 Found 232 hits with Last Name = 'whitefield' and Initial = 'b'
Found 232 hits with Last Name = 'whitefield' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM251460

(US9452998, 9)Show SMILES CC1(N)CCN(CC1)c1cccnc1NC(=O)c1nc(cnc1N)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C22H23F3N8O/c1-21(27)6-10-33(11-7-21)15-5-3-9-29-19(15)32-20(34)17-18(26)30-12-14(31-17)16-13(22(23,24)25)4-2-8-28-16/h2-5,8-9,12H,6-7,10-11,27H2,1H3,(H2,26,30)(H,29,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573043

(CHEMBL4849510)Show SMILES CC1(C)[C@@H](O)C[C@H]1Nc1nc(NCc2cncnc2OCC(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573038
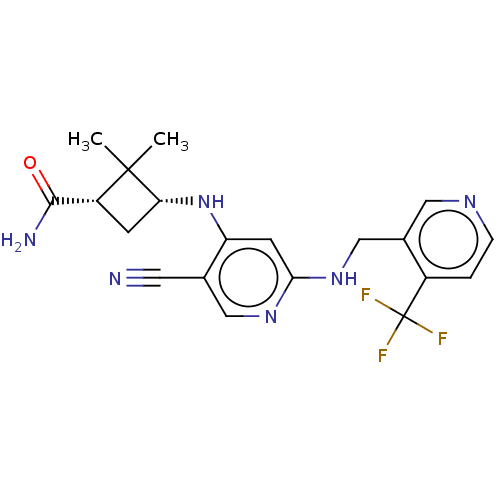
(CHEMBL4853353)Show SMILES CC1(C)[C@@H](C[C@@H]1C(N)=O)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM251460

(US9452998, 9)Show SMILES CC1(N)CCN(CC1)c1cccnc1NC(=O)c1nc(cnc1N)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C22H23F3N8O/c1-21(27)6-10-33(11-7-21)15-5-3-9-29-19(15)32-20(34)17-18(26)30-12-14(31-17)16-13(22(23,24)25)4-2-8-28-16/h2-5,8-9,12H,6-7,10-11,27H2,1H3,(H2,26,30)(H,29,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKC-alpha (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50431522

(CHEMBL2348865)Show SMILES O=c1[nH]c2ncc(nc2n1CCC1CCOCC1)-c1ccc(cc1)-c1nnc[nH]1 Show InChI InChI=1S/C20H21N7O2/c28-20-25-18-19(27(20)8-5-13-6-9-29-10-7-13)24-16(11-21-18)14-1-3-15(4-2-14)17-22-12-23-26-17/h1-4,11-13H,5-10H2,(H,21,25,28)(H,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (unknown origin) using GST-p70S6 as substrate after 60 mins by TR-FRET analysis |
Bioorg Med Chem Lett 23: 1588-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.110
BindingDB Entry DOI: 10.7270/Q21N82GM |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573042

(CHEMBL4854104)Show SMILES CC(C)(F)c1ncncc1CNc1ncc(C#N)c(N[C@@H]2C[C@H](O)C2(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093070
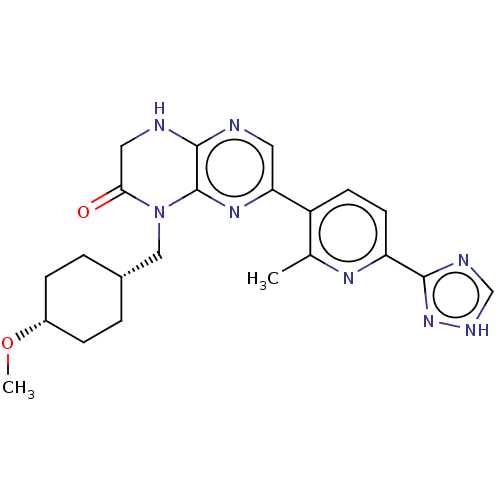
(CHEMBL3586561)Show SMILES CO[C@@H]1CC[C@H](CN2C(=O)CNc3ncc(nc23)-c2ccc(nc2C)-c2nc[nH]n2)CC1 |r,wD:5.5,2.1,(6.68,7.38,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;1.33,3.08,;1.33,1.54,;2.66,.77,;3.73,1.38,;2.66,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.35,.77,;-6.68,1.54,;-6.68,3.08,;-5.35,3.85,;-4.01,3.08,;-2.95,3.7,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,;2.68,5.39,;4.01,6.15,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573034

