 Found 323 hits with Last Name = 'wong' and Initial = 'jc'
Found 323 hits with Last Name = 'wong' and Initial = 'jc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM120996
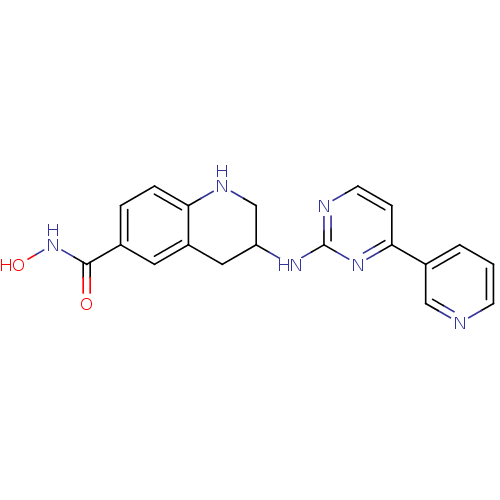
(US8716285, 53)Show SMILES ONC(=O)c1ccc2NCC(Cc2c1)Nc1nccc(n1)-c1cccnc1 Show InChI InChI=1S/C19H18N6O2/c26-18(25-27)12-3-4-16-14(8-12)9-15(11-22-16)23-19-21-7-5-17(24-19)13-2-1-6-20-10-13/h1-8,10,15,22,27H,9,11H2,(H,25,26)(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078748
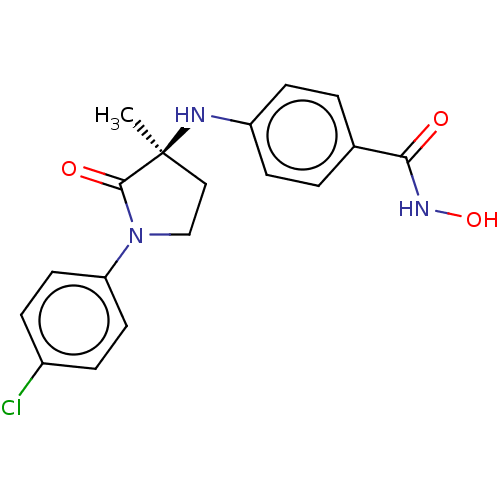
(CHEMBL3415629)Show SMILES C[C@@]1(CCN(C1=O)c1ccc(Cl)cc1)Nc1ccc(cc1)C(=O)NO |r| Show InChI InChI=1S/C18H18ClN3O3/c1-18(20-14-6-2-12(3-7-14)16(23)21-25)10-11-22(17(18)24)15-8-4-13(19)5-9-15/h2-9,20,25H,10-11H2,1H3,(H,21,23)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078747
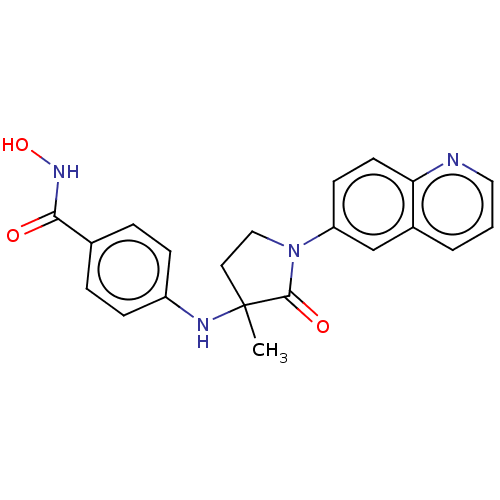
(CHEMBL3415628)Show SMILES CC1(CCN(C1=O)c1ccc2ncccc2c1)Nc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H20N4O3/c1-21(23-16-6-4-14(5-7-16)19(26)24-28)10-12-25(20(21)27)17-8-9-18-15(13-17)3-2-11-22-18/h2-9,11,13,23,28H,10,12H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078695
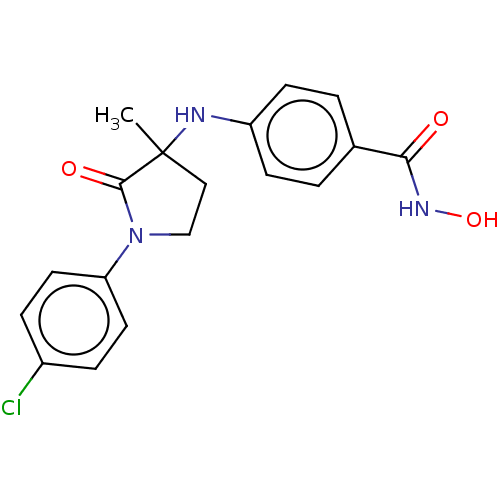
(CHEMBL3415627)Show SMILES CC1(CCN(C1=O)c1ccc(Cl)cc1)Nc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C18H18ClN3O3/c1-18(20-14-6-2-12(3-7-14)16(23)21-25)10-11-22(17(18)24)15-8-4-13(19)5-9-15/h2-9,20,25H,10-11H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Transcriptional enhancer factor TEF-1
(Homo sapiens) | BDBM50497300
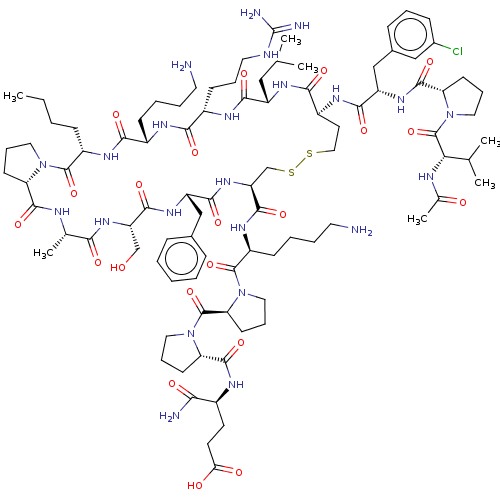
(CHEMBL3335461)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CCCC)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC2=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(N)=O)NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C93H144ClN23O21S2/c1-8-9-27-63-89(135)114-41-19-31-70(114)86(132)101-54(6)77(123)112-68(50-118)84(130)110-66(48-56-23-11-10-12-24-56)83(129)113-69(85(131)108-64(29-14-16-39-96)90(136)117-44-22-34-73(117)91(137)115-42-20-32-71(115)87(133)103-59(76(97)122)35-36-74(120)121)51-140-139-45-37-62(80(126)109-65(46-52(2)3)81(127)105-61(30-18-40-100-93(98)99)78(124)104-60(79(125)107-63)28-13-15-38-95)106-82(128)67(49-57-25-17-26-58(94)47-57)111-88(134)72-33-21-43-116(72)92(138)75(53(4)5)102-55(7)119/h10-12,17,23-26,47,52-54,59-73,75,118H,8-9,13-16,18-22,27-46,48-51,95-96H2,1-7H3,(H2,97,122)(H,101,132)(H,102,119)(H,103,133)(H,104,124)(H,105,127)(H,106,128)(H,107,125)(H,108,131)(H,109,126)(H,110,130)(H,111,134)(H,112,123)(H,113,129)(H,120,121)(H4,98,99,100)/t54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,75-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of YAP/ GST-TEAD1 (unknown origin) interaction by surface plasmon resonance assay |
