

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50177898 (2-Fluoro-N-[4-methoxymethyl-1-(2-thiophen-2-yl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Binding constant for Opioid receptor mu 1 in mouse | Bioorg Med Chem Lett 15: 1773-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.049 BindingDB Entry DOI: 10.7270/Q28916M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300442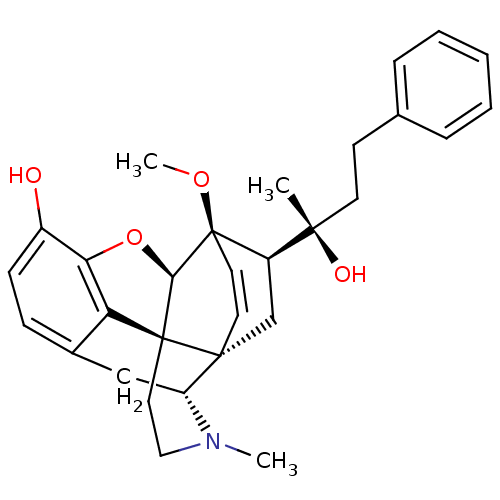 ((20R)-4,5-alpha-Epoxy-17-methyl-3-hydroxy-6-methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biomedizinische Forschungsreagenzien GmbH Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting | J Med Chem 52: 5586-9 (2009) Article DOI: 10.1021/jm900892x BindingDB Entry DOI: 10.7270/Q2SQ90F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177895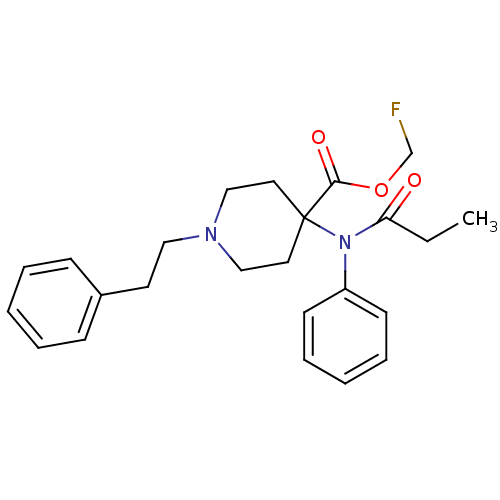 (CHEMBL200456 | fluoromethyl 1-(2-phenylethyl)-4-(N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177898 (2-Fluoro-N-[4-methoxymethyl-1-(2-thiophen-2-yl-eth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50331552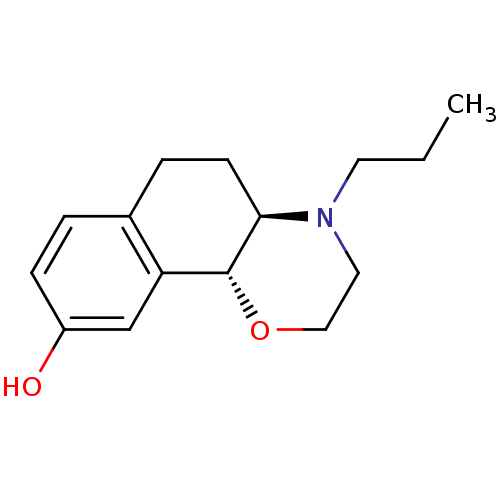 (CHEMBL1288585 | [11C]-(+)-(4aR,10bR)-4-propyl-3,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Binding affinity to human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300442 ((20R)-4,5-alpha-Epoxy-17-methyl-3-hydroxy-6-methox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biomedizinische Forschungsreagenzien GmbH Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting | J Med Chem 52: 5586-9 (2009) Article DOI: 10.1021/jm900892x BindingDB Entry DOI: 10.7270/Q2SQ90F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132057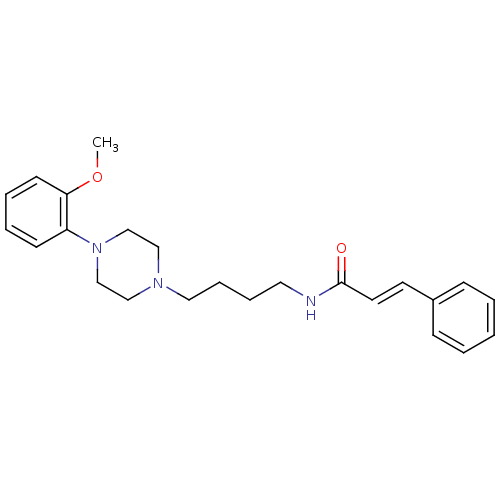 (CHEMBL1180430 | CHEMBL126128 | N-{4-[4-(2-Methoxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132069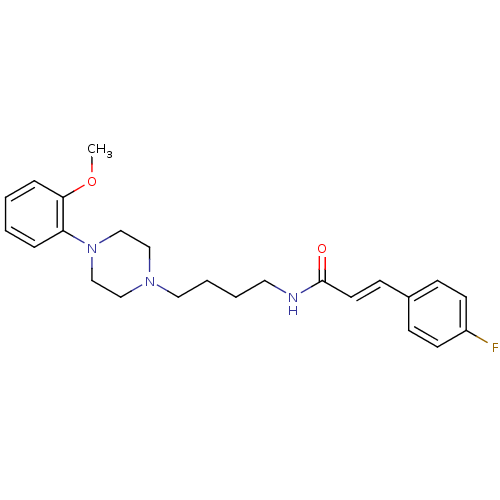 (3-(4-Fluoro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132047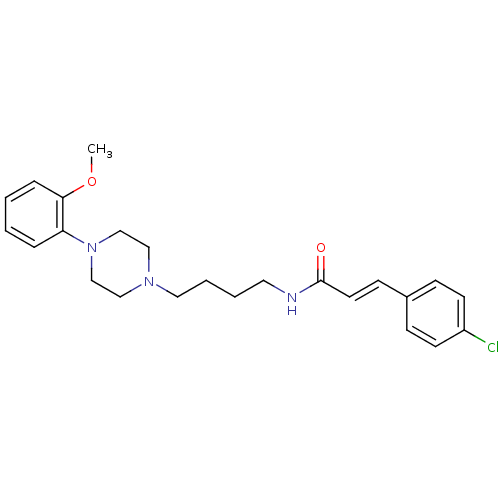 (3-(4-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50331549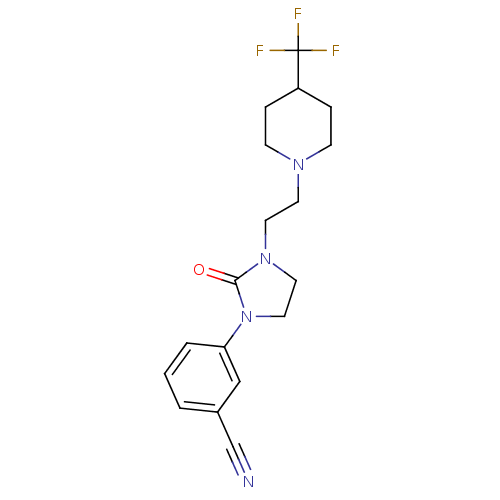 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177899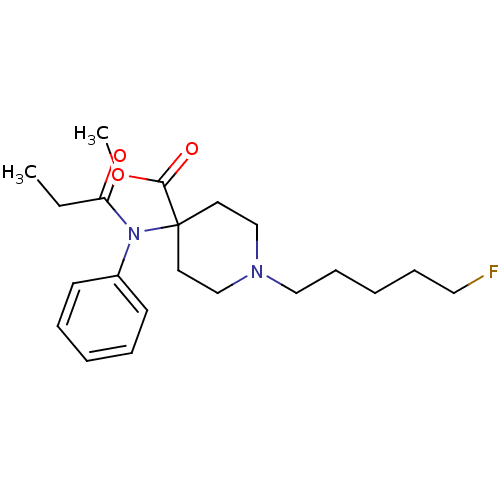 (CHEMBL371710 | methyl 4-[(N-1-oxopropyl)-N-phenyla...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177897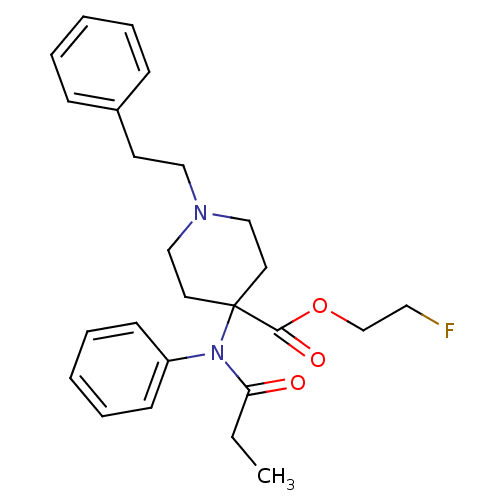 (2-fluoroethyl 1-(2-phenylethyl)-4-(N-phenylpropana...