(CHEMBL4859091)Show SMILES CC1(C)[C@@H](O)C[C@H]1Nc1nc(NCc2cnccc2C(F)(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092784
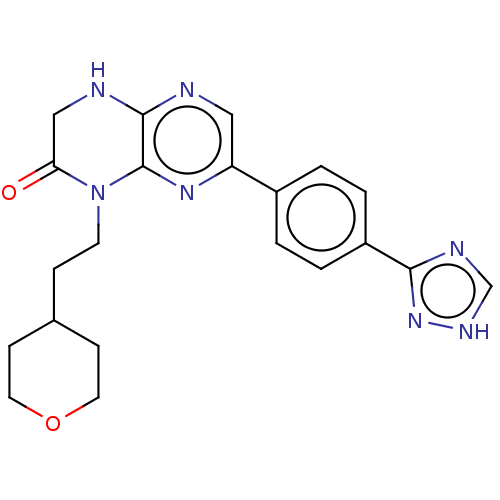
(CHEMBL3586382)Show SMILES O=C1CNc2ncc(nc2N1CCC1CCOCC1)-c1ccc(cc1)-c1nc[nH]n1 Show InChI InChI=1S/C21H23N7O2/c29-18-12-23-20-21(28(18)8-5-14-6-9-30-10-7-14)26-17(11-22-20)15-1-3-16(4-2-15)19-24-13-25-27-19/h1-4,11,13-14H,5-10,12H2,(H,22,23)(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092785
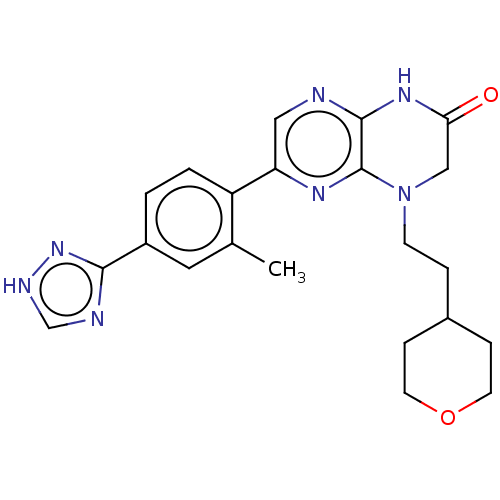
(CHEMBL3586383)Show SMILES Cc1cc(ccc1-c1cnc2NC(=O)CN(CCC3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C22H25N7O2/c1-14-10-16(20-24-13-25-28-20)2-3-17(14)18-11-23-21-22(26-18)29(12-19(30)27-21)7-4-15-5-8-31-9-6-15/h2-3,10-11,13,15H,4-9,12H2,1H3,(H,23,27,30)(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50572994

(CHEMBL4854326)Show SMILES CC1(C)CC(CC(C)(C)N1)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093070

(CHEMBL3586561)Show SMILES CO[C@@H]1CC[C@H](CN2C(=O)CNc3ncc(nc23)-c2ccc(nc2C)-c2nc[nH]n2)CC1 |r,wD:5.5,2.1,(6.68,7.38,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;1.33,3.08,;1.33,1.54,;2.66,.77,;3.73,1.38,;2.66,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.35,.77,;-6.68,1.54,;-6.68,3.08,;-5.35,3.85,;-4.01,3.08,;-2.95,3.7,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,;2.68,5.39,;4.01,6.15,)| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of p70S6K phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092786

(CHEMBL3586384)Show SMILES Cc1cc(ccc1-c1cnc2NCC(=O)N(CCC3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C22H25N7O2/c1-14-10-16(20-25-13-26-28-20)2-3-17(14)18-11-23-21-22(27-18)29(19(30)12-24-21)7-4-15-5-8-31-9-6-15/h2-3,10-11,13,15H,4-9,12H2,1H3,(H,23,24)(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093075