ACS Med Chem Lett 5: 993-8 (2014)
Article DOI: 10.1021/ml500160m
BindingDB Entry DOI: 10.7270/Q28P63H7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50027664
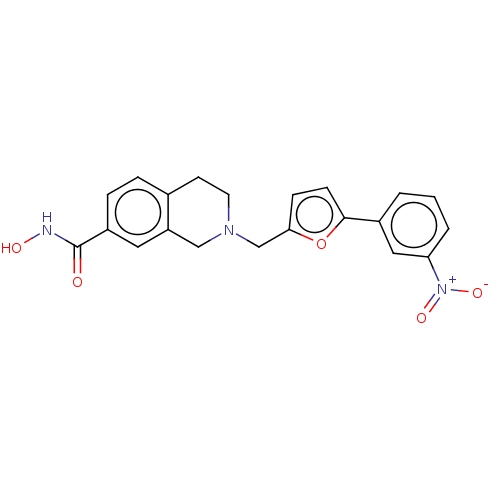
(CHEMBL3338404)Show SMILES ONC(=O)c1ccc2CCN(Cc3ccc(o3)-c3cccc(c3)[N+]([O-])=O)Cc2c1 Show InChI InChI=1S/C21H19N3O5/c25-21(22-26)16-5-4-14-8-9-23(12-17(14)10-16)13-19-6-7-20(29-19)15-2-1-3-18(11-15)24(27)28/h1-7,10-11,26H,8-9,12-13H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using fluorogenic peptide as substrate by fluorescence assay |
J Med Chem 57: 8026-34 (2014)
Article DOI: 10.1021/jm5008962
BindingDB Entry DOI: 10.7270/Q29C700V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50027661
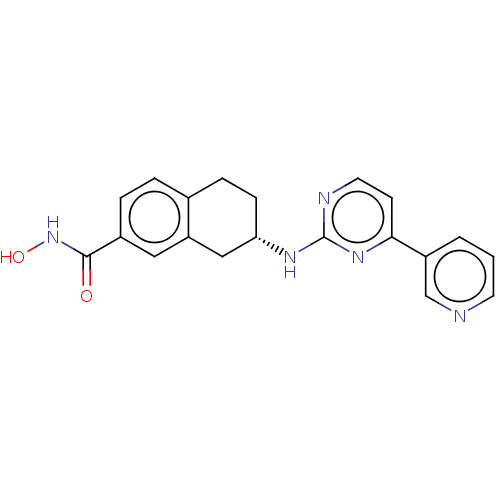
(CHEMBL3338417)Show SMILES ONC(=O)c1ccc2CC[C@@H](Cc2c1)Nc1nccc(n1)-c1cccnc1 |r| Show InChI InChI=1S/C20H19N5O2/c26-19(25-27)14-4-3-13-5-6-17(11-16(13)10-14)23-20-22-9-7-18(24-20)15-2-1-8-21-12-15/h1-4,7-10,12,17,27H,5-6,11H2,(H,25,26)(H,22,23,24)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using fluorogenic peptide as substrate by fluorescence assay |
J Med Chem 57: 8026-34 (2014)
Article DOI: 10.1021/jm5008962
BindingDB Entry DOI: 10.7270/Q29C700V |
More data for this
Ligand-Target Pair | |
Transcriptional enhancer factor TEF-1
(Homo sapiens) | BDBM50497290
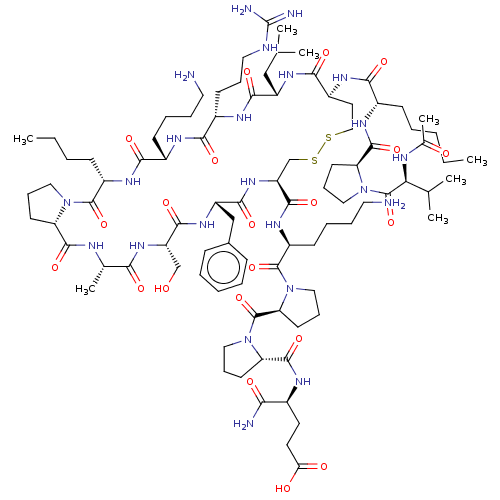
(CHEMBL3335458)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CCCC)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC2=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(N)=O)NC(=O)[C@H](CCCCC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C91H149N23O21S2/c1-9-11-14-29-59(104-86(131)70-35-24-45-113(70)90(135)73(53(5)6)99-55(8)116)76(121)103-61-39-47-136-137-51-67(83(128)106-63(31-18-20-41-93)88(133)114-46-25-36-71(114)89(134)112-44-23-34-69(112)85(130)100-57(74(94)119)37-38-72(117)118)110-81(126)65(49-56-26-15-13-16-27-56)108-82(127)66(50-115)109-75(120)54(7)98-84(129)68-33-22-43-111(68)87(132)62(28-12-10-2)105-78(123)58(30-17-19-40-92)101-77(122)60(32-21-42-97-91(95)96)102-80(125)64(48-52(3)4)107-79(61)124/h13,15-16,26-27,52-54,57-71,73,115H,9-12,14,17-25,28-51,92-93H2,1-8H3,(H2,94,119)(H,98,129)(H,99,116)(H,100,130)(H,101,122)(H,102,125)(H,103,121)(H,104,131)(H,105,123)(H,106,128)(H,107,124)(H,108,127)(H,109,120)(H,110,126)(H,117,118)(H4,95,96,97)/t54-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,73-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of YAP/ GST-TEAD1 (unknown origin) interaction by surface plasmon resonance assay |
ACS Med Chem Lett 5: 993-8 (2014)
Article DOI: 10.1021/ml500160m
BindingDB Entry DOI: 10.7270/Q28P63H7 |
More data for this
Ligand-Target Pair | |
Transcriptional enhancer factor TEF-1
(Homo sapiens) | BDBM50497304

