 Found 186 hits with Last Name = 'woodruff' and Initial = 'gn'
Found 186 hits with Last Name = 'woodruff' and Initial = 'gn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to rat pancreas cholecystokinin type A receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50033600

(Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2 | CHEMBL...)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@@](C)(Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1)[C@@]([H])(C2)C3 |wU:14.16,29.32,41.45,1.0,wD:14.15,6.6,3.3,TLB:8:6:44:1.43.2,8:1:9.6.5:44,10:9:1.8.43:3.5.44,THB:2:3:9:1.8.43,2:1:9:3.5.44,(.78,-3.99,;.75,-5.53,;-.6,-6,;-.58,-7.49,;-2.01,-6.93,;.74,-7.97,;2.13,-7.64,;3.09,-4.97,;2.15,-6.11,;1.12,-8.9,;2.61,-9.32,;3.7,-8.22,;3.41,-6.97,;5.18,-8.64,;6.27,-7.55,;4.94,-6.78,;6.3,-6.01,;6.78,-4.56,;5.87,-3.32,;6.78,-2.06,;8.25,-2.55,;9.57,-1.76,;10.91,-2.53,;10.91,-4.09,;9.57,-4.86,;8.25,-4.09,;7.6,-8.32,;8.93,-7.55,;7.6,-9.86,;8.93,-10.63,;10.25,-9.86,;11.79,-9.85,;12.56,-11.18,;12.56,-8.5,;8.93,-12.17,;10.27,-12.94,;10.25,-14.48,;11.58,-15.25,;12.91,-14.48,;12.91,-12.94,;11.59,-12.17,;-.28,-8.34,;-.37,-9.86,;-.29,-6.75,;-1.79,-8.77,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21-,22+,23-,24+,26-,30?,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type A receptor in the rat pancreas. |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type A receptor in the rat pancreas. |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to rat pancreas cholecystokinin type A receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type A receptor in the rat pancreas. |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033586

(3-[2-[(Adamantane-2-carbonyl)-amino]-3-(1H-indol-3...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)C1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CC(O)=O)Cc1ccccc1 |wU:1.0,wD:1.13,28.32,TLB:13:15:22:18.24.19,17:18:22:15.16.21,THB:17:16:22:18.24.19,19:20:15:18.17.24,19:18:15:20.22.21,(4.56,-2.63,;5.88,-3.4,;5.9,-1.86,;6.38,-.4,;5.47,.83,;6.38,2.09,;7.84,1.6,;9.18,2.4,;10.5,1.63,;10.5,.06,;9.18,-.71,;7.84,.06,;4.79,-4.47,;3.3,-4.07,;2.92,-2.58,;2,-4.89,;1.89,-6.4,;2.74,-7.71,;1.17,-6.96,;-.25,-7.42,;-1.11,-6.29,;.39,-6.9,;-.98,-4.78,;.46,-4.31,;1.23,-5.55,;7.22,-4.17,;8.53,-3.4,;7.2,-5.71,;8.53,-6.48,;9.86,-5.69,;11.4,-5.69,;12.17,-4.36,;12.17,-7.03,;8.53,-8.02,;9.86,-8.79,;9.85,-10.33,;11.18,-11.08,;12.51,-10.33,;12.51,-8.79,;11.23,-7.99,)| Show