(CHEMBL3586566)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N([C@@H]3CC[C@H](O)CC3)c2n1)-c1nc[nH]n1 |r,wU:16.16,19.20,(-2.95,3.7,;-4.01,3.08,;-5.35,3.85,;-6.68,3.08,;-6.68,1.54,;-5.35,.77,;-4.01,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.66,3.85,;2.66,5.39,;1.33,6.16,;1.32,7.39,;-.01,5.39,;-0,3.85,;,.77,;-1.33,1.54,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,)| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of p70S6K phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573033
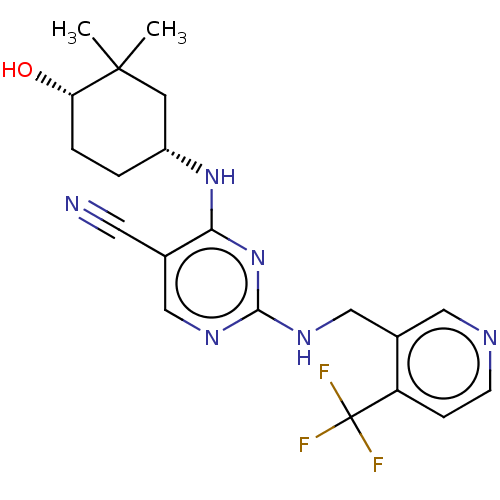
(CHEMBL4851695)Show SMILES CC1(C)C[C@@H](CC[C@@H]1O)Nc1nc(NCc2cnccc2C(F)(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573029
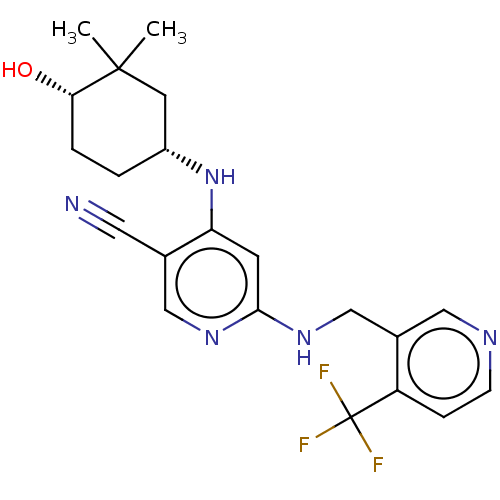
(CHEMBL4861883)Show SMILES CC1(C)C[C@@H](CC[C@@H]1O)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573015
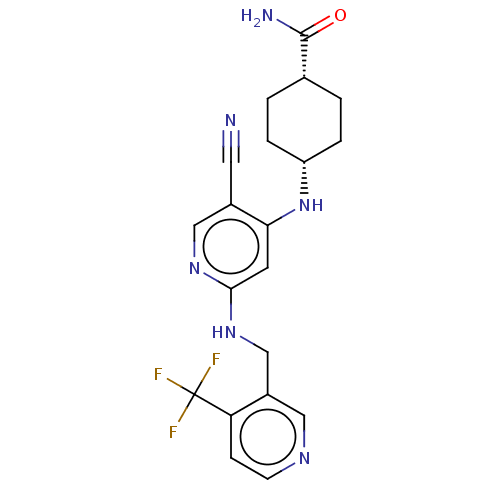
(CHEMBL4870774)Show SMILES NC(=O)[C@@H]1CC[C@@H](CC1)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r,wU:6.9,3.2,(38.42,-10.66,;37.11,-9.86,;37.14,-8.32,;35.76,-10.6,;34.44,-9.8,;33.08,-10.54,;33.06,-12.08,;34.37,-12.87,;35.72,-12.14,;31.71,-12.82,;31.68,-14.36,;30.33,-15.1,;30.31,-16.64,;28.96,-17.39,;28.93,-18.93,;27.58,-19.67,;27.56,-21.21,;26.21,-21.96,;24.89,-21.17,;24.92,-19.63,;26.27,-18.88,;26.29,-17.34,;24.75,-17.34,;25.52,-16,;27.64,-16.6,;31.62,-17.44,;32.97,-16.69,;33,-15.15,;34.35,-14.41,;35.69,-13.66,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50431521

(CHEMBL2348864)Show SMILES O=C1CN(CCC2CCOCC2)c2nc(cnc2N1)-c1ccc(cc1)-c1nnc[nH]1 Show InChI InChI=1S/C21H23N7O2/c29-18-12-28(8-5-14-6-9-30-10-7-14)21-20(26-18)22-11-17(25-21)15-1-3-16(4-2-15)19-23-13-24-27-19/h1-4,11,13-14H,5-10,12H2,(H,22,26,29)(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50092782

(CHEMBL3586387)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N(CCC3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C21H24N8O2/c1-13-15(2-3-16(26-13)19-24-12-25-28-19)17-10-22-20-21(27-17)29(18(30)11-23-20)7-4-14-5-8-31-9-6-14/h2-3,10,12,14H,4-9,11H2,1H3,(H,22,23)(H,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of p70S6K phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50431521

(CHEMBL2348864)Show SMILES O=C1CN(CCC2CCOCC2)c2nc(cnc2N1)-c1ccc(cc1)-c1nnc[nH]1 Show InChI InChI=1S/C21H23N7O2/c29-18-12-28(8-5-14-6-9-30-10-7-14)21-20(26-18)22-11-17(25-21)15-1-3-16(4-2-15)19-23-13-24-27-19/h1-4,11,13-14H,5-10,12H2,(H,22,26,29)(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (unknown origin) using GST-p70S6 as substrate after 60 mins by TR-FRET analysis |
Bioorg Med Chem Lett 23: 1588-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.110
BindingDB Entry DOI: 10.7270/Q21N82GM |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573044

(CHEMBL4873647)Show SMILES CC(F)(F)COc1ncncc1CNc1ncc(C#N)c(N[C@@H]2CC[C@H](O)C(C)(C)C2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093075

(CHEMBL3586566)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N([C@@H]3CC[C@H](O)CC3)c2n1)-c1nc[nH]n1 |r,wU:16.16,19.20,(-2.95,3.7,;-4.01,3.08,;-5.35,3.85,;-6.68,3.08,;-6.68,1.54,;-5.35,.77,;-4.01,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.66,3.85,;2.66,5.39,;1.33,6.16,;1.32,7.39,;-.01,5.39,;-0,3.85,;,.77,;-1.33,1.54,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093080
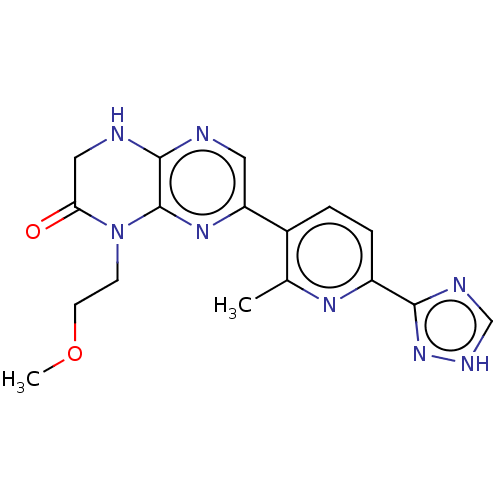
(CHEMBL3586571)Show SMILES COCCN1C(=O)CNc2ncc(nc12)-c1ccc(nc1C)-c1nc[nH]n1 Show InChI InChI=1S/C17H18N8O2/c1-10-11(3-4-12(22-10)15-20-9-21-24-15)13-7-18-16-17(23-13)25(5-6-27-2)14(26)8-19-16/h3-4,7,9H,5-6,8H2,1-2H3,(H,18,19)(H,20,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of p70S6K phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093077