(CHEMBL3335453)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CCCCC)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC2=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C90H147N23O21S3/c1-9-10-12-27-61-86(131)110-41-20-30-67(110)83(128)97-53(6)74(119)108-65(49-114)81(126)107-64(48-55-24-13-11-14-25-55)80(125)109-66(82(127)105-62(28-16-18-39-92)87(132)113-44-23-33-70(113)88(133)111-42-21-31-68(111)84(129)99-56(73(93)118)34-35-71(116)117)50-137-136-46-37-60(102-77(122)59(36-45-135-8)103-85(130)69-32-22-43-112(69)89(134)72(52(4)5)98-54(7)115)78(123)106-63(47-51(2)3)79(124)101-58(29-19-40-96-90(94)95)75(120)100-57(76(121)104-61)26-15-17-38-91/h11,13-14,24-25,51-53,56-70,72,114H,9-10,12,15-23,26-50,91-92H2,1-8H3,(H2,93,118)(H,97,128)(H,98,115)(H,99,129)(H,100,120)(H,101,124)(H,102,122)(H,103,130)(H,104,121)(H,105,127)(H,106,123)(H,107,126)(H,108,119)(H,109,125)(H,116,117)(H4,94,95,96)/t53-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,72-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of YAP/ GST-TEAD1 (unknown origin) interaction by surface plasmon resonance assay |
ACS Med Chem Lett 5: 993-8 (2014)
Article DOI: 10.1021/ml500160m
BindingDB Entry DOI: 10.7270/Q28P63H7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50027662
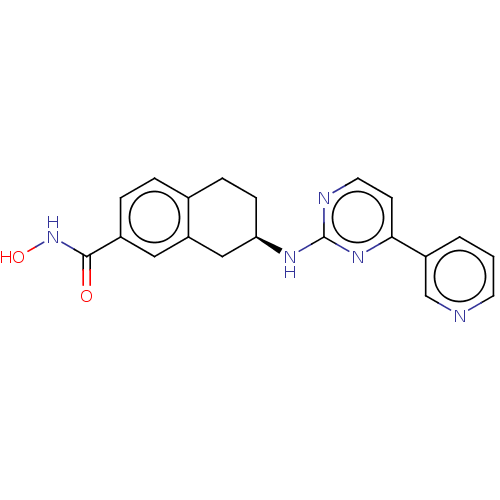
(CHEMBL3338418)Show SMILES ONC(=O)c1ccc2CC[C@H](Cc2c1)Nc1nccc(n1)-c1cccnc1 |r| Show InChI InChI=1S/C20H19N5O2/c26-19(25-27)14-4-3-13-5-6-17(11-16(13)10-14)23-20-22-9-7-18(24-20)15-2-1-8-21-12-15/h1-4,7-10,12,17,27H,5-6,11H2,(H,25,26)(H,22,23,24)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50334368