InChI InChI=1S/C33H39N3O4/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,32(40)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-31(39)30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,40)(H,36,39)(H,37,38)/t21?,22?,23?,24?,26-,30?,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033600

(Asp-Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2 | CHEMBL...)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@@](C)(Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1)[C@@]([H])(C2)C3 |wU:14.16,29.32,41.45,1.0,wD:14.15,6.6,3.3,TLB:8:6:44:1.43.2,8:1:9.6.5:44,10:9:1.8.43:3.5.44,THB:2:3:9:1.8.43,2:1:9:3.5.44,(.78,-3.99,;.75,-5.53,;-.6,-6,;-.58,-7.49,;-2.01,-6.93,;.74,-7.97,;2.13,-7.64,;3.09,-4.97,;2.15,-6.11,;1.12,-8.9,;2.61,-9.32,;3.7,-8.22,;3.41,-6.97,;5.18,-8.64,;6.27,-7.55,;4.94,-6.78,;6.3,-6.01,;6.78,-4.56,;5.87,-3.32,;6.78,-2.06,;8.25,-2.55,;9.57,-1.76,;10.91,-2.53,;10.91,-4.09,;9.57,-4.86,;8.25,-4.09,;7.6,-8.32,;8.93,-7.55,;7.6,-9.86,;8.93,-10.63,;10.25,-9.86,;11.79,-9.85,;12.56,-11.18,;12.56,-8.5,;8.93,-12.17,;10.27,-12.94,;10.25,-14.48,;11.58,-15.25,;12.91,-14.48,;12.91,-12.94,;11.59,-12.17,;-.28,-8.34,;-.37,-9.86,;-.29,-6.75,;-1.79,-8.77,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21-,22+,23-,24+,26-,30?,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50456331

(CHEMBL2111203)Show SMILES O.CC1CCCCC1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)NC(CO)Cc1ccccc1 Show InChI InChI=1S/C29H37N3O4.H2O/c1-20-10-6-9-15-26(20)36-28(35)32-29(2,17-22-18-30-25-14-8-7-13-24(22)25)27(34)31-23(19-33)16-21-11-4-3-5-12-21;/h3-5,7-8,11-14,18,20,23,26,30,33H,6,9-10,15-17,19H2,1-2H3,(H,31,34)(H,32,35);1H2/t20?,23?,26?,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50456329
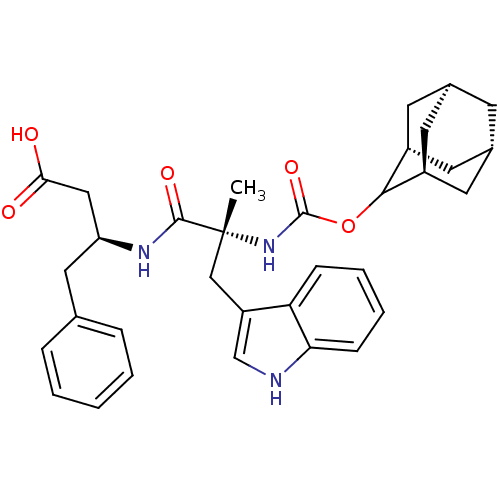
(CHEMBL2111261)Show SMILES O.[H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)N[C@H](CC(O)=O)Cc1ccccc1)[C@@]([H])(C2)C3 |wU:15.15,42.45,2.0,wD:15.16,30.32,7.6,4.3,TLB:9:7:45:2.44.3,9:2:10.7.6:45,11:10:2.9.44:4.6.45,THB:3:4:10:2.9.44,3:2:10:4.6.45,(18.02,-11.65,;.78,-3.99,;.75,-5.53,;-.6,-6,;-.58,-7.49,;-2.01,-6.93,;.74,-7.97,;2.13,-7.64,;3.09,-4.97,;2.15,-6.11,;1.12,-8.9,;2.61,-9.32,;3.7,-8.22,;3.41,-6.97,;5.18,-8.64,;6.27,-7.55,;4.94,-6.78,;6.3,-6.01,;6.78,-4.56,;5.87,-3.32,;6.78,-2.06,;8.25,-2.55,;9.57,-1.76,;10.91,-2.53,;10.91,-4.09,;9.57,-4.86,;8.25,-4.09,;7.6,-8.32,;8.93,-7.55,;7.6,-9.86,;8.93,-10.63,;10.25,-9.86,;11.79,-9.85,;12.56,-11.18,;12.56,-8.5,;8.93,-12.17,;10.27,-12.94,;10.25,-14.48,;11.58,-15.25,;12.91,-14.48,;12.91,-12.94,;11.59,-12.17,;-.28,-8.34,;-.37,-9.86,;-.29,-6.75,;-1.79,-8.77,)| Show