(CHEMBL3586568)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N(C3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C19H20N8O2/c1-11-13(2-3-14(24-11)17-22-10-23-26-17)15-8-20-18-19(25-15)27(16(28)9-21-18)12-4-6-29-7-5-12/h2-3,8,10,12H,4-7,9H2,1H3,(H,20,21)(H,22,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of p70S6K phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092783

(CHEMBL3586404)Show SMILES CO[C@H]1CC[C@@H](CC1)N1C(=O)CNc2ncc(nc12)-c1ccc(nc1)C(C)(C)O |r,wU:2.1,wD:5.8,(2.39,-8.32,;1.33,-7.7,;1.33,-6.16,;-.01,-5.39,;-0,-3.85,;1.33,-3.08,;2.66,-3.85,;2.66,-5.39,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;-4.01,-1.54,;-4.01,-3.08,;-5.35,-3.85,;-6.68,-3.08,;-6.68,-1.54,;-5.35,-.77,;-8.02,-3.85,;-9.08,-3.23,;-9.08,-4.47,;-8.02,-5.08,)| Show InChI InChI=1S/C21H27N5O3/c1-21(2,28)17-9-4-13(10-22-17)16-11-23-19-20(25-16)26(18(27)12-24-19)14-5-7-15(29-3)8-6-14/h4,9-11,14-15,28H,5-8,12H2,1-3H3,(H,23,24)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092769
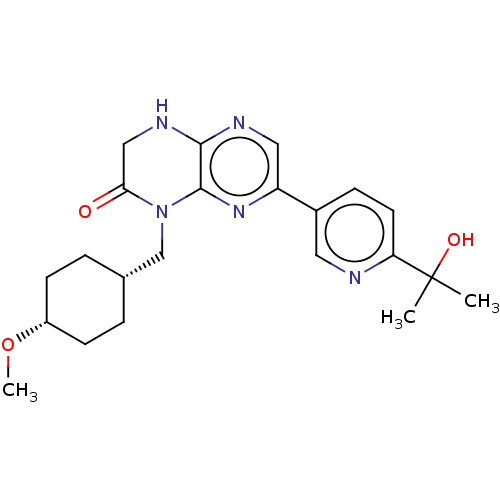
(CHEMBL3586397)Show SMILES CO[C@@H]1CC[C@H](CN2C(=O)CNc3ncc(nc23)-c2ccc(nc2)C(C)(C)O)CC1 |r,wU:5.5,2.1,(6.68,-7.38,;6.68,-6.15,;5.34,-5.38,;5.34,-3.84,;4,-3.07,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;-4.01,-1.54,;-4.01,-3.08,;-5.35,-3.85,;-6.68,-3.08,;-6.68,-1.54,;-5.35,-.77,;-8.02,-3.85,;-9.08,-3.23,;-9.08,-4.47,;-8.02,-5.08,;2.68,-5.39,;4.01,-6.15,)| Show InChI InChI=1S/C20H22ClFN2/c21-17-2-1-3-19(13-17)24-10-8-23(9-11-24)14-16-12-20(16)15-4-6-18(22)7-5-15/h1-7,13,16,20H,8-12,14H2/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573027

(CHEMBL4867906)Show SMILES CC1(C)C[C@@H](CC(C)(C)[C@@H]1O)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r,wD:9.10,4.11,(16.5,-7.5,;15.98,-6.06,;17.5,-5.78,;14.65,-6.83,;13.31,-6.06,;13.31,-4.52,;14.65,-3.75,;15.63,-2.56,;13.65,-2.57,;15.98,-4.52,;17.31,-3.74,;11.98,-6.83,;11.98,-8.37,;10.65,-9.14,;10.65,-10.68,;9.32,-11.45,;9.32,-12.99,;7.98,-13.75,;7.98,-15.29,;6.64,-16.06,;5.32,-15.29,;5.32,-13.75,;6.64,-12.98,;6.64,-11.44,;5.15,-11.04,;6.25,-9.95,;7.98,-10.67,;11.98,-11.45,;13.32,-10.68,;13.32,-9.14,;14.65,-8.37,;15.98,-7.6,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093076