(CHEMBL1643325 | N-(2-aminophenyl)-3-(4-(2-(4'-cycl...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCCCC1)C(=O)Nc1ccc(cc1)C1CC1 Show InChI InChI=1S/C33H39N5O2/c34-29-6-2-3-7-30(29)37-31(39)19-10-24-8-11-27(12-9-24)32(35-20-23-38-21-4-1-5-22-38)33(40)36-28-17-15-26(16-18-28)25-13-14-25/h2-3,6-12,15-19,25,32,35H,1,4-5,13-14,20-23,34H2,(H,36,40)(H,37,39)/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50027664

(CHEMBL3338404)Show SMILES ONC(=O)c1ccc2CCN(Cc3ccc(o3)-c3cccc(c3)[N+]([O-])=O)Cc2c1 Show InChI InChI=1S/C21H19N3O5/c25-21(22-26)16-5-4-14-8-9-23(12-17(14)10-16)13-19-6-7-20(29-19)15-2-1-3-18(11-15)24(27)28/h1-7,10-11,26H,8-9,12-13H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using fluorogenic peptide as substrate by fluorescence assay |
J Med Chem 57: 8026-34 (2014)
Article DOI: 10.1021/jm5008962
BindingDB Entry DOI: 10.7270/Q29C700V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50027662

(CHEMBL3338418)Show SMILES ONC(=O)c1ccc2CC[C@H](Cc2c1)Nc1nccc(n1)-c1cccnc1 |r| Show InChI InChI=1S/C20H19N5O2/c26-19(25-27)14-4-3-13-5-6-17(11-16(13)10-14)23-20-22-9-7-18(24-20)15-2-1-8-21-12-15/h1-4,7-10,12,17,27H,5-6,11H2,(H,25,26)(H,22,23,24)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using fluorogenic peptide as substrate by fluorescence assay |
J Med Chem 57: 8026-34 (2014)
Article DOI: 10.1021/jm5008962
BindingDB Entry DOI: 10.7270/Q29C700V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50334366
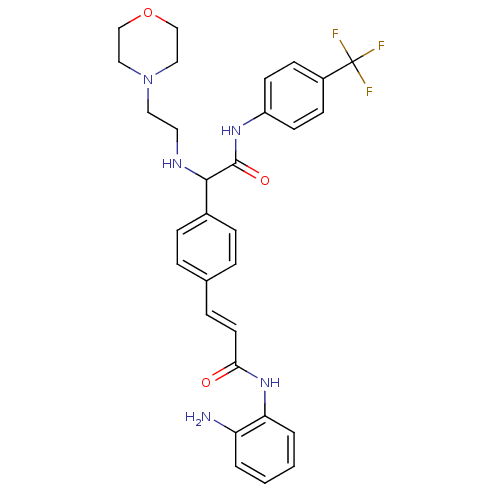
(CHEMBL1643308 | N-(2-aminophenyl)-3-(4-(1-(2-morph...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-10-12-24(13-11-23)36-29(40)28(35-15-16-38-17-19-41-20-18-38)22-8-5-21(6-9-22)7-14-27(39)37-26-4-2-1-3-25(26)34/h1-14,28,35H,15-20,34H2,(H,36,40)(H,37,39)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50399006
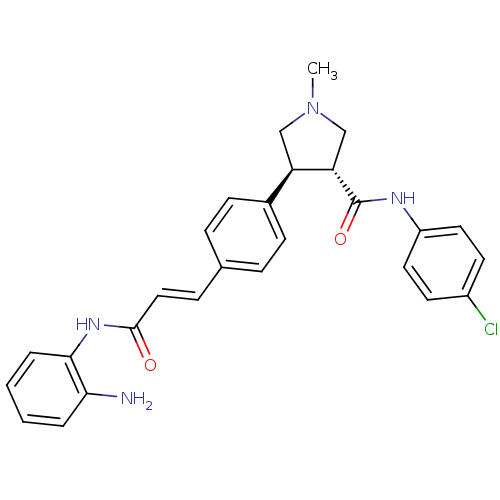
(CHEMBL2177587)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50334366