InChI InChI=1S/C33H39N3O5.H2O/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21;/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38);1H2/t21-,22+,23-,24+,26-,30?,33+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033587
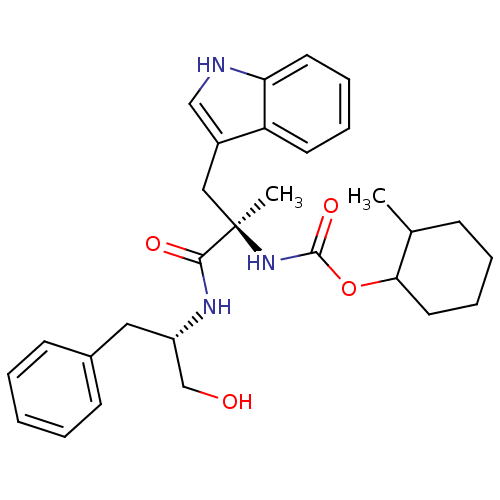
((1R,2R)-[1-(1-Hydroxymethyl-2-phenyl-ethylcarbamoy...)Show SMILES CC1CCCCC1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)N[C@H](CO)Cc1ccccc1 Show InChI InChI=1S/C29H37N3O4/c1-20-10-6-9-15-26(20)36-28(35)32-29(2,17-22-18-30-25-14-8-7-13-24(22)25)27(34)31-23(19-33)16-21-11-4-3-5-12-21/h3-5,7-8,11-14,18,20,23,26,30,33H,6,9-10,15-17,19H2,1-2H3,(H,31,34)(H,32,35)/t20?,23-,26?,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007439
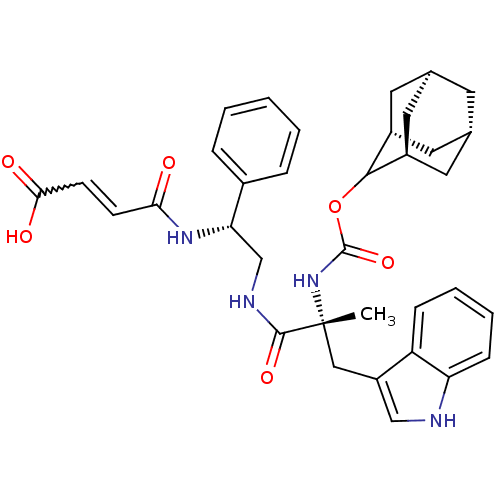
(3-{2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-ind...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)NC[C@H](NC(=O)C=CC(O)=O)c1ccccc1 |w:35.40,wU:21.28,17.19,1.0,wD:1.13,30.35,19.20,23.24,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,(7.42,-12.42,;7.33,-10.91,;7.31,-9.36,;8.4,-8.29,;7.5,-7.05,;8.4,-5.8,;9.88,-6.28,;11.19,-5.5,;12.54,-6.27,;12.54,-7.82,;11.19,-8.59,;9.88,-7.82,;5.99,-11.7,;4.64,-10.94,;4.64,-9.4,;3.32,-11.71,;1.78,-11.71,;1.07,-13.06,;-.42,-13.72,;-1.41,-12.58,;-2.95,-12.26,;-1.31,-11.68,;-.63,-10.35,;.78,-10.54,;-.7,-11.16,;-.34,-12.73,;8.66,-11.67,;9.99,-10.87,;8.68,-13.21,;10.03,-13.96,;11.36,-13.19,;12.67,-12.38,;14.22,-12.38,;14.22,-10.84,;15.53,-11.61,;16.88,-12.37,;18.22,-11.58,;19.55,-12.35,;18.2,-10.04,;12.69,-13.95,;14.01,-13.16,;15.34,-13.92,;15.37,-15.47,;14.04,-16.24,;12.69,-15.49,)| Show InChI InChI=1S/C35H40N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-12,19,21-22,24-25,29,32,36H,13-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21-,22+,24-,25+,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex Cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033606