(CHEMBL3586567)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N([C@H]3CC[C@H](O)CC3)c2n1)-c1nc[nH]n1 |r,wU:16.16,wD:19.20,(-2.95,3.7,;-4.01,3.08,;-5.35,3.85,;-6.68,3.08,;-6.68,1.54,;-5.35,.77,;-4.01,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;-0,3.85,;-.01,5.39,;1.33,6.16,;1.32,7.39,;2.66,5.39,;2.66,3.85,;,.77,;-1.33,1.54,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,)| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of p70S6K phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093069
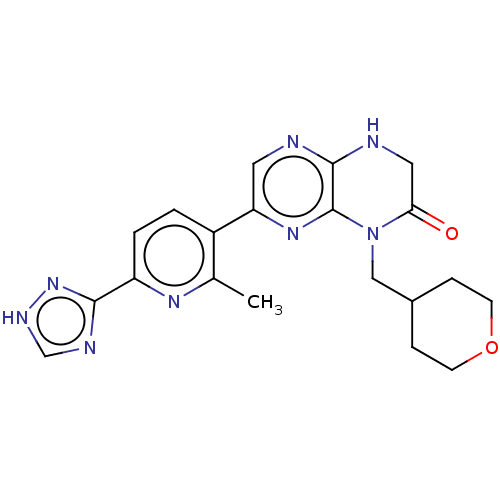
(CHEMBL3586560)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N(CC3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C20H22N8O2/c1-12-14(2-3-15(25-12)18-23-11-24-27-18)16-8-21-19-20(26-16)28(17(29)9-22-19)10-13-4-6-30-7-5-13/h2-3,8,11,13H,4-7,9-10H2,1H3,(H,21,22)(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092782

(CHEMBL3586387)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N(CCC3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C21H24N8O2/c1-13-15(2-3-16(26-13)19-24-12-25-28-19)17-10-22-20-21(27-17)29(18(30)11-23-20)7-4-14-5-8-31-9-6-14/h2-3,10,12,14H,4-9,11H2,1H3,(H,22,23)(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092782

(CHEMBL3586387)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N(CCC3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C21H24N8O2/c1-13-15(2-3-16(26-13)19-24-12-25-28-19)17-10-22-20-21(27-17)29(18(30)11-23-20)7-4-14-5-8-31-9-6-14/h2-3,10,12,14H,4-9,11H2,1H3,(H,22,23)(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50545275

(CHEMBL4645677)Show SMILES Fc1ccc(N2CCN(Cc3ccc(COc4cccc5C(=O)N(Cc45)C4CCC(=O)NC4=O)cc3)CC2)c(F)c1 Show InChI InChI=1S/C31H30F2N4O4/c32-22-8-9-26(25(33)16-22)36-14-12-35(13-15-36)17-20-4-6-21(7-5-20)19-41-28-3-1-2-23-24(28)18-37(31(23)40)27-10-11-29(38)34-30(27)39/h1-9,16,27H,10-15,17-19H2,(H,34,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of adrenergic receptor alpha1 (unknown origin) |
J Med Chem 63: 6648-6676 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01928
BindingDB Entry DOI: 10.7270/Q2KS6W4N |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093074

(CHEMBL3586565)Show SMILES CO[C@H]1CC[C@@H](CC1)N1C(=O)CNc2ncc(nc12)-c1ccc(nc1C)-c1nc[nH]n1 |r,wU:5.8,wD:2.1,(2.39,8.32,;1.33,7.7,;1.33,6.16,;-.01,5.39,;-0,3.85,;1.33,3.08,;2.66,3.85,;2.66,5.39,;1.33,1.54,;2.66,.77,;3.73,1.38,;2.66,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.35,.77,;-6.68,1.54,;-6.68,3.08,;-5.35,3.85,;-4.01,3.08,;-2.95,3.7,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093069

(CHEMBL3586560)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N(CC3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C20H22N8O2/c1-12-14(2-3-15(25-12)18-23-11-24-27-18)16-8-21-19-20(26-16)28(17(29)9-22-19)10-13-4-6-30-7-5-13/h2-3,8,11,13H,4-7,9-10H2,1H3,(H,21,22)(H,23,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of S6 phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50093082
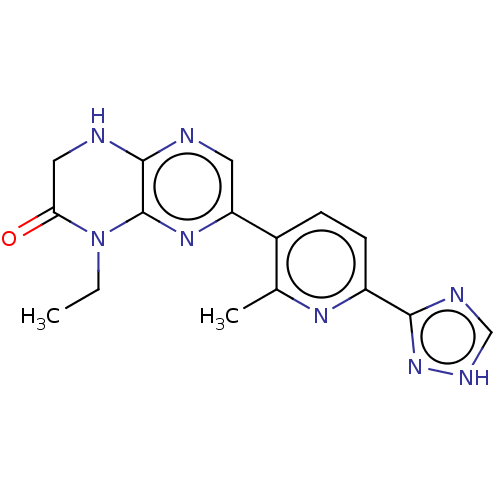
(CHEMBL3586573)Show SMILES CCN1C(=O)CNc2ncc(nc12)-c1ccc(nc1C)-c1nc[nH]n1 Show InChI InChI=1S/C16H16N8O/c1-3-24-13(25)7-18-15-16(24)22-12(6-17-15)10-4-5-11(21-9(10)2)14-19-8-20-23-14/h4-6,8H,3,7H2,1-2H3,(H,17,18)(H,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human DNA PK |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092780