(CHEMBL1643308 | N-(2-aminophenyl)-3-(4-(1-(2-morph...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-10-12-24(13-11-23)36-29(40)28(35-15-16-38-17-19-41-20-18-38)22-8-5-21(6-9-22)7-14-27(39)37-26-4-2-1-3-25(26)34/h1-14,28,35H,15-20,34H2,(H,36,40)(H,37,39)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078687
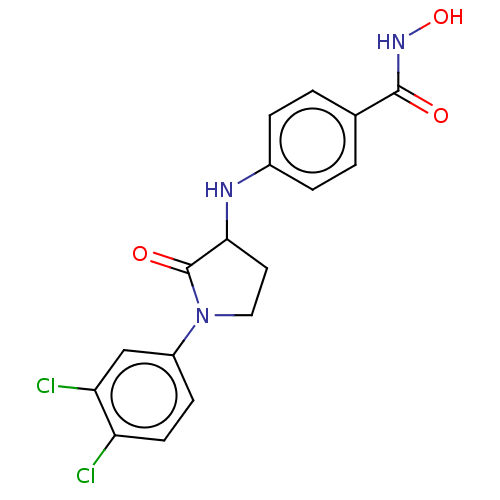
(CHEMBL3415619)Show SMILES ONC(=O)c1ccc(NC2CCN(C2=O)c2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C17H15Cl2N3O3/c18-13-6-5-12(9-14(13)19)22-8-7-15(17(22)24)20-11-3-1-10(2-4-11)16(23)21-25/h1-6,9,15,20,25H,7-8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Transcriptional enhancer factor TEF-1
(Homo sapiens) | BDBM50497306
(CHEMBL3335463)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CCCC)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSSC[C@H](NC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC2=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C89H144FN23O21S3/c1-9-10-20-60-85(131)110-39-16-24-66(110)82(128)97-51(6)73(119)108-64(47-114)80(126)107-63(46-53-28-30-54(90)31-29-53)79(125)109-65(81(127)105-61(22-12-14-37-92)86(132)113-42-19-27-69(113)87(133)111-40-17-25-67(111)83(129)99-55(72(93)118)32-33-70(116)117)48-137-136-44-35-59(102-76(122)58(34-43-135-8)103-84(130)68-26-18-41-112(68)88(134)71(50(4)5)98-52(7)115)77(123)106-62(45-49(2)3)78(124)101-57(23-15-38-96-89(94)95)74(120)100-56(75(121)104-60)21-11-13-36-91/h28-31,49-51,55-69,71,114H,9-27,32-48,91-92H2,1-8H3,(H2,93,118)(H,97,128)(H,98,115)(H,99,129)(H,100,120)(H,101,124)(H,102,122)(H,103,130)(H,104,121)(H,105,127)(H,106,123)(H,107,126)(H,108,119)(H,109,125)(H,116,117)(H4,94,95,96)/t51-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,71-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of YAP/ GST-TEAD1 (unknown origin) interaction by surface plasmon resonance assay |
ACS Med Chem Lett 5: 993-8 (2014)
Article DOI: 10.1021/ml500160m
BindingDB Entry DOI: 10.7270/Q28P63H7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078747

(CHEMBL3415628)Show SMILES CC1(CCN(C1=O)c1ccc2ncccc2c1)Nc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H20N4O3/c1-21(23-16-6-4-14(5-7-16)19(26)24-28)10-12-25(20(21)27)17-8-9-18-15(13-17)3-2-11-22-18/h2-9,11,13,23,28H,10,12H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50027662

(CHEMBL3338418)Show SMILES ONC(=O)c1ccc2CC[C@H](Cc2c1)Nc1nccc(n1)-c1cccnc1 |r| Show InChI InChI=1S/C20H19N5O2/c26-19(25-27)14-4-3-13-5-6-17(11-16(13)10-14)23-20-22-9-7-18(24-20)15-2-1-8-21-12-15/h1-4,7-10,12,17,27H,5-6,11H2,(H,25,26)(H,22,23,24)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using fluorogenic peptide as substrate by fluorescence assay |
J Med Chem 57: 8026-34 (2014)
Article DOI: 10.1021/jm5008962
BindingDB Entry DOI: 10.7270/Q29C700V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50027662