(3-{2-[2-[(Adamantane-2-carbonyl)-amino]-3-(1H-indo...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)C1C2CC3CC(C2)CC1C3)C(=O)NC[C@H](NC(=O)\C=C\C(O)=O)c1ccccc1 |wU:29.34,1.0,wD:1.13,TLB:21:16:24:20.22.19,21:20:15.16.17:24,THB:13:15:24:20.22.19,19:18:15:20.22.21,19:20:15:18.17.24,(7.9,-1.29,;9.25,-2.06,;9.25,-.52,;9.73,.93,;8.83,2.18,;9.73,3.43,;11.21,2.95,;12.52,3.72,;13.87,2.95,;13.87,1.41,;12.52,.64,;11.21,1.41,;8.16,-3.14,;6.67,-2.74,;6.27,-1.25,;5.26,-3.38,;3.83,-2.58,;4.41,-3.89,;4.14,-5.29,;2.67,-5.55,;1.99,-4.3,;2.32,-2.82,;3.37,-5.11,;4.94,-4.86,;5.59,-6.27,;10.57,-2.83,;11.9,-2.06,;10.57,-4.37,;11.9,-5.14,;11.88,-6.67,;10.56,-7.45,;10.56,-8.99,;11.88,-9.76,;9.22,-9.75,;7.89,-8.98,;6.56,-9.75,;6.54,-11.29,;5.21,-8.98,;13.23,-7.45,;14.58,-6.65,;15.88,-7.45,;15.88,-8.99,;14.54,-9.76,;13.22,-8.99,)| Show InChI InChI=1S/C35H40N4O5/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-33(43)32-24-14-21-13-22(16-24)17-25(32)15-21)34(44)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-12,19,21-22,24-25,29,32,36H,13-18,20H2,1H3,(H,37,44)(H,38,40)(H,39,43)(H,41,42)/b12-11+/t21?,22?,24?,25?,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50452998
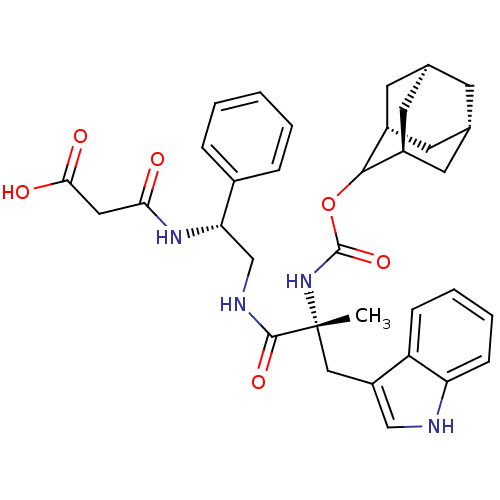
(CHEMBL2112693)Show SMILES O.[H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1[C@@]2([H])C[C@]3([H])C[C@@]([H])(C[C@@]1([H])C3)C2)(NC(=O)CC(O)=O)c1ccccc1 |wU:7.6,24.25,30.31,2.0,wD:2.40,33.35,27.28,TLB:22:23:26:30.36.29,THB:32:33:26:30.36.29,32:30:23.33.35:26,29:30:23:27.35.26,29:27:23:30.36.32,(21.23,-10.12,;15.18,-14.74,;15.19,-16.28,;13.87,-17.07,;12.52,-16.31,;12.51,-14.77,;13.84,-13.99,;11.18,-14,;11.27,-15.53,;11.17,-12.48,;12.26,-11.4,;11.34,-10.15,;12.26,-8.9,;13.71,-9.38,;15.05,-8.61,;16.37,-9.38,;16.37,-10.92,;15.05,-11.69,;13.71,-10.92,;9.85,-14.8,;8.5,-14.03,;8.48,-12.49,;7.17,-14.82,;5.63,-14.8,;4.65,-13.65,;5.37,-12.3,;3.24,-13.45,;2.57,-14.77,;1.55,-13.61,;.91,-15.37,;2.45,-15.67,;1.67,-17,;3.44,-16.82,;4.92,-16.15,;5.98,-17.27,;3.51,-15.83,;3.16,-14.26,;16.52,-15.5,;18.06,-15.5,;18.04,-13.96,;19.38,-14.73,;20.73,-15.48,;22.05,-14.69,;20.74,-17.02,;16.53,-17.05,;17.85,-16.27,;19.19,-17.02,;19.2,-18.56,;17.88,-19.35,;16.53,-18.59,)| Show InChI InChI=1S/C34H40N4O6.H2O/c1-34(17-25-18-35-27-10-6-5-9-26(25)27,38-33(43)44-31-23-12-20-11-21(14-23)15-24(31)13-20)32(42)36-19-28(22-7-3-2-4-8-22)37-29(39)16-30(40)41;/h2-10,18,20-21,23-24,28,31,35H,11-17,19H2,1H3,(H,36,42)(H,37,39)(H,38,43)(H,40,41);1H2/t20-,21+,23-,24+,28-,31?,34+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex Cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033604
