 Found 2797 hits with Last Name = 'westlin' and Initial = 'w'
Found 2797 hits with Last Name = 'westlin' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332992
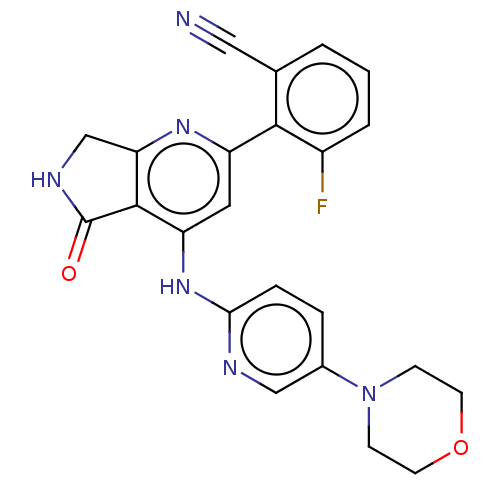
(US10196390, Compound I-281)Show SMILES Fc1cccc(C#N)c1-c1cc(Nc2ccc(cn2)N2CCOCC2)c2C(=O)NCc2n1 Show InChI InChI=1S/C23H19FN6O2/c24-16-3-1-2-14(11-25)21(16)17-10-18(22-19(28-17)13-27-23(22)31)29-20-5-4-15(12-26-20)30-6-8-32-9-7-30/h1-5,10,12H,6-9,13H2,(H,27,31)(H,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332893
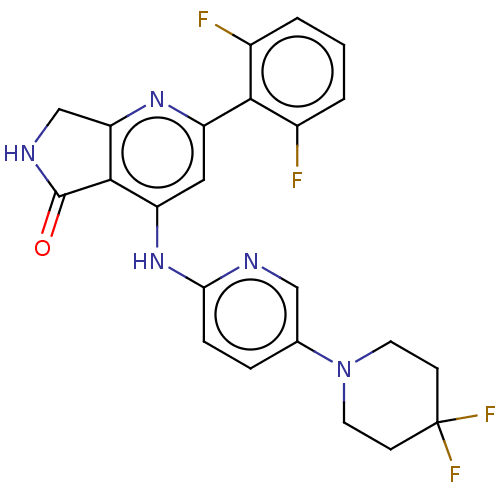
(US10196390, Compound I-182)Show SMILES Fc1cccc(F)c1-c1cc(Nc2ccc(cn2)N2CCC(F)(F)CC2)c2C(=O)NCc2n1 Show InChI InChI=1S/C23H19F4N5O/c24-14-2-1-3-15(25)20(14)16-10-17(21-18(30-16)12-29-22(21)33)31-19-5-4-13(11-28-19)32-8-6-23(26,27)7-9-32/h1-5,10-11H,6-9,12H2,(H,29,33)(H,28,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332847
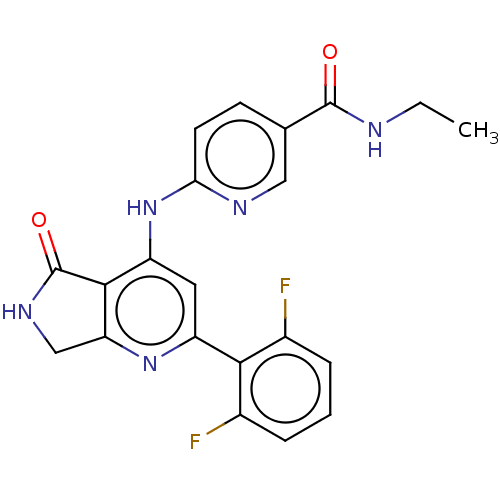
(US10196390, Compound I-136)Show SMILES CCNC(=O)c1ccc(Nc2cc(nc3CNC(=O)c23)-c2c(F)cccc2F)nc1 Show InChI InChI=1S/C21H17F2N5O2/c1-2-24-20(29)11-6-7-17(25-9-11)28-15-8-14(18-12(22)4-3-5-13(18)23)27-16-10-26-21(30)19(15)16/h3-9H,2,10H2,1H3,(H,24,29)(H,26,30)(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50589369

(CHEMBL5197513)Show SMILES Fc1cccc(F)c1-c1cc(Nc2ccc(cn2)C(=O)N2CCCC2)c2C(=O)NCc2n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332858