(CHEMBL3338418)Show SMILES ONC(=O)c1ccc2CC[C@H](Cc2c1)Nc1nccc(n1)-c1cccnc1 |r| Show InChI InChI=1S/C20H19N5O2/c26-19(25-27)14-4-3-13-5-6-17(11-16(13)10-14)23-20-22-9-7-18(24-20)15-2-1-8-21-12-15/h1-4,7-10,12,17,27H,5-6,11H2,(H,25,26)(H,22,23,24)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078680
(CHEMBL3415454)Show InChI InChI=1S/C17H16ClN3O3/c18-12-2-1-3-14(10-12)21-9-8-15(17(21)23)19-13-6-4-11(5-7-13)16(22)20-24/h1-7,10,15,19,24H,8-9H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50399004
(CHEMBL2177582)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1F)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H26ClFN4O2/c1-33-15-21(22(16-33)27(35)31-19-10-8-18(28)9-11-19)20-12-6-17(14-23(20)29)7-13-26(34)32-25-5-3-2-4-24(25)30/h2-14,21-22H,15-16,30H2,1H3,(H,31,35)(H,32,34)/b13-7+/t21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078690
(CHEMBL3415621)Show InChI InChI=1S/C18H16N4O3/c19-11-12-1-7-15(8-2-12)22-10-9-16(18(22)24)20-14-5-3-13(4-6-14)17(23)21-25/h1-8,16,20,25H,9-10H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078682
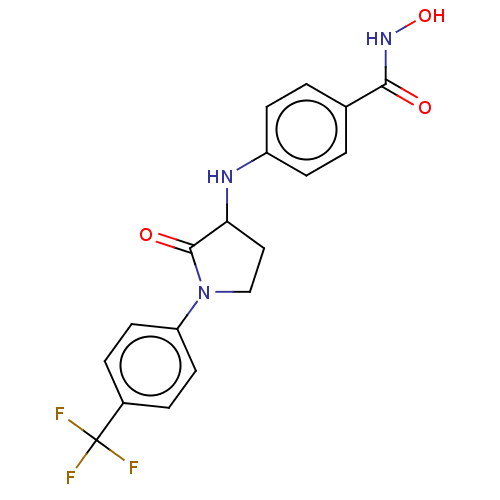
(CHEMBL3415618)Show SMILES ONC(=O)c1ccc(NC2CCN(C2=O)c2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H16F3N3O3/c19-18(20,21)12-3-7-14(8-4-12)24-10-9-15(17(24)26)22-13-5-1-11(2-6-13)16(25)23-27/h1-8,15,22,27H,9-10H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078689
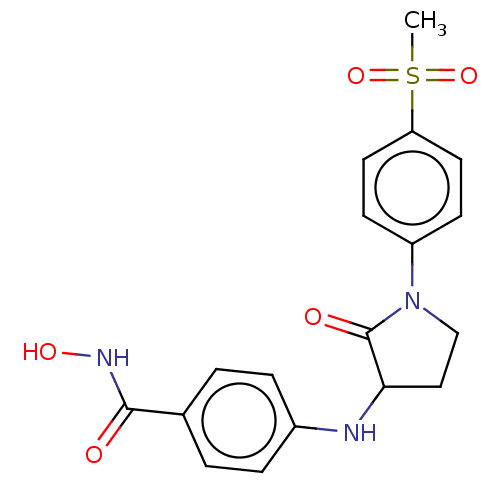
(CHEMBL3415620)Show SMILES CS(=O)(=O)c1ccc(cc1)N1CCC(Nc2ccc(cc2)C(=O)NO)C1=O Show InChI InChI=1S/C18H19N3O5S/c1-27(25,26)15-8-6-14(7-9-15)21-11-10-16(18(21)23)19-13-4-2-12(3-5-13)17(22)20-24/h2-9,16,19,24H,10-11H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078757
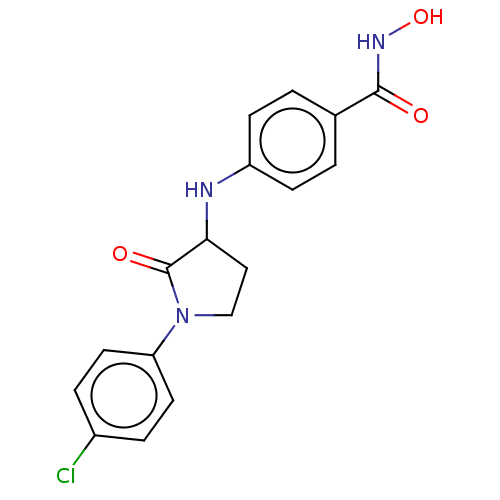
(CHEMBL3415453)Show InChI InChI=1S/C17H16ClN3O3/c18-12-3-7-14(8-4-12)21-10-9-15(17(21)23)19-13-5-1-11(2-6-13)16(22)20-24/h1-8,15,19,24H,9-10H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) using fluorogenic peptide as substrate by fluorescence assay |
J Med Chem 57: 8026-34 (2014)
Article DOI: 10.1021/jm5008962
BindingDB Entry DOI: 10.7270/Q29C700V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078689

(CHEMBL3415620)Show SMILES CS(=O)(=O)c1ccc(cc1)N1CCC(Nc2ccc(cc2)C(=O)NO)C1=O Show InChI InChI=1S/C18H19N3O5S/c1-27(25,26)15-8-6-14(7-9-15)21-11-10-16(18(21)23)19-13-4-2-12(3-5-13)17(22)20-24/h2-9,16,19,24H,10-11H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50399005
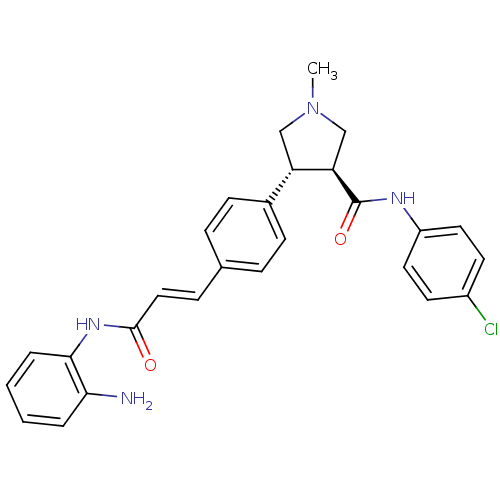
(CHEMBL2177588)Show SMILES CN1C[C@H]([C@@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Transcriptional enhancer factor TEF-1
(Homo sapiens) | BDBM50497296
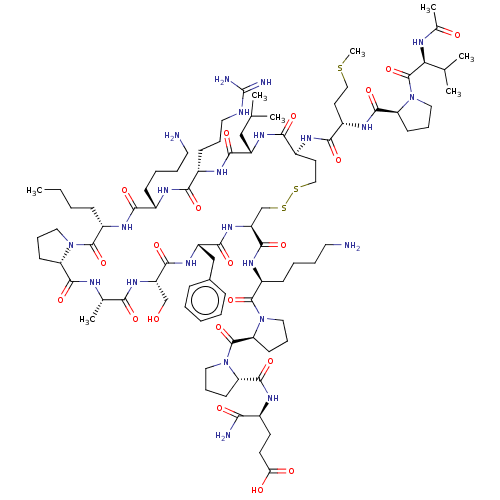
(CHEMBL3335452)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CCCC)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC2=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C89H145N23O21S3/c1-9-10-25-60-85(130)109-40-19-29-66(109)82(127)96-52(6)73(118)107-64(48-113)80(125)106-63(47-54-23-12-11-13-24-54)79(124)108-65(81(126)104-61(27-15-17-38-91)86(131)112-43-22-32-69(112)87(132)110-41-20-30-67(110)83(128)98-55(72(92)117)33-34-70(115)116)49-136-135-45-36-59(101-76(121)58(35-44-134-8)102-84(129)68-31-21-42-111(68)88(133)71(51(4)5)97-53(7)114)77(122)105-62(46-50(2)3)78(123)100-57(28-18-39-95-89(93)94)74(119)99-56(75(120)103-60)26-14-16-37-90/h11-13,23-24,50-52,55-69,71,113H,9-10,14-22,25-49,90-91H2,1-8H3,(H2,92,117)(H,96,127)(H,97,114)(H,98,128)(H,99,119)(H,100,123)(H,101,121)(H,102,129)(H,103,120)(H,104,126)(H,105,122)(H,106,125)(H,107,118)(H,108,124)(H,115,116)(H4,93,94,95)/t52-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,71-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of YAP/ GST-TEAD1 (unknown origin) interaction by surface plasmon resonance assay |
ACS Med Chem Lett 5: 993-8 (2014)
Article DOI: 10.1021/ml500160m
BindingDB Entry DOI: 10.7270/Q28P63H7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078681