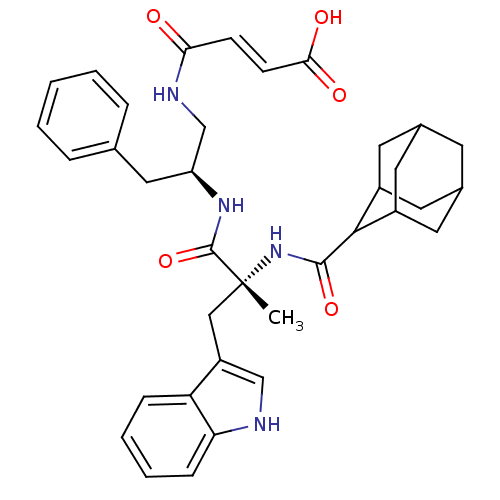
(3-{2-[2-[(Adamantane-2-carbonyl)-amino]-3-(1H-indo...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)C1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CNC(=O)\C=C\C(O)=O)Cc1ccccc1 |wU:1.0,wD:1.13,28.32,TLB:13:15:17:20.21.19,THB:22:23:17:20.21.19,22:20:17:15.23.24,19:18:15:20.21.22,19:20:15:18.17.24,(4.56,-2.64,;5.89,-3.41,;5.91,-1.86,;6.4,-.4,;5.48,.84,;6.4,2.09,;7.85,1.61,;9.19,2.4,;10.52,1.63,;10.52,.06,;9.19,-.71,;7.85,.06,;4.8,-4.48,;3.31,-4.08,;2.92,-2.58,;2,-4.9,;.46,-4.32,;-.99,-4.79,;-1.11,-6.3,;-.25,-7.44,;1.17,-6.98,;1.23,-5.56,;2.74,-7.73,;1.9,-6.41,;.39,-6.91,;7.23,-4.18,;8.55,-3.41,;7.21,-5.72,;8.55,-6.49,;9.88,-5.7,;11.22,-6.46,;12.55,-5.69,;12.54,-4.15,;13.89,-6.43,;15.21,-5.66,;16.56,-6.41,;17.9,-5.62,;16.59,-7.95,;8.55,-8.04,;9.88,-8.81,;11.25,-8,;12.54,-8.81,;12.54,-10.35,;11.2,-11.1,;9.87,-10.35,)| Show InChI InChI=1S/C36H42N4O5/c1-36(19-27-20-37-30-10-6-5-9-29(27)30,40-34(44)33-25-14-23-13-24(16-25)17-26(33)15-23)35(45)39-28(18-22-7-3-2-4-8-22)21-38-31(41)11-12-32(42)43/h2-12,20,23-26,28,33,37H,13-19,21H2,1H3,(H,38,41)(H,39,45)(H,40,44)(H,42,43)/b12-11+/t23?,24?,25?,26?,28-,33?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007449
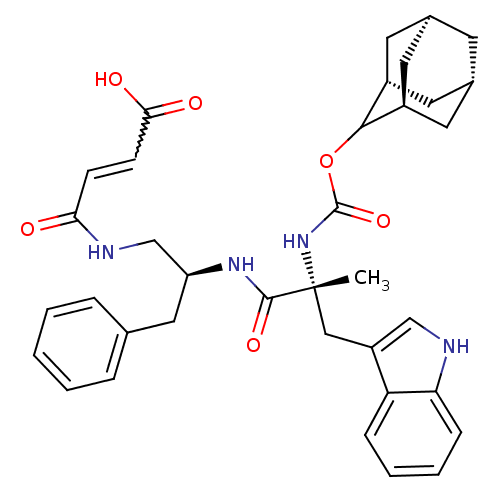
(3-{2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-ind...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N[C@H](CNC(=O)C=CC(O)=O)Cc1ccccc1 |w:35.40,wU:19.27,17.29,23.24,29.33,1.0,wD:1.13,21.23,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:18:16.23.24,20:21:16:19.18.24,(21.99,-13.05,;21.9,-11.54,;21.87,-10,;22.97,-8.93,;22.07,-7.68,;22.97,-6.43,;24.44,-6.91,;25.76,-6.13,;27.11,-6.9,;27.11,-8.45,;25.76,-9.23,;24.44,-8.45,;20.56,-12.33,;19.21,-11.57,;19.21,-10.03,;17.89,-12.34,;16.34,-12.33,;15.36,-11.17,;13.94,-10.98,;13.27,-12.31,;11.63,-12.89,;13.16,-13.21,;14.16,-14.35,;15.65,-13.69,;14.23,-13.36,;13.88,-11.79,;23.22,-12.3,;24.56,-11.5,;23.24,-13.84,;24.59,-14.59,;24.6,-16.13,;23.28,-16.92,;23.29,-18.46,;24.63,-19.21,;21.96,-19.24,;20.62,-18.49,;19.3,-19.27,;17.95,-18.5,;19.31,-20.82,;25.92,-13.82,;27.26,-14.58,;28.57,-13.78,;29.9,-14.54,;29.93,-16.1,;28.61,-16.87,;27.26,-16.12,)| Show InChI InChI=1S/C36H42N4O6/c1-36(19-27-20-37-30-10-6-5-9-29(27)30,40-35(45)46-33-25-14-23-13-24(16-25)17-26(33)15-23)34(44)39-28(18-22-7-3-2-4-8-22)21-38-31(41)11-12-32(42)43/h2-12,20,23-26,28,33,37H,13-19,21H2,1H3,(H,38,41)(H,39,44)(H,40,45)(H,42,43)/t23-,24+,25-,26+,28-,33?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50024321