(CHEMBL3586385)Show SMILES O=C1CN(CCC2CCOCC2)c2nc(cnc2N1)-c1ccc(nc1)-c1nc[nH]n1 Show InChI InChI=1S/C20H22N8O2/c29-17-11-28(6-3-13-4-7-30-8-5-13)20-19(26-17)22-10-16(25-20)14-1-2-15(21-9-14)18-23-12-24-27-18/h1-2,9-10,12-13H,3-8,11H2,(H,22,26,29)(H,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093080

(CHEMBL3586571)Show SMILES COCCN1C(=O)CNc2ncc(nc12)-c1ccc(nc1C)-c1nc[nH]n1 Show InChI InChI=1S/C17H18N8O2/c1-10-11(3-4-12(22-10)15-20-9-21-24-15)13-7-18-16-17(23-13)25(5-6-27-2)14(26)8-19-16/h3-4,7,9H,5-6,8H2,1-2H3,(H,18,19)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093076

(CHEMBL3586567)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N([C@H]3CC[C@H](O)CC3)c2n1)-c1nc[nH]n1 |r,wU:16.16,wD:19.20,(-2.95,3.7,;-4.01,3.08,;-5.35,3.85,;-6.68,3.08,;-6.68,1.54,;-5.35,.77,;-4.01,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;-0,3.85,;-.01,5.39,;1.33,6.16,;1.32,7.39,;2.66,5.39,;2.66,3.85,;,.77,;-1.33,1.54,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093074

(CHEMBL3586565)Show SMILES CO[C@H]1CC[C@@H](CC1)N1C(=O)CNc2ncc(nc12)-c1ccc(nc1C)-c1nc[nH]n1 |r,wU:5.8,wD:2.1,(2.39,8.32,;1.33,7.7,;1.33,6.16,;-.01,5.39,;-0,3.85,;1.33,3.08,;2.66,3.85,;2.66,5.39,;1.33,1.54,;2.66,.77,;3.73,1.38,;2.66,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.35,.77,;-6.68,1.54,;-6.68,3.08,;-5.35,3.85,;-4.01,3.08,;-2.95,3.7,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,)| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of S6 phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573028
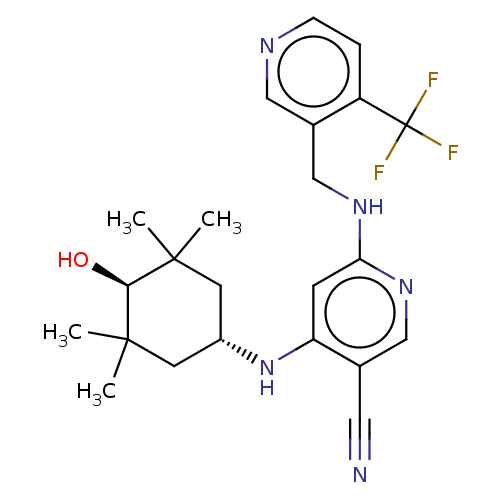
(CHEMBL4856779)Show SMILES CC1(C)C[C@@H](CC(C)(C)[C@H]1O)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r,wU:9.10,wD:4.11,(27,-8.26,;26.47,-6.82,;27.99,-6.54,;25.14,-7.59,;23.81,-6.82,;23.8,-5.28,;25.14,-4.51,;26.13,-3.33,;24.15,-3.33,;26.47,-5.28,;27.81,-4.51,;22.47,-7.59,;22.47,-9.13,;21.14,-9.9,;21.14,-11.44,;19.81,-12.21,;19.81,-13.75,;18.47,-14.51,;18.47,-16.05,;17.14,-16.82,;15.81,-16.05,;15.81,-14.51,;17.14,-13.74,;17.13,-12.2,;15.64,-11.8,;16.74,-10.71,;18.47,-11.43,;22.47,-12.21,;23.81,-11.44,;23.81,-9.9,;25.14,-9.13,;26.47,-8.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573014
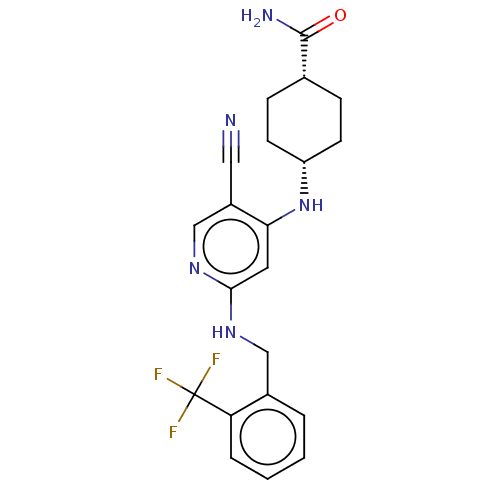
(CHEMBL4858914)Show SMILES NC(=O)[C@@H]1CC[C@@H](CC1)Nc1cc(NCc2ccccc2C(F)(F)F)ncc1C#N |r,wU:6.9,3.2,(23.15,-9.78,;21.84,-8.98,;21.87,-7.44,;20.49,-9.72,;19.17,-8.92,;17.81,-9.66,;17.79,-11.2,;19.1,-12,;20.45,-11.26,;16.44,-11.94,;16.41,-13.48,;15.06,-14.22,;15.04,-15.76,;13.69,-16.51,;13.66,-18.05,;12.31,-18.79,;12.29,-20.33,;10.94,-21.08,;9.62,-20.29,;9.65,-18.75,;11,-18,;11.02,-16.46,;9.48,-16.46,;10.25,-15.13,;12.37,-15.72,;16.35,-16.56,;17.7,-15.81,;17.73,-14.27,;19.08,-13.53,;20.43,-12.78,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093082