(US10196390, Compound I-147)Show SMILES OC1CCN(CC1)c1ccc(Nc2cc(nc3CNC(=O)c23)-c2c(F)cccc2F)nc1 Show InChI InChI=1S/C23H21F2N5O2/c24-15-2-1-3-16(25)21(15)17-10-18(22-19(28-17)12-27-23(22)32)29-20-5-4-13(11-26-20)30-8-6-14(31)7-9-30/h1-5,10-11,14,31H,6-9,12H2,(H,27,32)(H,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332993
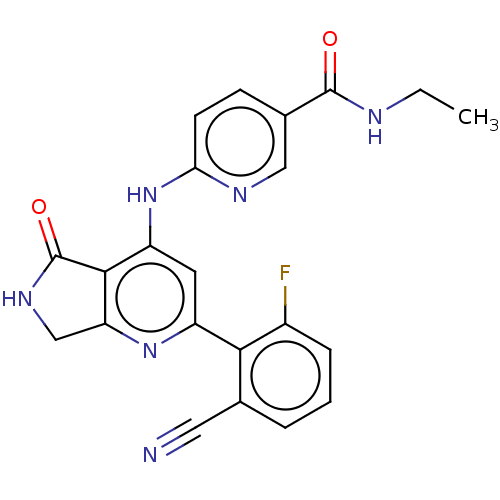
(US10196390, Compound I-282)Show SMILES CCNC(=O)c1ccc(Nc2cc(nc3CNC(=O)c23)-c2c(F)cccc2C#N)nc1 Show InChI InChI=1S/C22H17FN6O2/c1-2-25-21(30)13-6-7-18(26-10-13)29-16-8-15(28-17-11-27-22(31)20(16)17)19-12(9-24)4-3-5-14(19)23/h3-8,10H,2,11H2,1H3,(H,25,30)(H,27,31)(H,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM490686
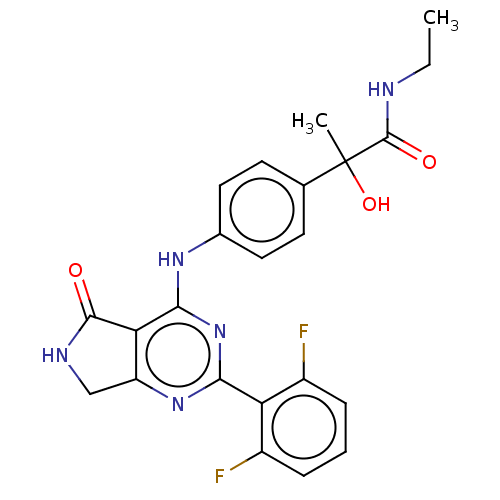
(US10968236, Compound I-14)Show SMILES CCNC(=O)C(C)(O)c1ccc(Nc2nc(nc3CNC(=O)c23)-c2c(F)cccc2F)cc1 Show InChI InChI=1S/C23H21F2N5O3/c1-3-26-22(32)23(2,33)12-7-9-13(10-8-12)28-20-18-16(11-27-21(18)31)29-19(30-20)17-14(24)5-4-6-15(17)25/h4-10,33H,3,11H2,1-2H3,(H,26,32)(H,27,31)(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM490686

(US10968236, Compound I-14)Show SMILES CCNC(=O)C(C)(O)c1ccc(Nc2nc(nc3CNC(=O)c23)-c2c(F)cccc2F)cc1 Show InChI InChI=1S/C23H21F2N5O3/c1-3-26-22(32)23(2,33)12-7-9-13(10-8-12)28-20-18-16(11-27-21(18)31)29-19(30-20)17-14(24)5-4-6-15(17)25/h4-10,33H,3,11H2,1-2H3,(H,26,32)(H,27,31)(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332959
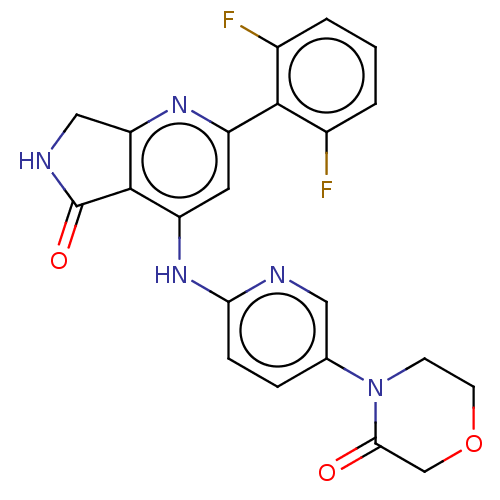
(US10196390, Compound I-248)Show SMILES Fc1cccc(F)c1-c1cc(Nc2ccc(cn2)N2CCOCC2=O)c2C(=O)NCc2n1 Show InChI InChI=1S/C22H17F2N5O3/c23-13-2-1-3-14(24)20(13)15-8-16(21-17(27-15)10-26-22(21)31)28-18-5-4-12(9-25-18)29-6-7-32-11-19(29)30/h1-5,8-9H,6-7,10-11H2,(H,26,31)(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332942