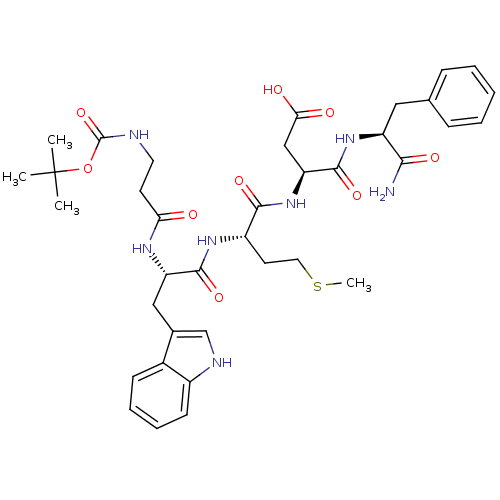
(3-{2-[2-(3-tert-Butoxycarbonylamino-propionylamino...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50024321

(3-{2-[2-(3-tert-Butoxycarbonylamino-propionylamino...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033592
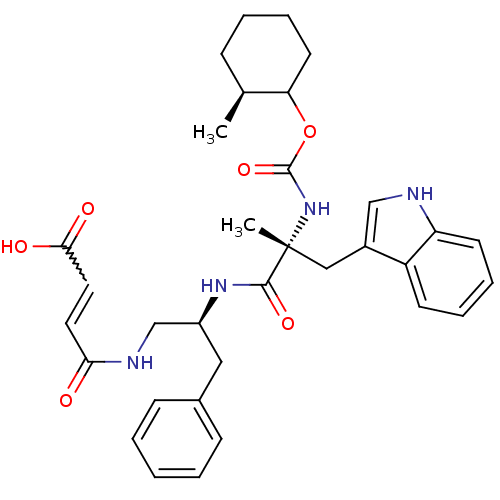
(3-{2-[3-(1H-Indol-3-yl)-2-methyl-2-(2-methyl-cyclo...)Show SMILES C[C@H]1CCCCC1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)N[C@H](CNC(=O)C=CC(O)=O)Cc1ccccc1 |w:32.35| Show InChI InChI=1S/C33H40N4O6/c1-22-10-6-9-15-28(22)43-32(42)37-33(2,19-24-20-34-27-14-8-7-13-26(24)27)31(41)36-25(18-23-11-4-3-5-12-23)21-35-29(38)16-17-30(39)40/h3-5,7-8,11-14,16-17,20,22,25,28,34H,6,9-10,15,18-19,21H2,1-2H3,(H,35,38)(H,36,41)(H,37,42)(H,39,40)/t22-,25-,28?,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033598

(CHEMBL345511 | N-{2-[2-[(Adamantane-2-carbonyl)-am...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)C1C2CC3CC(C2)CC1C3)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1 |wU:29.34,1.0,wD:1.13,TLB:24:23:21:18.17.19,THB:13:15:21:18.17.19,24:18:21:15.23.22,19:20:15:18.17.24,19:18:15:20.21.22,(7.9,-1.29,;9.25,-2.06,;9.25,-.52,;9.73,.93,;8.83,2.18,;9.73,3.43,;11.21,2.95,;12.52,3.72,;13.87,2.95,;13.87,1.41,;12.52,.64,;11.21,1.41,;8.16,-3.14,;6.67,-2.74,;6.27,-1.25,;5.26,-3.38,;4.94,-4.86,;3.37,-5.11,;1.99,-4.3,;2.67,-5.55,;4.14,-5.29,;5.59,-6.27,;4.41,-3.89,;3.83,-2.58,;2.32,-2.82,;10.57,-2.83,;11.9,-2.06,;10.57,-4.37,;11.9,-5.14,;11.88,-6.67,;10.56,-7.45,;10.56,-8.99,;11.88,-9.76,;9.22,-9.75,;7.89,-8.98,;6.56,-9.75,;6.54,-11.29,;5.21,-8.98,;13.23,-7.45,;14.58,-6.65,;15.88,-7.45,;15.88,-8.99,;14.54,-9.76,;13.22,-8.99,)| Show InChI InChI=1S/C35H42N4O5/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-33(43)32-24-14-21-13-22(16-24)17-25(32)15-21)34(44)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,44)(H,38,40)(H,39,43)(H,41,42)/t21?,22?,24?,25?,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50449787