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50484947 (CHEMBL2017621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [3H]Pittsburgh compound B from human Abeta1-40 after 3 hrs by liquid scintillation counting | ACS Med Chem Lett 2: 673-7 (2011) Article DOI: 10.1021/ml200123w BindingDB Entry DOI: 10.7270/Q29889V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177894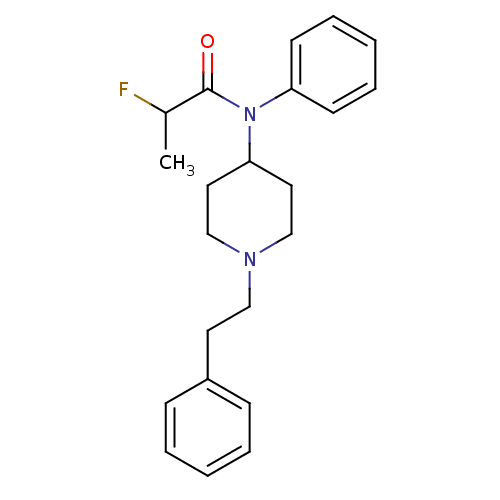 (2-Fluoro-N-(1-phenethyl-piperidin-4-yl)-N-phenyl-p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50177894 (2-Fluoro-N-(1-phenethyl-piperidin-4-yl)-N-phenyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Binding constant for Opioid receptor mu 1 in mouse | Bioorg Med Chem Lett 15: 1773-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.049 BindingDB Entry DOI: 10.7270/Q28916M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50484946 ((18F)AV-45 | 18F-AV-45 | AV-45 F-18 | Amyvid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Binding affinity to amyloid beta aggregates in postmortem human Alzheimer's disease brain homogenates after 2 hrs by gamma counting | ACS Med Chem Lett 2: 673-7 (2011) Article DOI: 10.1021/ml200123w BindingDB Entry DOI: 10.7270/Q29889V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50484947 (CHEMBL2017621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [3H]Pittsburgh compound B from human Abeta1-42 after 3 hrs by liquid scintillation counting | ACS Med Chem Lett 2: 673-7 (2011) Article DOI: 10.1021/ml200123w BindingDB Entry DOI: 10.7270/Q29889V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50483423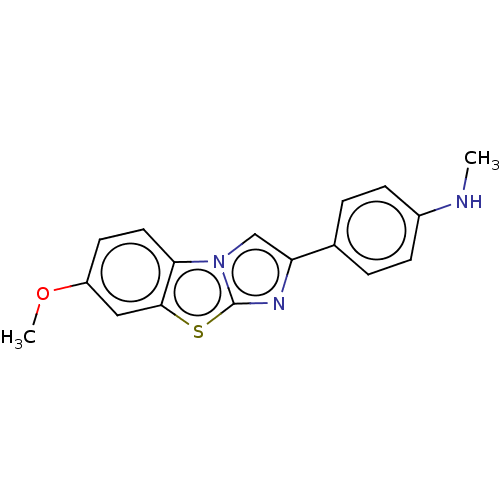 (CHEMBL1671878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [3H]PiB from human amyloid beta (1-40) at 100 nM after 180 mins by liquid scintillation counter | J Med Chem 54: 949-56 (2011) Article DOI: 10.1021/jm101129a BindingDB Entry DOI: 10.7270/Q2639SJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300442 ((20R)-4,5-alpha-Epoxy-17-methyl-3-hydroxy-6-methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biomedizinische Forschungsreagenzien GmbH Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human cloned delta opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting | J Med Chem 52: 5586-9 (2009) Article DOI: 10.1021/jm900892x BindingDB Entry DOI: 10.7270/Q2SQ90F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50483423 (CHEMBL1671878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [3H]PiB from human amyloid beta (1-42) at 100 nM after 180 mins by liquid scintillation counter | J Med Chem 54: 949-56 (2011) Article DOI: 10.1021/jm101129a BindingDB Entry DOI: 10.7270/Q2639SJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129793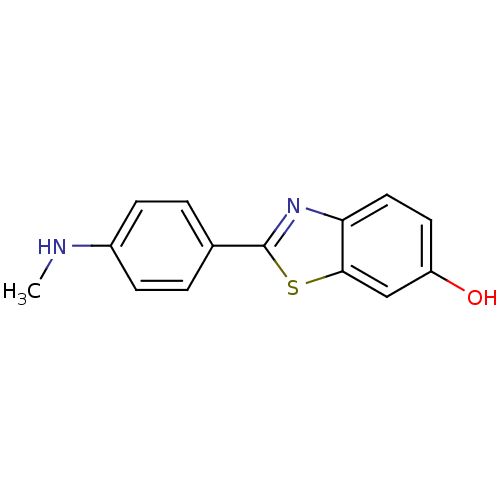 (2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [3H]PiB from human amyloid beta (1-40) at 100 nM after 180 mins by liquid scintillation counter | J Med Chem 54: 949-56 (2011) Article DOI: 10.1021/jm101129a BindingDB Entry DOI: 10.7270/Q2639SJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129793 (2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [3H]Pittsburgh compound B from human Abeta1-42 after 3 hrs by liquid scintillation counting | ACS Med Chem Lett 2: 673-7 (2011) Article DOI: 10.1021/ml200123w BindingDB Entry DOI: 10.7270/Q29889V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50331552 (CHEMBL1288585 | [11C]-(+)-(4aR,10bR)-4-propyl-3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Binding affinity to human dopamine D2 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129793 (2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [3H]Pittsburgh compound B from human Abeta1-40 after 3 hrs by liquid scintillation counting | ACS Med Chem Lett 2: 673-7 (2011) Article DOI: 10.1021/ml200123w BindingDB Entry DOI: 10.7270/Q29889V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129793 (2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [3H]PiB from human amyloid beta (1-42) at 100 nM after 180 mins by liquid scintillation counter | J Med Chem 54: 949-56 (2011) Article DOI: 10.1021/jm101129a BindingDB Entry DOI: 10.7270/Q2639SJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177896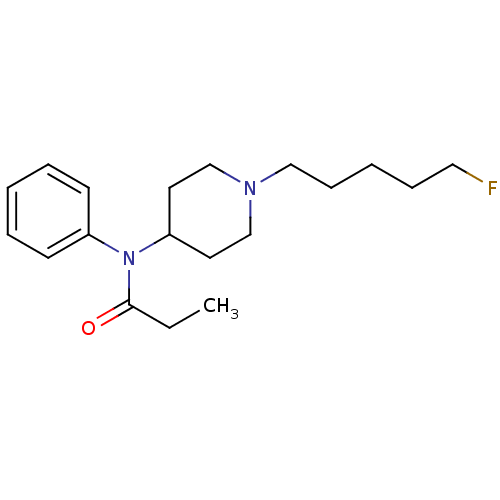 (CHEMBL371268 | N[1-(5-[18F]fluoropentyl)-4-piperid...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50132057 (CHEMBL1180430 | CHEMBL126128 | N-{4-[4-(2-Methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D2long receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50132069 (3-(4-Fluoro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D2short receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50132047 (3-(4-Chloro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D2long receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50132069 (3-(4-Fluoro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D2long receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50331549 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D2short receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50331549 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D2long receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (PIG) | BDBM50331549 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5-HT2 receptor in porcine cerebral cortex membranes | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Sus scrofa) | BDBM50331549 (CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]SCH 23990 from dopamine D1 receptor in porcine cerebral cortex membranes | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (PIG) | BDBM50132069 (3-(4-Fluoro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5-HT2 receptor in porcine cerebral cortex membranes | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Sus scrofa) | BDBM50132069 (3-(4-Fluoro-phenyl)-N-{4-[4-(2-methoxy-phenyl)-pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universität Erlangen-Nürnberg Curated by ChEMBL | Assay Description Displacement of [3H]SCH 23990 from dopamine D1 receptor in porcine cerebral cortex membranes | Bioorg Med Chem Lett 20: 6933-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.142 BindingDB Entry DOI: 10.7270/Q2474B38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-8 (Homo sapiens (Human)) | BDBM50530090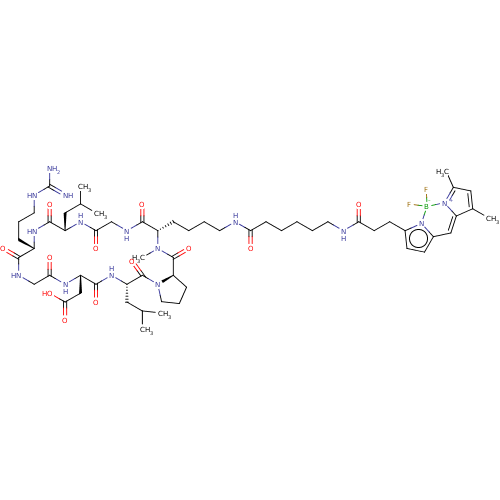 (CHEMBL4443665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of LAP binding to human integrin alphavbeta8 receptor after 1 hr by solid-phase binding assay | J Med Chem 62: 2024-2037 (2019) Article DOI: 10.1021/acs.jmedchem.8b01588 BindingDB Entry DOI: 10.7270/Q26T0R53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-8 (Homo sapiens (Human)) | BDBM50530090 (CHEMBL4443665) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of LAP binding to human integrin alphavbeta8 receptor after 1 hr by solid-phase binding assay | J Med Chem 62: 2024-2037 (2019) Article DOI: 10.1021/acs.jmedchem.8b01588 BindingDB Entry DOI: 10.7270/Q26T0R53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50165164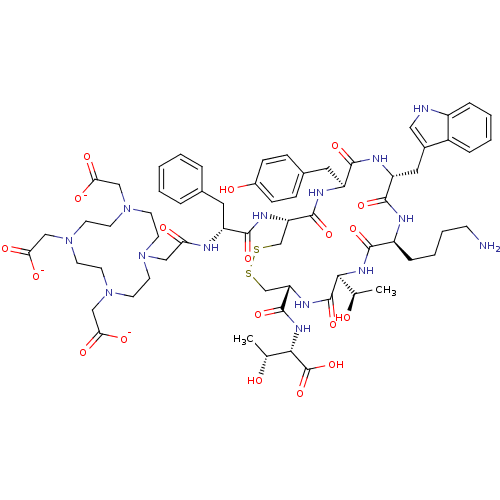 (CHEMBL262135 | CHEMBL430066 | Radiolabeled octreot...