(US10196390, Compound I-231 | US10336752, Compound ...)Show SMILES Fc1cccc(F)c1-c1cc(Nc2ccc(cc2)C(=O)N2CCOCC2)c2C(=O)NCc2n1 Show InChI InChI=1S/C24H20F2N4O3/c25-16-2-1-3-17(26)21(16)18-12-19(22-20(29-18)13-27-23(22)31)28-15-6-4-14(5-7-15)24(32)30-8-10-33-11-9-30/h1-7,12H,8-11,13H2,(H,27,31)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332882
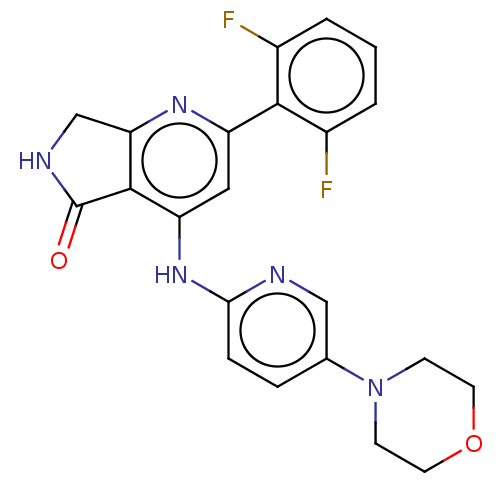
(US10196390, Compound I-171 | US10336752, Compound ...)Show SMILES Fc1cccc(F)c1-c1cc(Nc2ccc(cn2)N2CCOCC2)c2C(=O)NCc2n1 Show InChI InChI=1S/C22H19F2N5O2/c23-14-2-1-3-15(24)20(14)16-10-17(21-18(27-16)12-26-22(21)30)28-19-5-4-13(11-25-19)29-6-8-31-9-7-29/h1-5,10-11H,6-9,12H2,(H,26,30)(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50589370
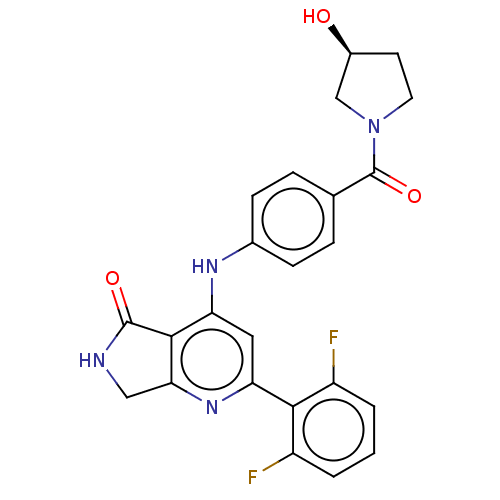
(CHEMBL5182922)Show SMILES O[C@H]1CCN(C1)C(=O)c1ccc(Nc2cc(nc3CNC(=O)c23)-c2c(F)cccc2F)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM406663
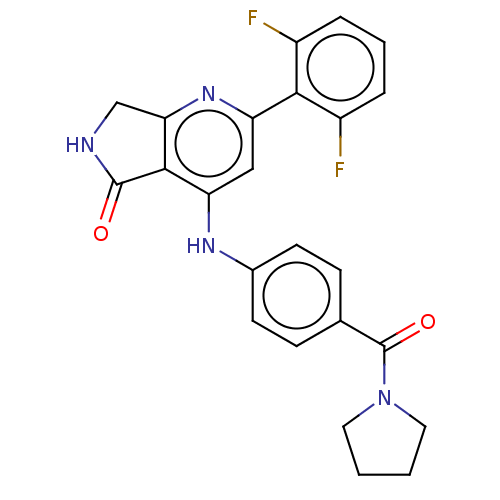
(US10336752, Compound 1 | US10336752, Compound 2 | ...)Show SMILES Fc1cccc(F)c1-c1cc(Nc2ccc(cc2)C(=O)N2CCCC2)c2C(=O)NCc2n1 Show InChI InChI=1S/C24H20F2N4O2/c25-16-4-3-5-17(26)21(16)18-12-19(22-20(29-18)13-27-23(22)31)28-15-8-6-14(7-9-15)24(32)30-10-1-2-11-30/h3-9,12H,1-2,10-11,13H2,(H,27,31)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332923
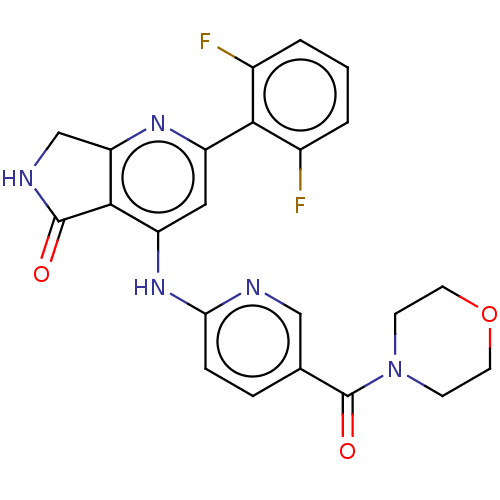
(US10196390, Compound I-212)Show SMILES Fc1cccc(F)c1-c1cc(Nc2ccc(cn2)C(=O)N2CCOCC2)c2C(=O)NCc2n1 Show InChI InChI=1S/C23H19F2N5O3/c24-14-2-1-3-15(25)20(14)16-10-17(21-18(28-16)12-27-22(21)31)29-19-5-4-13(11-26-19)23(32)30-6-8-33-9-7-30/h1-5,10-11H,6-9,12H2,(H,27,31)(H,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50589368

(CHEMBL5182121)Show SMILES Fc1cccc(F)c1-c1nc2CNC(=O)c2c(Nc2ccc(cc2)C2CCNC2=O)n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50589368

(CHEMBL5182121)Show SMILES Fc1cccc(F)c1-c1nc2CNC(=O)c2c(Nc2ccc(cc2)C2CCNC2=O)n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332805

(US10196390, Compound I-78)Show SMILES FC(F)CN1CCN(CC1)C(=O)Cc1ccc(Nc2nc(nc3CNC(=O)c23)-c2c(F)cccc2F)cc1 Show InChI InChI=1S/C26H24F4N6O2/c27-17-2-1-3-18(28)22(17)24-33-19-13-31-26(38)23(19)25(34-24)32-16-6-4-15(5-7-16)12-21(37)36-10-8-35(9-11-36)14-20(29)30/h1-7,20H,8-14H2,(H,31,38)(H,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332857
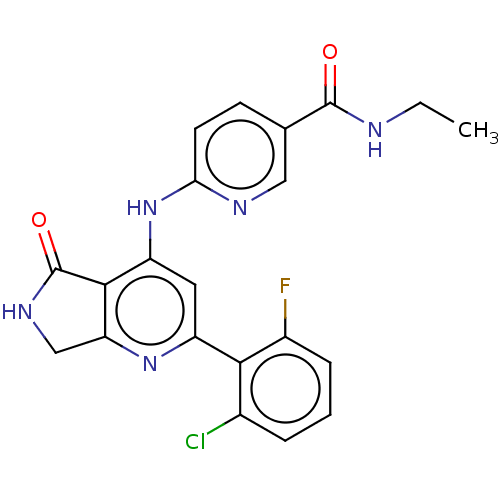
(US10196390, Compound I-146)Show SMILES CCNC(=O)c1ccc(Nc2cc(nc3CNC(=O)c23)-c2c(F)cccc2Cl)nc1 Show InChI InChI=1S/C21H17ClFN5O2/c1-2-24-20(29)11-6-7-17(25-9-11)28-15-8-14(18-12(22)4-3-5-13(18)23)27-16-10-26-21(30)19(15)16/h3-9H,2,10H2,1H3,(H,24,29)(H,26,30)(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332816

(US10196390, Compound I-88)Show SMILES CCNC(=O)Cc1ccc(Nc2cc(nc3CNC(=O)c23)-c2c(F)cccc2F)nc1 Show InChI InChI=1S/C22H19F2N5O2/c1-2-25-19(30)8-12-6-7-18(26-10-12)29-16-9-15(20-13(23)4-3-5-14(20)24)28-17-11-27-22(31)21(16)17/h3-7,9-10H,2,8,11H2,1H3,(H,25,30)(H,27,31)(H,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332864