(CHEMBL2062154 | PD-134308)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1)[C@@]([H])(C2)C3 |wU:30.41,14.15,45.49,3.3,wD:6.6,1.0,TLB:5:3:47:9.6.8,10:9:47:3.48.2,THB:5:6:47:3.48.2,10:9:1.47.8:3.5.48,2:3:9:1.47.8,2:1:9:3.5.48,(-14.99,-2.1,;-13.56,-2.66,;-14.77,-3.94,;-13.26,-3.53,;-13.35,-5.06,;-11.86,-4.09,;-10.83,-2.82,;-9.38,-3.33,;-12.24,-3.16,;-10.83,-1.28,;-9.29,-1.31,;-8.5,.01,;-9.25,1.36,;-6.96,-.01,;-6.19,1.3,;-5.42,-.02,;-7.44,2.2,;-7.28,3.74,;-8.44,4.76,;-7.81,6.18,;-6.29,6.03,;-5.14,7.07,;-3.69,6.6,;-3.34,5.08,;-4.49,4.06,;-5.94,4.52,;-4.66,1.42,;-3.99,2.8,;-3.79,.15,;-2.27,.27,;-1.4,-1.02,;-2.08,-2.4,;-1.22,-3.69,;.32,-3.58,;-1.9,-5.07,;-3.43,-5.16,;-4.1,-6.57,;-5.64,-6.67,;-3.25,-7.83,;.14,-.91,;.99,-2.2,;2.51,-2.08,;3.19,-.7,;2.32,.59,;.8,.47,;-12.23,-.7,;-12.2,.82,;-13.58,-1.18,;-13.27,-1.94,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21-,22+,24-,25+,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex Cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM50079412
((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C55H79N13O14S2/c1-30(2)21-38(50(77)62-36(47(57)74)17-19-83-5)61-43(69)28-59-55(82)46(31(3)4)68-54(81)40(23-33-15-11-8-12-16-33)65-51(78)39(22-32-13-9-7-10-14-32)64-53(80)42(26-45(72)73)67-52(79)41(24-34-27-58-29-60-34)66-49(76)37(18-20-84-6)63-48(75)35(56)25-44(70)71/h7-16,27,29-31,35-42,46H,17-26,28,56H2,1-6H3,(H2,57,74)(H,58,60)(H,59,82)(H,61,69)(H,62,77)(H,63,75)(H,64,80)(H,65,78)(H,66,76)(H,67,79)(H,68,81)(H,70,71)(H,72,73)/t35-,36-,37-,38-,39-,40-,41-,42-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 3 in guinea pig cortical membranes labeled with [125 I][MePhe7]-NKB |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50079412

((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C55H79N13O14S2/c1-30(2)21-38(50(77)62-36(47(57)74)17-19-83-5)61-43(69)28-59-55(82)46(31(3)4)68-54(81)40(23-33-15-11-8-12-16-33)65-51(78)39(22-32-13-9-7-10-14-32)64-53(80)42(26-45(72)73)67-52(79)41(24-34-27-58-29-60-34)66-49(76)37(18-20-84-6)63-48(75)35(56)25-44(70)71/h7-16,27,29-31,35-42,46H,17-26,28,56H2,1-6H3,(H2,57,74)(H,58,60)(H,59,82)(H,61,69)(H,62,77)(H,63,75)(H,64,80)(H,65,78)(H,66,76)(H,67,79)(H,68,81)(H,70,71)(H,72,73)/t35-,36-,37-,38-,39-,40-,41-,42-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 3 in guinea pig cortical membranes labeled with [125 I][MePhe7]-NKB |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007448

