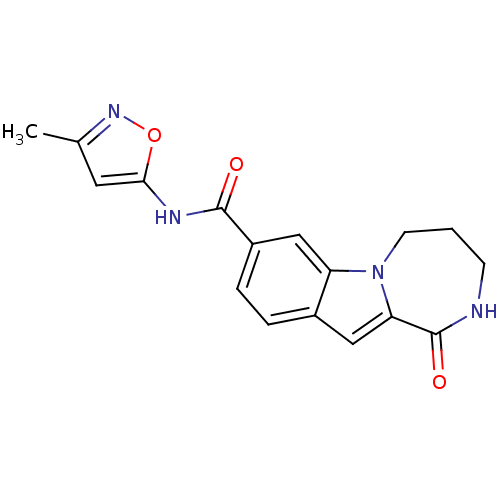 (CHEMBL1933143 | US9150577, 193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435459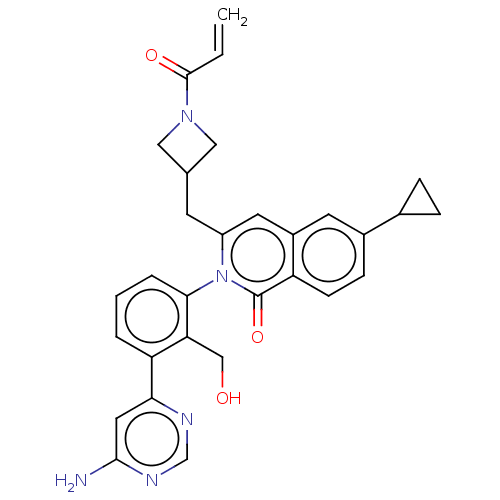 (US10570118, Example 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435461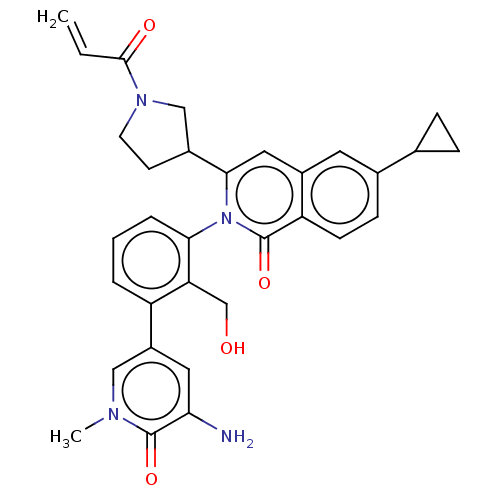 (US10570118, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361841 (CHEMBL1938786 | US9150577, 215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361845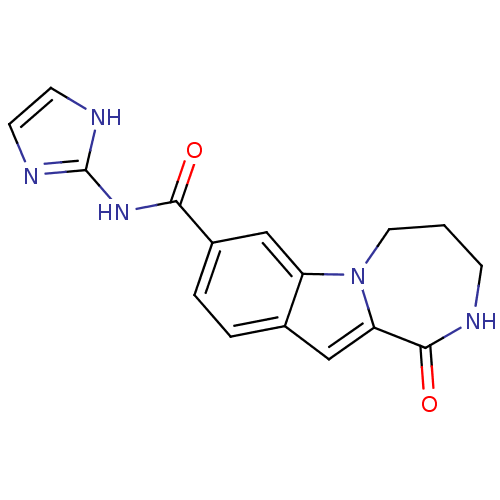 (CHEMBL1938792 | US9150577, 108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361847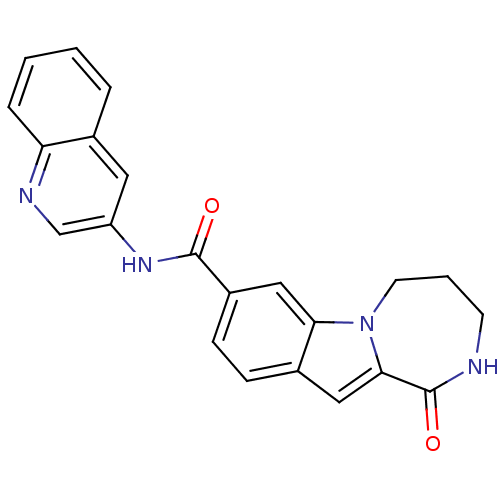 (CHEMBL1938798 | US9150577, 190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361842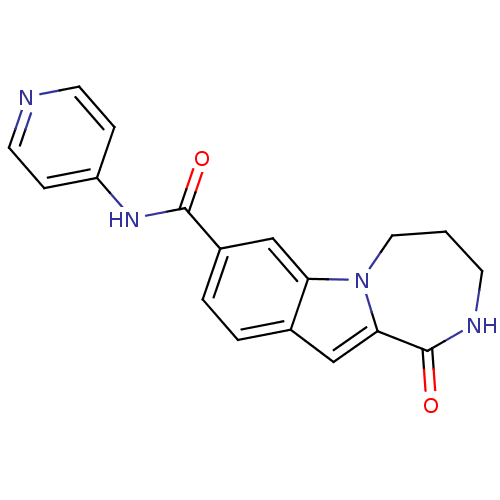 (CHEMBL1938787 | US9150577, 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435468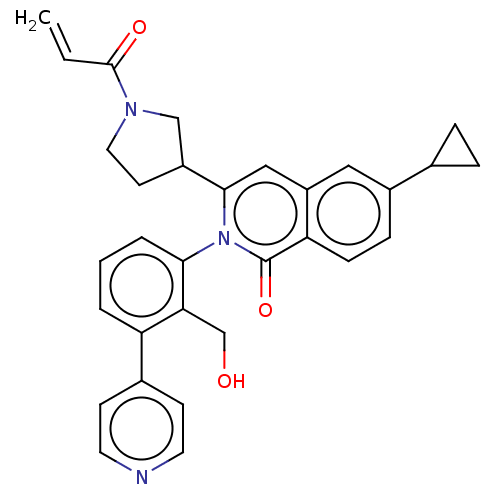 (US10570118, Example 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361835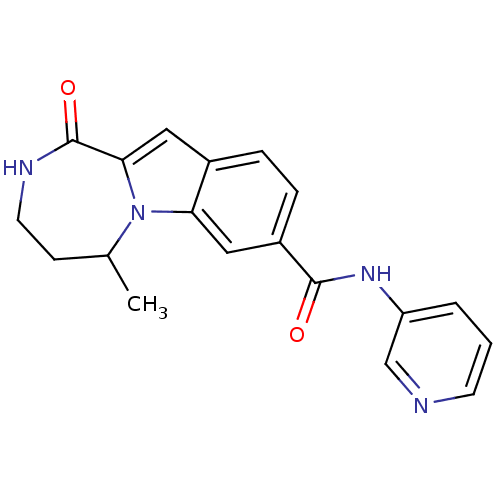 (CHEMBL1938765 | US9150577, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361846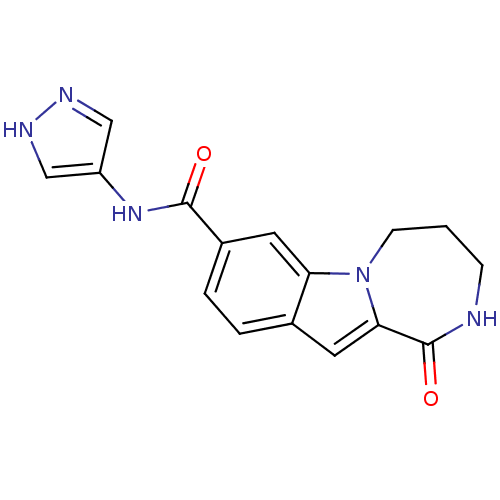 (CHEMBL1938794 | US9150577, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361839 (CHEMBL1938776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361867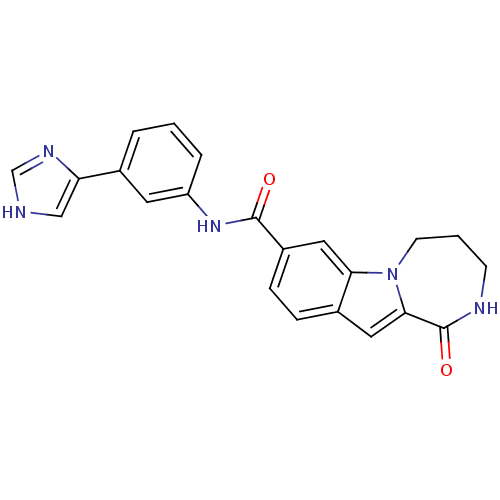 (CHEMBL1938791 | US9150577, 226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361848 (CHEMBL1938799 | US9150577, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435464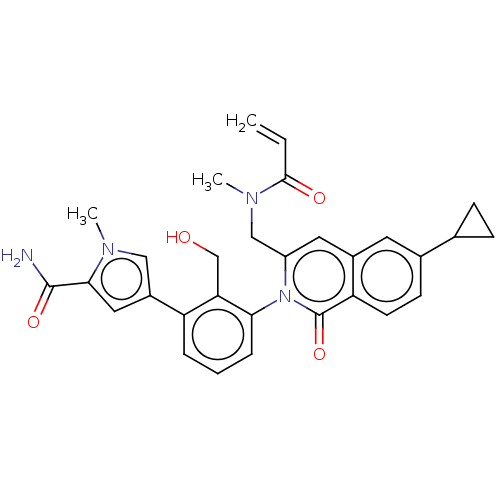 (US10570118, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435465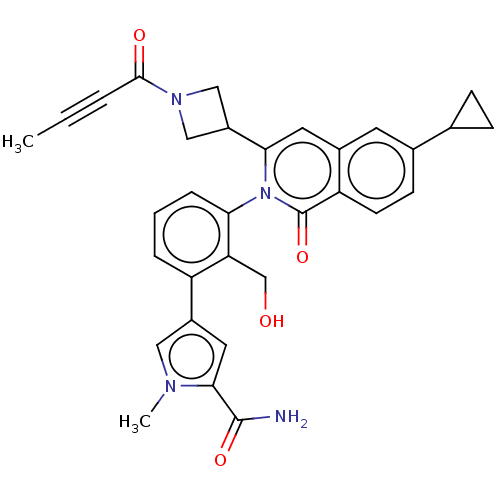 (US10570118, Example 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361849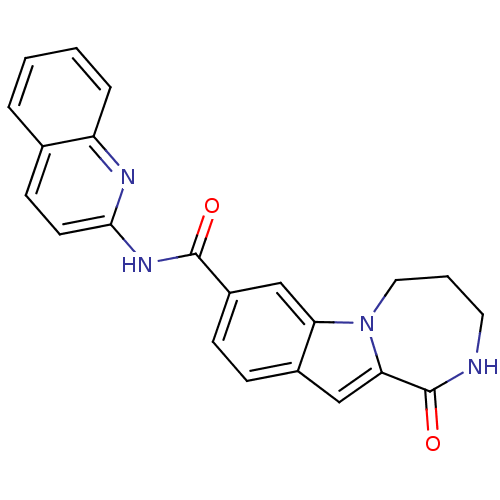 (CHEMBL1938800 | US9150577, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361870 (CHEMBL1938796) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435484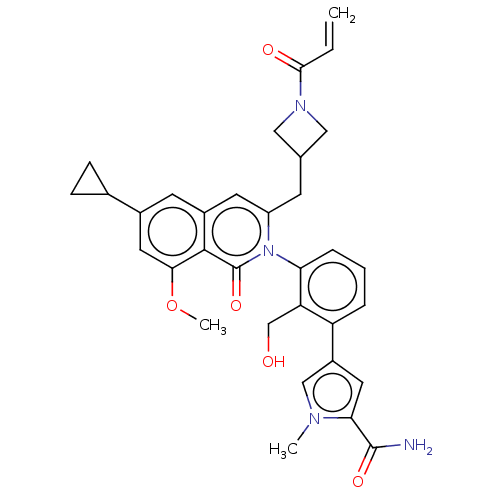 (US10570118, Example 38 | US10570118, Example 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435469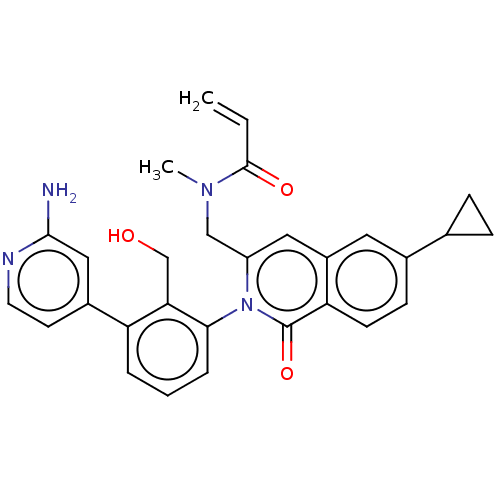 (US10570118, Example 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435470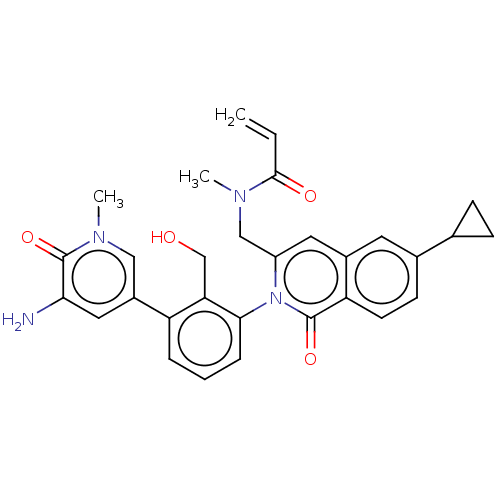 (US10570118, Example 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM435471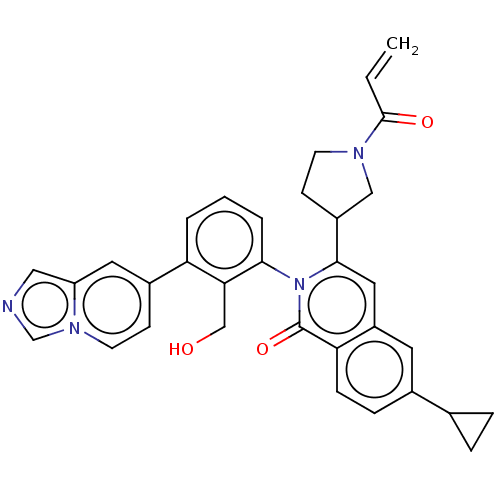 (US10570118, Example 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description A Lanthscreen Eu Kinase Binding assay (Life Technologies) was performed to quantitate the ability of test compounds to bind to BTK. The assay is base... | US Patent US10570118 (2020) BindingDB Entry DOI: 10.7270/Q2DJ5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361837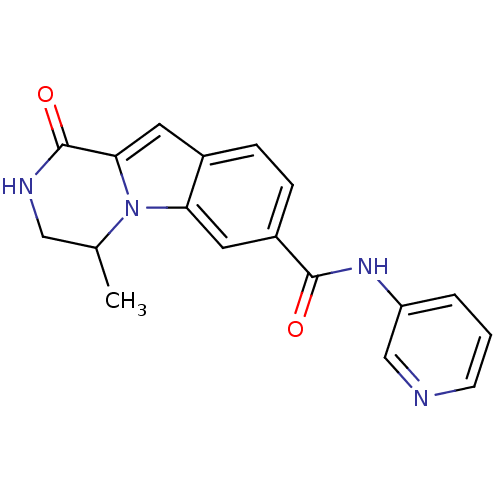 (CHEMBL1938774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361873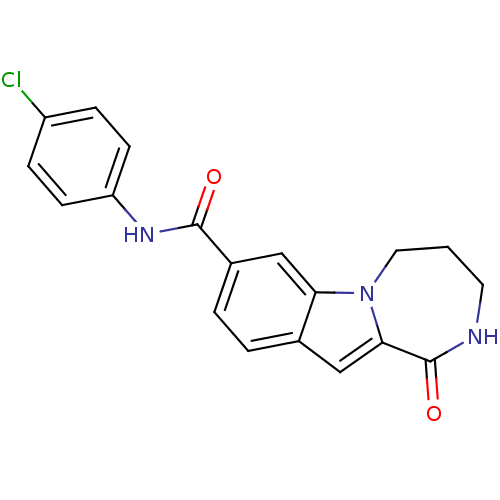 (CHEMBL1938782 | US9150577, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50360282 (CHEMBL1933143 | US9150577, 193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2-mediated CREB phosphorylation in HeLa cells expressing luciferase reporter gene | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50360292 (CHEMBL1933279 | US9150577, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2-mediated CREB phosphorylation in HeLa cells expressing luciferase reporter gene | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361838 (CHEMBL1938775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-3 (Homo sapiens (Human)) | BDBM50361843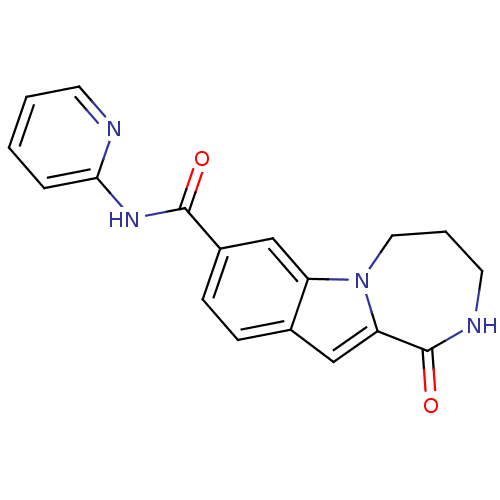 (CHEMBL1938788 | US9150577, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of RSK2 phosphorylation by luminescence assay | Bioorg Med Chem Lett 22: 733-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.030 BindingDB Entry DOI: 10.7270/Q2XW4K7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 444 total ) | Next | Last >> |