(US10196390, Compound I-153)Show SMILES Fc1cccc(F)c1-c1nc2CNC(=O)c2c(Nc2ccc(cc2)C2CCCNC2=O)n1 Show InChI InChI=1S/C23H19F2N5O2/c24-15-4-1-5-16(25)18(15)20-29-17-11-27-23(32)19(17)21(30-20)28-13-8-6-12(7-9-13)14-3-2-10-26-22(14)31/h1,4-9,14H,2-3,10-11H2,(H,26,31)(H,27,32)(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332864

(US10196390, Compound I-153)Show SMILES Fc1cccc(F)c1-c1nc2CNC(=O)c2c(Nc2ccc(cc2)C2CCCNC2=O)n1 Show InChI InChI=1S/C23H19F2N5O2/c24-15-4-1-5-16(25)18(15)20-29-17-11-27-23(32)19(17)21(30-20)28-13-8-6-12(7-9-13)14-3-2-10-26-22(14)31/h1,4-9,14H,2-3,10-11H2,(H,26,31)(H,27,32)(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332794
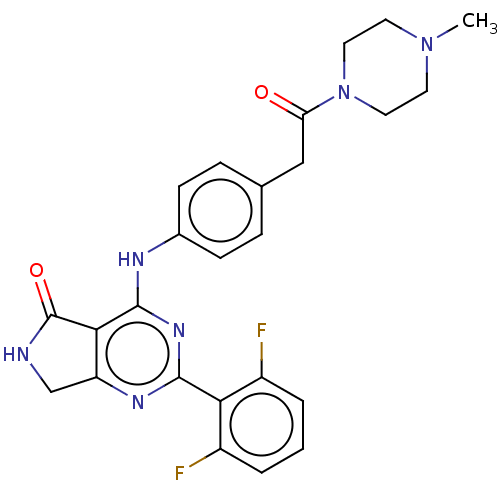
(US10196390, Compound I-67)Show SMILES CN1CCN(CC1)C(=O)Cc1ccc(Nc2nc(nc3CNC(=O)c23)-c2c(F)cccc2F)cc1 Show InChI InChI=1S/C25H24F2N6O2/c1-32-9-11-33(12-10-32)20(34)13-15-5-7-16(8-6-15)29-24-22-19(14-28-25(22)35)30-23(31-24)21-17(26)3-2-4-18(21)27/h2-8H,9-14H2,1H3,(H,28,35)(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50589366
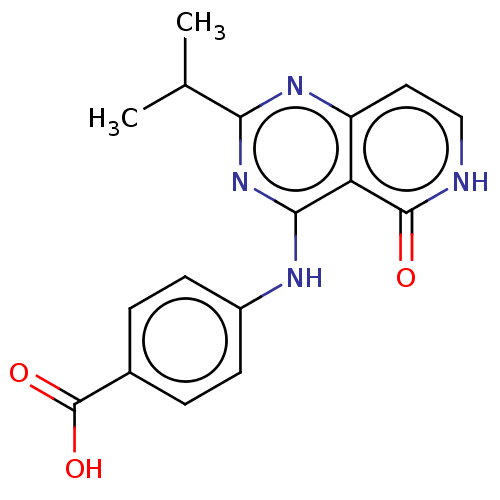
(CHEMBL5203504)Show SMILES CC(C)c1nc(Nc2ccc(cc2)C(O)=O)c2c(cc[nH]c2=O)n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332793
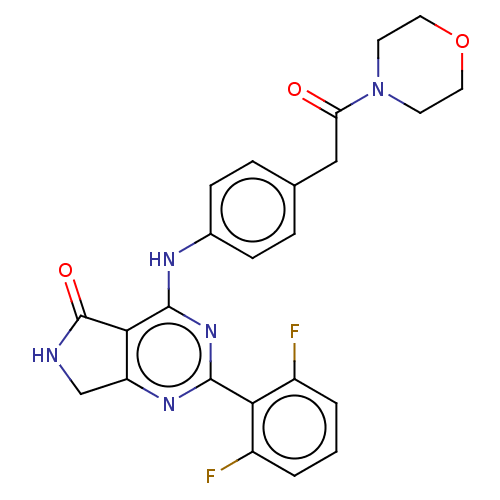
(US10196390, Compound I-66)Show SMILES Fc1cccc(F)c1-c1nc2CNC(=O)c2c(Nc2ccc(CC(=O)N3CCOCC3)cc2)n1 Show InChI InChI=1S/C24H21F2N5O3/c25-16-2-1-3-17(26)20(16)22-29-18-13-27-24(33)21(18)23(30-22)28-15-6-4-14(5-7-15)12-19(32)31-8-10-34-11-9-31/h1-7H,8-13H2,(H,27,33)(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50589367
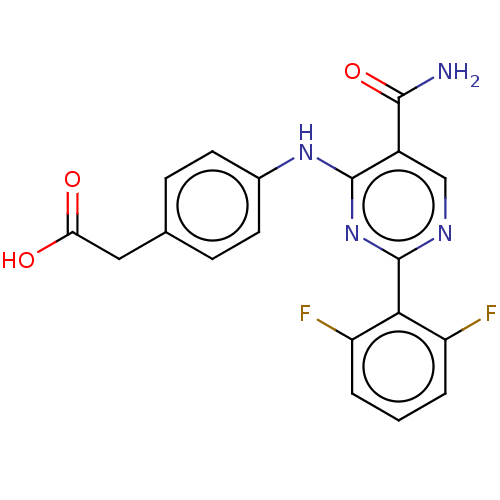
(CHEMBL5170799)Show SMILES NC(=O)c1cnc(nc1Nc1ccc(CC(O)=O)cc1)-c1c(F)cccc1F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332807