(CHEMBL3586573)Show SMILES CCN1C(=O)CNc2ncc(nc12)-c1ccc(nc1C)-c1nc[nH]n1 Show InChI InChI=1S/C16H16N8O/c1-3-24-13(25)7-18-15-16(24)22-12(6-17-15)10-4-5-11(21-9(10)2)14-19-8-20-23-14/h4-6,8H,3,7H2,1-2H3,(H,17,18)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093082

(CHEMBL3586573)Show SMILES CCN1C(=O)CNc2ncc(nc12)-c1ccc(nc1C)-c1nc[nH]n1 Show InChI InChI=1S/C16H16N8O/c1-3-24-13(25)7-18-15-16(24)22-12(6-17-15)10-4-5-11(21-9(10)2)14-19-8-20-23-14/h4-6,8H,3,7H2,1-2H3,(H,17,18)(H,19,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of S6 phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50092793
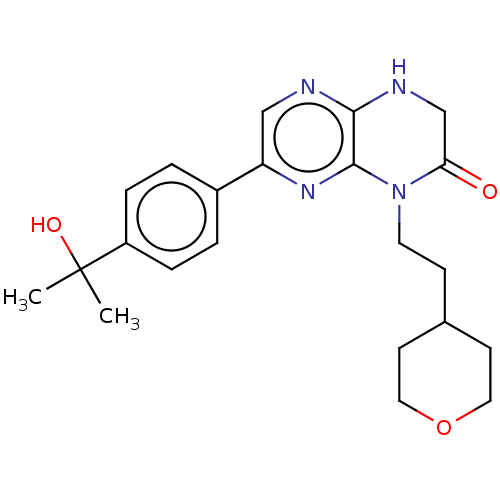
(CHEMBL3586380)Show SMILES CC(C)(O)c1ccc(cc1)-c1cnc2NCC(=O)N(CCC3CCOCC3)c2n1 Show InChI InChI=1S/C22H28N4O3/c1-22(2,28)17-5-3-16(4-6-17)18-13-23-20-21(25-18)26(19(27)14-24-20)10-7-15-8-11-29-12-9-15/h3-6,13,15,28H,7-12,14H2,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093077

(CHEMBL3586568)Show SMILES Cc1nc(ccc1-c1cnc2NCC(=O)N(C3CCOCC3)c2n1)-c1nc[nH]n1 Show InChI InChI=1S/C19H20N8O2/c1-11-13(2-3-14(24-11)17-22-10-23-26-17)15-8-20-18-19(25-15)27(16(28)9-21-18)12-4-6-29-7-5-12/h2-3,8,10,12H,4-7,9H2,1H3,(H,20,21)(H,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573016
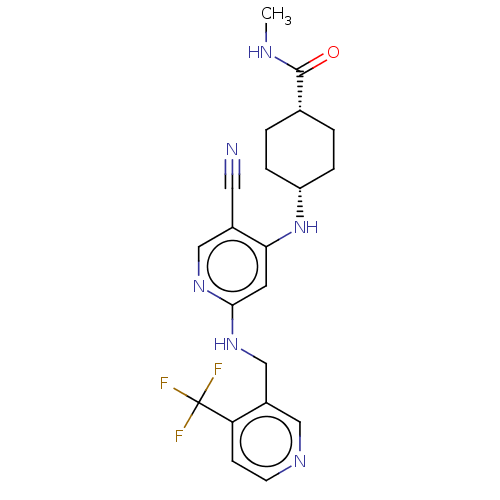
(CHEMBL4876360)Show SMILES CNC(=O)[C@@H]1CC[C@@H](CC1)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r,wU:7.10,4.3,(50.96,-14.24,;51,-12.7,;49.68,-11.9,;49.72,-10.36,;48.33,-12.64,;47.01,-11.84,;45.66,-12.58,;45.64,-14.12,;46.95,-14.92,;48.3,-14.18,;44.29,-14.87,;44.26,-16.41,;42.91,-17.15,;42.88,-18.69,;41.54,-19.44,;41.51,-20.98,;40.16,-21.72,;40.13,-23.26,;38.79,-24.01,;37.47,-23.22,;37.5,-21.68,;38.84,-20.93,;38.87,-19.38,;37.33,-19.38,;38.1,-18.05,;40.22,-18.64,;44.2,-19.49,;45.55,-18.74,;45.57,-17.2,;46.92,-16.45,;48.27,-15.71,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50093071
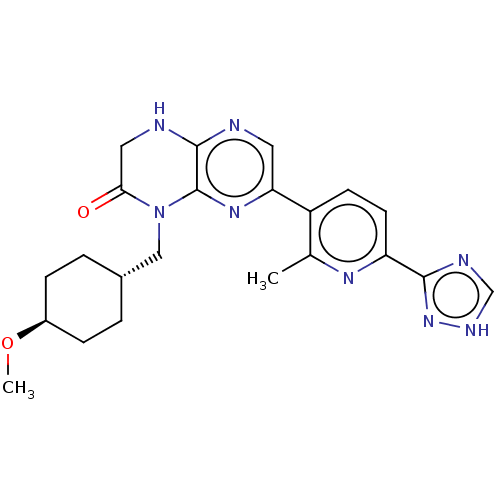
(CHEMBL3586562)Show SMILES CO[C@H]1CC[C@H](CN2C(=O)CNc3ncc(nc23)-c2ccc(nc2C)-c2nc[nH]n2)CC1 |r,wU:2.1,wD:5.5,(6.68,7.38,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;1.33,3.08,;1.33,1.54,;2.66,.77,;3.73,1.38,;2.66,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.35,.77,;-6.68,1.54,;-6.68,3.08,;-5.35,3.85,;-4.01,3.08,;-2.95,3.7,;-8.02,3.85,;-8.16,5.37,;-9.66,5.69,;-10.43,4.36,;-9.4,3.21,;2.68,5.39,;4.01,6.15,)| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of p70S6K phosphorylation after 1 hr |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50092783