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description In vitro inhibition of [125I][Leu8,D-Trp22,Tyr25] somatostatin 28 binding to human somatostatin receptor type 2 expressed in CCL39 cells; (n=3) | J Med Chem 48: 2778-89 (2005) Article DOI: 10.1021/jm040794i BindingDB Entry DOI: 10.7270/Q26T0ND0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50530099 (CHEMBL4441624) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of LAP binding to human integrin alphavbeta6 receptor after 1 hr by solid-phase binding assay | J Med Chem 62: 2024-2037 (2019) Article DOI: 10.1021/acs.jmedchem.8b01588 BindingDB Entry DOI: 10.7270/Q26T0R53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50530099 (CHEMBL4441624) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of LAP binding to human integrin alphavbeta6 receptor after 1 hr by solid-phase binding assay | J Med Chem 62: 2024-2037 (2019) Article DOI: 10.1021/acs.jmedchem.8b01588 BindingDB Entry DOI: 10.7270/Q26T0R53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50165167 (4-[(4R,7S,10S,13R,16S)-19-(2-azaniumyl-3-phenylpro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description In vitro inhibition of [125I][Leu8,D-Trp22,Tyr25] somatostatin 28 binding to human somatostatin receptor type 2 expressed in CCL39 cells; (n=3) | J Med Chem 48: 2778-89 (2005) Article DOI: 10.1021/jm040794i BindingDB Entry DOI: 10.7270/Q26T0ND0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50530110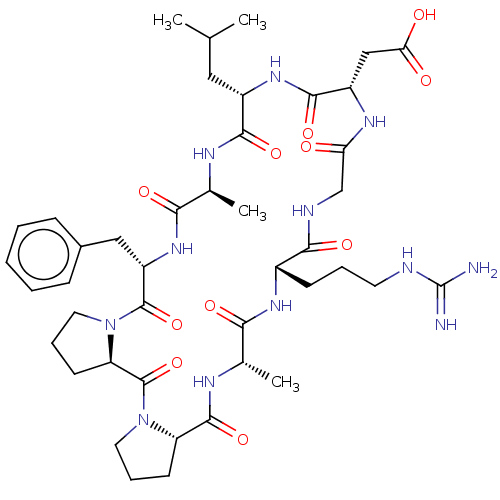 (CHEMBL4575627) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of LAP binding to human integrin alphavbeta6 receptor after 1 hr by solid-phase binding assay | J Med Chem 62: 2024-2037 (2019) Article DOI: 10.1021/acs.jmedchem.8b01588 BindingDB Entry DOI: 10.7270/Q26T0R53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50530110 (CHEMBL4575627) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of LAP binding to human integrin alphavbeta6 receptor after 1 hr by solid-phase binding assay | J Med Chem 62: 2024-2037 (2019) Article DOI: 10.1021/acs.jmedchem.8b01588 BindingDB Entry DOI: 10.7270/Q26T0R53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50165159 (4-[(4R,7S,10S,13R,16S)-4-{[(1S)-1-carboxy-2-hydrox...