(US10196390, Compound I-80)Show SMILES CCNC(=O)Cc1ccc(Nc2nc(nc3CNC(=O)c23)-c2c(F)cccc2F)cc1 Show InChI InChI=1S/C22H19F2N5O2/c1-2-25-17(30)10-12-6-8-13(9-7-12)27-21-19-16(11-26-22(19)31)28-20(29-21)18-14(23)4-3-5-15(18)24/h3-9H,2,10-11H2,1H3,(H,25,30)(H,26,31)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM332820
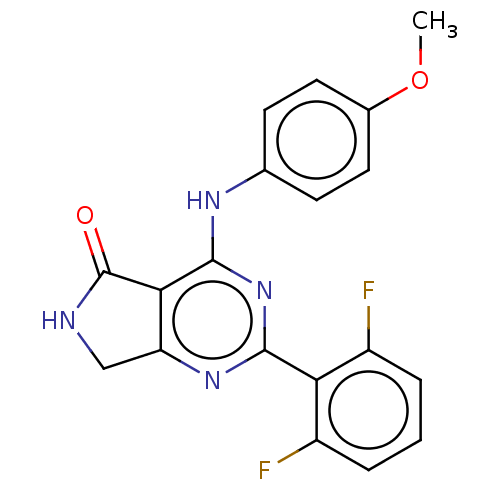
(US10196390, Compound I-110)Show SMILES COc1ccc(Nc2nc(nc3CNC(=O)c23)-c2c(F)cccc2F)cc1 Show InChI InChI=1S/C19H14F2N4O2/c1-27-11-7-5-10(6-8-11)23-18-16-14(9-22-19(16)26)24-17(25-18)15-12(20)3-2-4-13(15)21/h2-8H,9H2,1H3,(H,22,26)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50589365

(CHEMBL5196418) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc.
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 983-7 (2005)
Article DOI: 10.1016/j.bmcl.2022.128891
BindingDB Entry DOI: 10.7270/Q2P84GV3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088527
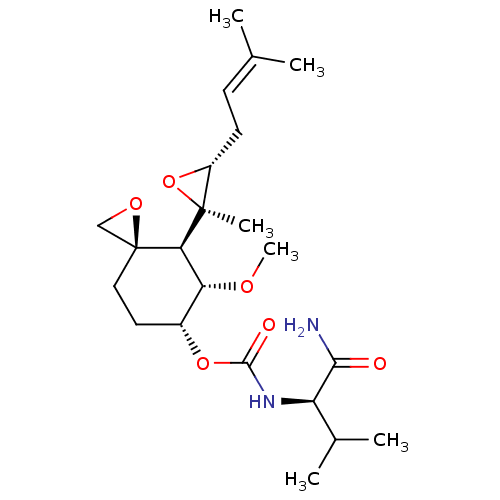
(CHEMBL3527358)Show SMILES [H][C@@]1([#6@H](-[#8]-[#6])-[#6@@H](-[#6]-[#6][C@]11[#6]-[#8]1)-[#8]-[#6](=O)-[#7]-[#6@H](-[#6](-[#6])-[#6])-[#6](-[#7])=O)[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C22H36N2O6/c1-12(2)7-8-15-21(5,30-15)18-17(27-6)14(9-10-22(18)11-28-22)29-20(26)24-16(13(3)4)19(23)25/h7,13-18H,8-11H2,1-6H3,(H2,23,25)(H,24,26)/t14-,15-,16-,17-,18-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088527

(CHEMBL3527358)Show SMILES [H][C@@]1([#6@H](-[#8]-[#6])-[#6@@H](-[#6]-[#6][C@]11[#6]-[#8]1)-[#8]-[#6](=O)-[#7]-[#6@H](-[#6](-[#6])-[#6])-[#6](-[#7])=O)[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C22H36N2O6/c1-12(2)7-8-15-21(5,30-15)18-17(27-6)14(9-10-22(18)11-28-22)29-20(26)24-16(13(3)4)19(23)25/h7,13-18H,8-11H2,1-6H3,(H2,23,25)(H,24,26)/t14-,15-,16-,17-,18-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088527

(CHEMBL3527358)Show SMILES [H][C@@]1([#6@H](-[#8]-[#6])-[#6@@H](-[#6]-[#6][C@]11[#6]-[#8]1)-[#8]-[#6](=O)-[#7]-[#6@H](-[#6](-[#6])-[#6])-[#6](-[#7])=O)[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C22H36N2O6/c1-12(2)7-8-15-21(5,30-15)18-17(27-6)14(9-10-22(18)11-28-22)29-20(26)24-16(13(3)4)19(23)25/h7,13-18H,8-11H2,1-6H3,(H2,23,25)(H,24,26)/t14-,15-,16-,17-,18-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277149
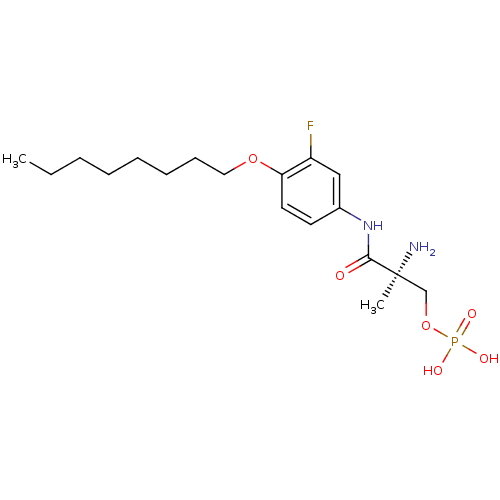
((S)-2-amino-3-(3-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)cc1F |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-16-10-9-14(12-15(16)19)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249294