(CHEMBL3586404)Show SMILES CO[C@H]1CC[C@@H](CC1)N1C(=O)CNc2ncc(nc12)-c1ccc(nc1)C(C)(C)O |r,wU:2.1,wD:5.8,(2.39,-8.32,;1.33,-7.7,;1.33,-6.16,;-.01,-5.39,;-0,-3.85,;1.33,-3.08,;2.66,-3.85,;2.66,-5.39,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;-4.01,-1.54,;-4.01,-3.08,;-5.35,-3.85,;-6.68,-3.08,;-6.68,-1.54,;-5.35,-.77,;-8.02,-3.85,;-9.08,-3.23,;-9.08,-4.47,;-8.02,-5.08,)| Show InChI InChI=1S/C21H27N5O3/c1-21(2,28)17-9-4-13(10-22-17)16-11-23-19-20(25-16)26(18(27)12-24-19)14-5-7-15(29-3)8-6-14/h4,9-11,14-15,28H,5-8,12H2,1-3H3,(H,23,24)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of cFMS (unknown origin) |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50093078
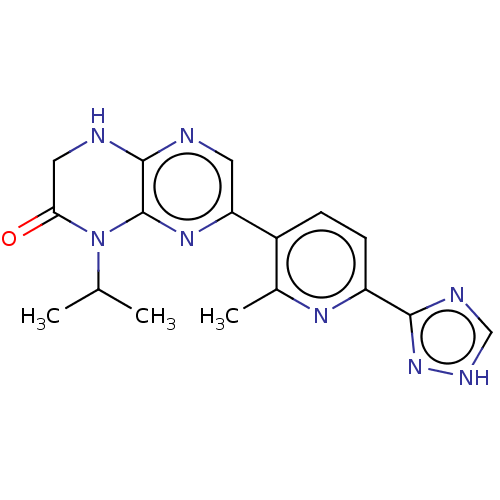
(CHEMBL3586569)Show SMILES CC(C)N1C(=O)CNc2ncc(nc12)-c1ccc(nc1C)-c1nc[nH]n1 Show InChI InChI=1S/C17H18N8O/c1-9(2)25-14(26)7-19-16-17(25)23-13(6-18-16)11-4-5-12(22-10(11)3)15-20-8-21-24-15/h4-6,8-9H,7H2,1-3H3,(H,18,19)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) by HTR-FRET substrate phosphorylation assay |
J Med Chem 58: 5599-608 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00627
BindingDB Entry DOI: 10.7270/Q2BG2QRV |
More data for this
Ligand-Target Pair | |
FKBP12A/mTOR
(Homo sapiens (Human)) | BDBM50092783

(CHEMBL3586404)Show SMILES CO[C@H]1CC[C@@H](CC1)N1C(=O)CNc2ncc(nc12)-c1ccc(nc1)C(C)(C)O |r,wU:2.1,wD:5.8,(2.39,-8.32,;1.33,-7.7,;1.33,-6.16,;-.01,-5.39,;-0,-3.85,;1.33,-3.08,;2.66,-3.85,;2.66,-5.39,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;-4.01,-1.54,;-4.01,-3.08,;-5.35,-3.85,;-6.68,-3.08,;-6.68,-1.54,;-5.35,-.77,;-8.02,-3.85,;-9.08,-3.23,;-9.08,-4.47,;-8.02,-5.08,)| Show InChI InChI=1S/C21H27N5O3/c1-21(2,28)17-9-4-13(10-22-17)16-11-23-19-20(25-16)26(18(27)12-24-19)14-5-7-15(29-3)8-6-14/h4,9-11,14-15,28H,5-8,12H2,1-3H3,(H,23,24)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human PC3 cells assessed as inhibition of S6 phosphorylation after 1 hr |
J Med Chem 58: 5323-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00626
BindingDB Entry DOI: 10.7270/Q208672F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data