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description In vitro inhibition of [125I][Leu8,D-Trp22,Tyr25] somatostatin 28 binding to human somatostatin receptor type 2 expressed in CCL39 cells; (n=3) | J Med Chem 48: 2778-89 (2005) Article DOI: 10.1021/jm040794i BindingDB Entry DOI: 10.7270/Q26T0ND0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50165180 (4-[(4R,7S,10S,13R,16S)-4-{[(1S)-1-carboxy-2-hydrox...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description In vitro inhibition of [125I][Leu8,D-Trp22,Tyr25] somatostatin 28 binding to human somatostatin receptor type 2 expressed in CCL39 cells; (n=3) | J Med Chem 48: 2778-89 (2005) Article DOI: 10.1021/jm040794i BindingDB Entry DOI: 10.7270/Q26T0ND0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50165174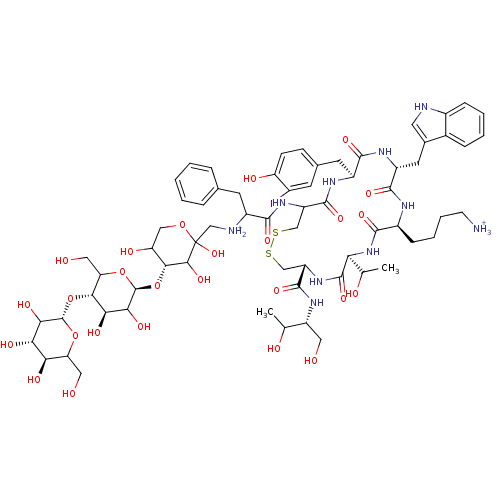 (4-[(4R,7S,10S,13R,16S)-19-[2-({[(4R)-4-{[(2S,4R,5S...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description In vitro inhibition of [125I][Leu8,D-Trp22,Tyr25] somatostatin 28 binding to human somatostatin receptor type 2 expressed in CCL39 cells; (n=3) | J Med Chem 48: 2778-89 (2005) Article DOI: 10.1021/jm040794i BindingDB Entry DOI: 10.7270/Q26T0ND0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50165160 (4-[(4R,7S,10S,13R,16S)-4-{[(2R)-1,3-dihydroxybutan...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description In vitro inhibition of [125I][Leu8,D-Trp22,Tyr25] somatostatin 28 binding to human somatostatin receptor type 2 expressed in CCL39 cells; (n=3) | J Med Chem 48: 2778-89 (2005) Article DOI: 10.1021/jm040794i BindingDB Entry DOI: 10.7270/Q26T0ND0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-8 (Homo sapiens (Human)) | BDBM50530119 (CHEMBL4449438) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of LAP binding to human integrin alphavbeta8 receptor after 1 hr by solid-phase binding assay | J Med Chem 62: 2024-2037 (2019) Article DOI: 10.1021/acs.jmedchem.8b01588 BindingDB Entry DOI: 10.7270/Q26T0R53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-8 (Homo sapiens (Human)) | BDBM50530119 (CHEMBL4449438) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Inhibition of LAP binding to human integrin alphavbeta8 receptor after 1 hr by solid-phase binding assay | J Med Chem 62: 2024-2037 (2019) Article DOI: 10.1021/acs.jmedchem.8b01588 BindingDB Entry DOI: 10.7270/Q26T0R53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 420 total ) | Next | Last >> |