((R)-2-amino-2-(4-(4-(5-phenylpentyloxy)phenyl)-1H-...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C23H30N3O5P/c1-23(24,17-31-32(27,28)29)22-25-16-21(26-22)19-11-13-20(14-12-19)30-15-7-3-6-10-18-8-4-2-5-9-18/h2,4-5,8-9,11-14,16H,3,6-7,10,15,17,24H2,1H3,(H,25,26)(H2,27,28,29)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50185450
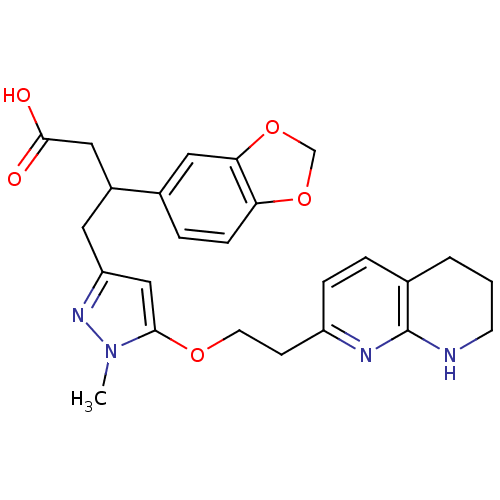
(3-(benzo[d][1,3]dioxol-5-yl)-4-(1-methyl-5-(2-(5,6...)Show SMILES Cn1nc(CC(CC(O)=O)c2ccc3OCOc3c2)cc1OCCc1ccc2CCCNc2n1 Show InChI InChI=1S/C25H28N4O5/c1-29-23(32-10-8-19-6-4-16-3-2-9-26-25(16)27-19)14-20(28-29)11-18(13-24(30)31)17-5-7-21-22(12-17)34-15-33-21/h4-7,12,14,18H,2-3,8-11,13,15H2,1H3,(H,26,27)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of alphavbeta3 integrin in 293 cells |
Bioorg Med Chem Lett 16: 3156-61 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.045
BindingDB Entry DOI: 10.7270/Q29023CB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50249114
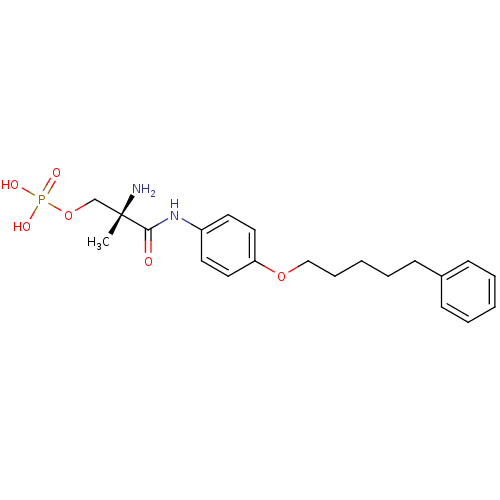
((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C21H29N2O6P/c1-21(22,16-29-30(25,26)27)20(24)23-18-11-13-19(14-12-18)28-15-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,11-14H,3,6-7,10,15-16,22H2,1H3,(H,23,24)(H2,25,26,27)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315562
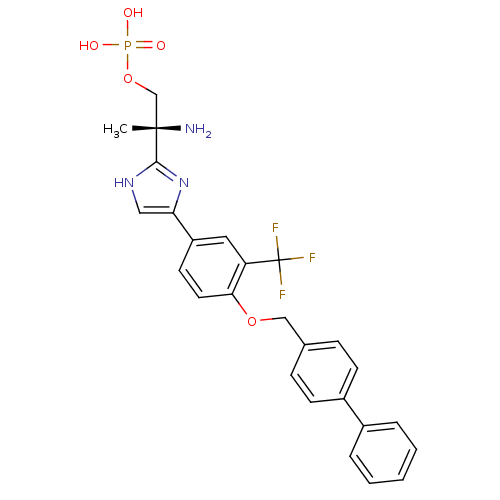
((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...)Show SMILES C[C@](N)(COP(O)(O)=O)c1nc(c[nH]1)-c1ccc(OCc2ccc(cc2)-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C26H25F3N3O5P/c1-25(30,16-37-38(33,34)35)24-31-14-22(32-24)20-11-12-23(21(13-20)26(27,28)29)36-15-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-14H,15-16,30H2,1H3,(H,31,32)(H2,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
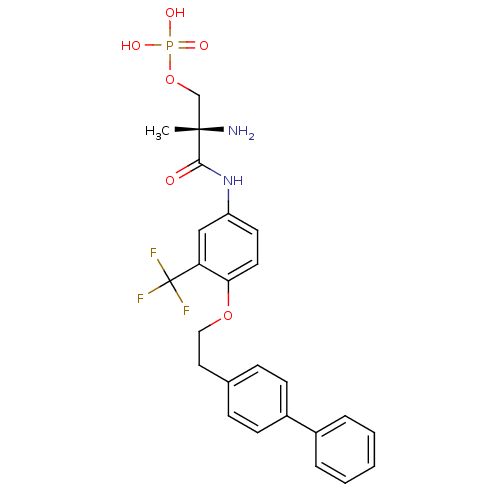
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50315559
((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCc2ccc(cc2)-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C25H26F3N2O6P/c1-24(29,16-36-37(32,33)34)23(31)30-20-11-12-22(21(15-20)25(26,27)28)35-14-13-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h2-12,15H,13-14,16,29H2,1H3,(H,30,31)(H2,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2520-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.098
BindingDB Entry DOI: 10.7270/Q2XS5VJB |
More data for this
Ligand-Target Pair | |
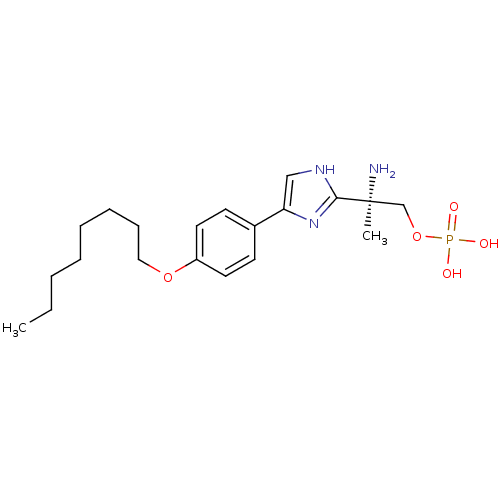
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277186
((R)-2-amino-2-(4-(4-(octyloxy)phenyl)-1H-imidazol-...)Show SMILES CCCCCCCCOc1ccc(cc1)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H32N3O5P/c1-3-4-5-6-7-8-13-27-17-11-9-16(10-12-17)18-14-22-19(23-18)20(2,21)15-28-29(24,25)26/h9-12,14H,3-8,13,15,21H2,1-2H3,(H,22,23)(H2,24,25,26)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
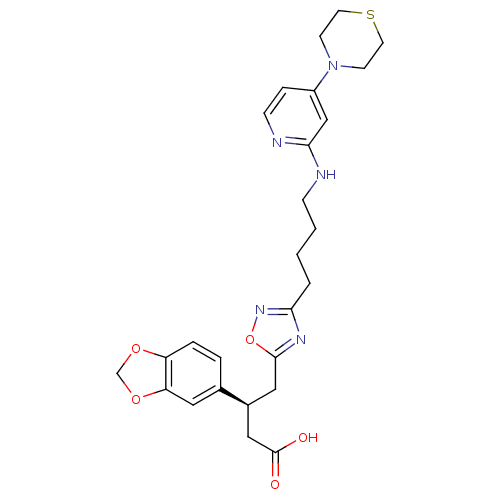
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50177621
((S)-3-(benzo[d][1,3]dioxol-5-yl)-4-(3-(4-(4-thiomo...)Show SMILES OC(=O)C[C@H](Cc1nc(CCCCNc2cc(ccn2)N2CCSCC2)no1)c1ccc2OCOc2c1 Show InChI InChI=1S/C26H31N5O5S/c32-26(33)15-19(18-4-5-21-22(13-18)35-17-34-21)14-25-29-23(30-36-25)3-1-2-7-27-24-16-20(6-8-28-24)31-9-11-37-12-10-31/h4-6,8,13,16,19H,1-3,7,9-12,14-15,17H2,(H,27,28)(H,32,33)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Integrin alphav-beta3 receptor by SPRA assay |
Bioorg Med Chem Lett 16: 839-44 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.008
BindingDB Entry DOI: 10.7270/Q28K78NF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50177626