(CHEMBL3415456)Show InChI InChI=1S/C17H16ClN3O3/c18-13-3-1-2-4-15(13)21-10-9-14(17(21)23)19-12-7-5-11(6-8-12)16(22)20-24/h1-8,14,19,24H,9-10H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078687

(CHEMBL3415619)Show SMILES ONC(=O)c1ccc(NC2CCN(C2=O)c2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C17H15Cl2N3O3/c18-13-6-5-12(9-14(13)19)22-8-7-15(17(22)24)20-11-3-1-10(2-4-11)16(23)21-25/h1-6,9,15,20,25H,7-8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078757

(CHEMBL3415453)Show InChI InChI=1S/C17H16ClN3O3/c18-12-3-7-14(8-4-12)21-10-9-15(17(21)23)19-13-5-1-11(2-6-13)16(22)20-24/h1-8,15,19,24H,9-10H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078690

(CHEMBL3415621)Show InChI InChI=1S/C18H16N4O3/c19-11-12-1-7-15(8-2-12)22-10-9-16(18(22)24)20-14-5-3-13(4-6-14)17(23)21-25/h1-8,16,20,25H,9-10H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM120985
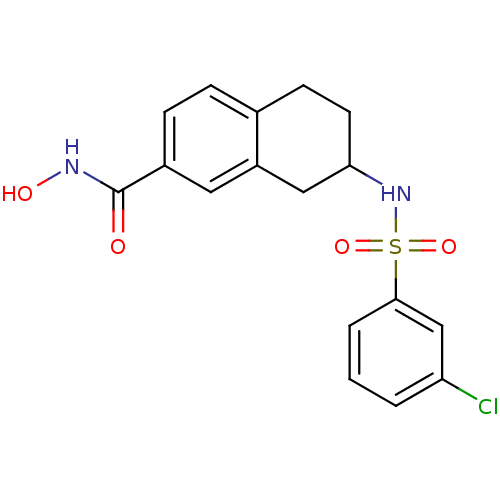
(US8716285, 42)Show SMILES ONC(=O)c1ccc2CCC(Cc2c1)NS(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C17H17ClN2O4S/c18-14-2-1-3-16(10-14)25(23,24)20-15-7-6-11-4-5-12(17(21)19-22)8-13(11)9-15/h1-5,8,10,15,20,22H,6-7,9H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit histone deacetylase 8 using an in vitro deacetylation assay. In a detailed procedure, 8 μl o... |
US Patent US8716285 (2014)
BindingDB Entry DOI: 10.7270/Q2K072XG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078680

(CHEMBL3415454)Show InChI InChI=1S/C17H16ClN3O3/c18-12-2-1-3-14(10-12)21-9-8-15(17(21)23)19-13-6-4-11(5-7-13)16(22)20-24/h1-7,10,15,19,24H,8-9H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078748

(CHEMBL3415629)Show SMILES C[C@@]1(CCN(C1=O)c1ccc(Cl)cc1)Nc1ccc(cc1)C(=O)NO |r| Show InChI InChI=1S/C18H18ClN3O3/c1-18(20-14-6-2-12(3-7-14)16(23)21-25)10-11-22(17(18)24)15-8-4-13(19)5-9-15/h2-9,20,25H,10-11H2,1H3,(H,21,23)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM120989