(3-(benzo[d]oxazol-6-yl)-4-(3-(3-(5,6,7,8-tetrahydr...)Show SMILES OC(=O)CC(Cc1nc(CCCc2ccc3CCCNc3n2)no1)c1ccc2ncoc2c1 Show InChI InChI=1S/C24H25N5O4/c30-23(31)13-17(16-7-9-19-20(11-16)32-14-26-19)12-22-28-21(29-33-22)5-1-4-18-8-6-15-3-2-10-25-24(15)27-18/h6-9,11,14,17H,1-5,10,12-13H2,(H,25,27)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Integrin alphav-beta3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 839-44 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.008
BindingDB Entry DOI: 10.7270/Q28K78NF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50185464
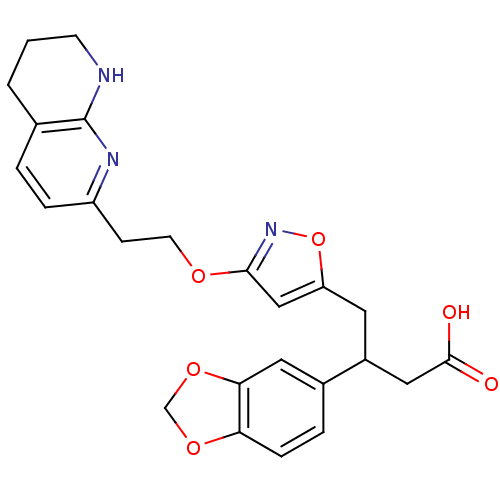
(3-(benzo[d][1,3]dioxol-5-yl)-4-(3-(2-(5,6,7,8-tetr...)Show SMILES OC(=O)CC(Cc1cc(OCCc2ccc3CCCNc3n2)no1)c1ccc2OCOc2c1 Show InChI InChI=1S/C24H25N3O6/c28-23(29)12-17(16-4-6-20-21(11-16)32-14-31-20)10-19-13-22(27-33-19)30-9-7-18-5-3-15-2-1-8-25-24(15)26-18/h3-6,11,13,17H,1-2,7-10,12,14H2,(H,25,26)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of alphavbeta3 integrin in 293 cells |
Bioorg Med Chem Lett 16: 3156-61 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.045
BindingDB Entry DOI: 10.7270/Q29023CB |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277185
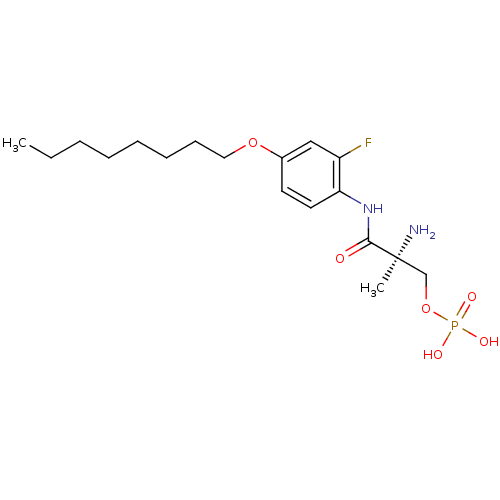
((S)-2-amino-3-(2-fluoro-4-(octyloxy)phenylamino)-2...)Show SMILES CCCCCCCCOc1ccc(NC(=O)[C@@](C)(N)COP(O)(O)=O)c(F)c1 |r| Show InChI InChI=1S/C18H30FN2O6P/c1-3-4-5-6-7-8-11-26-14-9-10-16(15(19)12-14)21-17(22)18(2,20)13-27-28(23,24)25/h9-10,12H,3-8,11,13,20H2,1-2H3,(H,21,22)(H2,23,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50277187
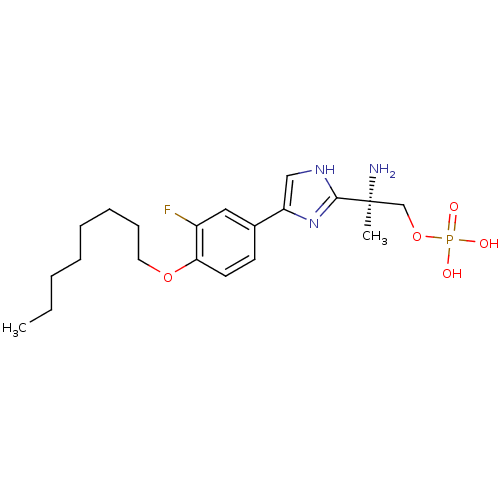
((R)-2-amino-2-(4-(3-fluoro-4-(octyloxy)phenyl)-1H-...)Show SMILES CCCCCCCCOc1ccc(cc1F)-c1c[nH]c(n1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H31FN3O5P/c1-3-4-5-6-7-8-11-28-18-10-9-15(12-16(18)21)17-13-23-19(24-17)20(2,22)14-29-30(25,26)27/h9-10,12-13H,3-8,11,14,22H2,1-2H3,(H,23,24)(H2,25,26,27)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor |
Bioorg Med Chem Lett 19: 369-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.072
BindingDB Entry DOI: 10.7270/Q2H70FNK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50249114

((S)-2-amino-2-methyl-3-oxo-3-(4-(5-phenylpentyloxy...)Show SMILES C[C@](N)(COP(O)(O)=O)C(=O)Nc1ccc(OCCCCCc2ccccc2)cc1 |r| Show InChI InChI=1S/C21H29N2O6P/c1-21(22,16-29-30(25,26)27)20(24)23-18-11-13-19(14-12-18)28-15-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,11-14H,3,6-7,10,15-16,22H2,1H3,(H,23,24)(H2,25,26,27)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor |
Bioorg Med Chem Lett 19: 2315-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.073
BindingDB Entry DOI: 10.7270/Q2XD11KJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50177628
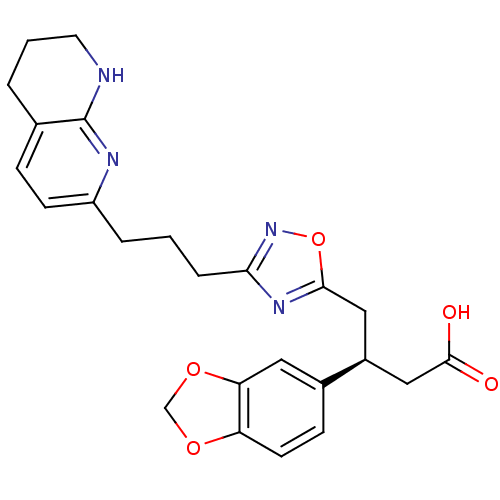
((S)-3-(benzo[d][1,3]dioxol-5-yl)-4-(3-(3-(5,6,7,8-...)Show SMILES OC(=O)C[C@H](Cc1nc(CCCc2ccc3CCCNc3n2)no1)c1ccc2OCOc2c1 Show InChI InChI=1S/C24H26N4O5/c29-23(30)13-17(16-7-9-19-20(11-16)32-14-31-19)12-22-27-21(28-33-22)5-1-4-18-8-6-15-3-2-10-25-24(15)26-18/h6-9,11,17H,1-5,10,12-14H2,(H,25,26)(H,29,30)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Integrin alphav-beta3 receptor by SPRA assay |
Bioorg Med Chem Lett 16: 839-44 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.008
BindingDB Entry DOI: 10.7270/Q28K78NF |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50209124

((2,2-dimethyl-3-{4-[2-(5,6,7,8-tetrahydro-1,8-naph...)Show SMILES CC1(C)C(CC(O)=O)C1c1ccc(OCCc2ccc3CCCNc3n2)cc1 |w:8.9,3.3| Show InChI InChI=1S/C23H28N2O3/c1-23(2)19(14-20(26)27)21(23)15-6-9-18(10-7-15)28-13-11-17-8-5-16-4-3-12-24-22(16)25-17/h5-10,19,21H,3-4,11-14H2,1-2H3,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human intergrin alphavbeta3 receptor expressed in HEK293 cells |
Bioorg Med Chem 15: 3390-412 (2007)
Article DOI: 10.1016/j.bmc.2007.03.020
BindingDB Entry DOI: 10.7270/Q2057FK0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50209124

((2,2-dimethyl-3-{4-[2-(5,6,7,8-tetrahydro-1,8-naph...)Show SMILES CC1(C)C(CC(O)=O)C1c1ccc(OCCc2ccc3CCCNc3n2)cc1 |w:8.9,3.3| Show InChI InChI=1S/C23H28N2O3/c1-23(2)19(14-20(26)27)21(23)15-6-9-18(10-7-15)28-13-11-17-8-5-16-4-3-12-24-22(16)25-17/h5-10,19,21H,3-4,11-14H2,1-2H3,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human intergrin alphavbeta3 receptor expressed in HEK293 cells |
Bioorg Med Chem 15: 3390-412 (2007)
Article DOI: 10.1016/j.bmc.2007.03.020
BindingDB Entry DOI: 10.7270/Q2057FK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data