(US8716285, 46)Show SMILES ONC(=O)c1ccc2CCC(Cc2c1)NS(=O)(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C17H16Cl2N2O4S/c18-13-4-6-16(15(19)9-13)26(24,25)21-14-5-3-10-1-2-11(17(22)20-23)7-12(10)8-14/h1-2,4,6-7,9,14,21,23H,3,5,8H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit histone deacetylase 8 using an in vitro deacetylation assay. In a detailed procedure, 8 μl o... |
US Patent US8716285 (2014)
BindingDB Entry DOI: 10.7270/Q2K072XG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50334367
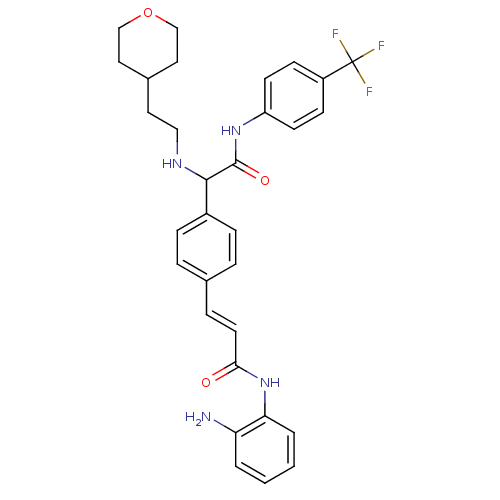
(CHEMBL1643315 | N-(2-aminophenyl)-3-(4-(2-oxo-1-(2...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCC1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C31H33F3N4O3/c32-31(33,34)24-10-12-25(13-11-24)37-30(40)29(36-18-15-22-16-19-41-20-17-22)23-8-5-21(6-9-23)7-14-28(39)38-27-4-2-1-3-26(27)35/h1-14,22,29,36H,15-20,35H2,(H,37,40)(H,38,39)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using fluorogenic peptide as substrate by fluorescence assay |
J Med Chem 57: 8026-34 (2014)
Article DOI: 10.1021/jm5008962
BindingDB Entry DOI: 10.7270/Q29C700V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of HDAC11 (unknown origin) using fluorogenic peptide as substrate by fluorescence assay |
J Med Chem 57: 8026-34 (2014)
Article DOI: 10.1021/jm5008962
BindingDB Entry DOI: 10.7270/Q29C700V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50399005

(CHEMBL2177588)Show SMILES CN1C[C@H]([C@@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM120987

(US8716285, 44)Show SMILES ONC(=O)c1ccc2CCC(Cc2c1)NS(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C17H16F2N2O4S/c18-13-4-6-16(15(19)9-13)26(24,25)21-14-5-3-10-1-2-11(17(22)20-23)7-12(10)8-14/h1-2,4,6-7,9,14,21,23H,3,5,8H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit histone deacetylase 8 using an in vitro deacetylation assay. In a detailed procedure, 8 μl o... |
US Patent US8716285 (2014)
BindingDB Entry DOI: 10.7270/Q2K072XG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50078750
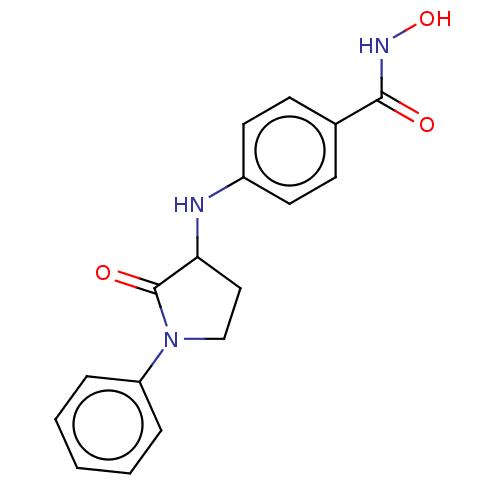
(CHEMBL3415446)Show InChI InChI=1S/C17H17N3O3/c21-16(19-23)12-6-8-13(9-7-12)18-15-10-11-20(17(15)22)14-4-2-1-3-5-14/h1-9,15,18,23H,10-11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using RHK(Ac)K(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50399004

(CHEMBL2177582)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1F)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H26ClFN4O2/c1-33-15-21(22(16-33)27(35)31-19-10-8-18(28)9-11-19)20-12-6-17(14-23(20)29)7-13-26(34)32-25-5-3-2-4-24(25)30/h2-14,21-22H,15-16,30H2,1H3,(H,31,35)(H,32,34)/b13-7+/t21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50399006

(CHEMBL2177587)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50399004

(CHEMBL2177582)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1F)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H26ClFN4O2/c1-33-15-21(22(16-33)27(35)31-19-10-8-18(28)9-11-19)20-12-6-17(14-23(20)29)7-13-26(34)32-25-5-3-2-4-24(25)30/h2-14,21-22H,15-16,30H2,1H3,(H,31,35)(H,32,34)/b13-7+/t21-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50078756
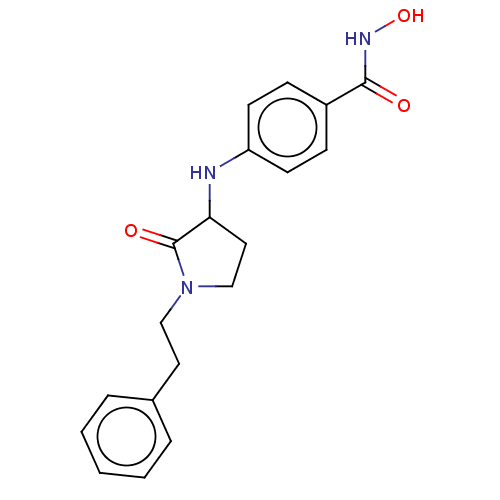
(CHEMBL3415452)Show InChI InChI=1S/C19H21N3O3/c23-18(21-25)15-6-8-16(9-7-15)20-17-11-13-22(19(17)24)12-10-14-4-2-1-3-5-14/h1-9,17,20,25H,10-13H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Innovation Center Shanghai
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using RHKK(Ac)AMC as substrate |
J Med Chem 58: 2809-20 (2015)
Article DOI: 10.1021/jm502011f
BindingDB Entry DOI: 10.7270/Q2B56MF0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data