(CHEMBL131754 | N-{2-[2-(Adamantan-2-yloxycarbonyla...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N[C@H](CNC(=O)CC(O)=O)Cc1ccccc1 |wU:19.27,17.29,29.33,1.0,wD:1.13,21.23,23.26,TLB:15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:16.23.24:18,(17.95,-9.98,;17.85,-8.45,;17.85,-6.93,;18.94,-5.85,;18.03,-4.6,;18.94,-3.35,;20.41,-3.83,;21.73,-3.06,;23.07,-3.83,;23.07,-5.37,;21.73,-6.14,;20.41,-5.37,;16.53,-9.25,;15.19,-8.48,;15.18,-6.94,;13.86,-9.27,;12.32,-9.25,;11.34,-8.1,;9.92,-7.9,;9.25,-9.22,;7.6,-9.82,;9.12,-10.12,;10.12,-11.27,;11.61,-10.6,;10.2,-10.28,;9.85,-8.71,;19.2,-9.22,;20.51,-8.44,;19.2,-10.76,;20.54,-11.52,;20.57,-13.06,;19.23,-13.84,;19.25,-15.38,;20.58,-16.14,;17.91,-16.15,;16.59,-15.41,;15.25,-16.18,;16.57,-13.87,;21.88,-10.73,;23.21,-11.5,;23.21,-13.04,;24.55,-13.8,;25.87,-13,;25.87,-11.46,;24.52,-10.72,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-29-10-6-5-9-28(26)29,39-34(44)45-32-24-12-22-11-23(14-24)15-25(32)13-22)33(43)38-27(16-21-7-3-2-4-8-21)20-37-30(40)17-31(41)42/h2-10,19,22-25,27,32,36H,11-18,20H2,1H3,(H,37,40)(H,38,43)(H,39,44)(H,41,42)/t22-,23+,24-,25+,27-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007436
(CHEMBL334346 | Succinic acid mono-{2-[2-(adamantan...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N[C@H](COC(=O)CCC(O)=O)Cc1ccccc1 |wU:21.28,23.26,17.19,29.33,1.0,wD:1.13,19.20,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,20:19:16:21.22.25,(16.6,-9.44,;16.5,-7.94,;16.49,-6.4,;17.58,-5.3,;16.66,-4.07,;17.58,-2.82,;19.04,-3.3,;20.37,-2.51,;21.7,-3.28,;21.7,-4.84,;20.37,-5.61,;19.04,-4.84,;15.18,-8.71,;13.83,-7.96,;13.81,-6.42,;12.49,-8.74,;10.95,-8.71,;10.25,-10.07,;8.77,-10.73,;7.78,-9.59,;6.24,-9.28,;7.89,-8.69,;8.57,-7.36,;9.98,-7.55,;8.48,-8.18,;8.83,-9.73,;17.84,-8.67,;19.16,-7.9,;17.85,-10.21,;19.2,-10.98,;19.2,-12.52,;17.88,-13.29,;17.88,-14.83,;19.23,-15.6,;16.57,-15.62,;15.22,-14.87,;13.9,-15.66,;12.56,-14.9,;13.9,-17.2,;20.51,-10.2,;21.86,-10.95,;23.18,-10.18,;24.51,-10.92,;24.52,-12.48,;23.21,-13.26,;21.86,-12.49,)| Show InChI InChI=1S/C36H43N3O7/c1-36(19-27-20-37-30-10-6-5-9-29(27)30,39-35(44)46-33-25-14-23-13-24(16-25)17-26(33)15-23)34(43)38-28(18-22-7-3-2-4-8-22)21-45-32(42)12-11-31(40)41/h2-10,20,23-26,28,33,37H,11-19,21H2,1H3,(H,38,43)(H,39,44)(H,40,41)/t23-,24+,25-,26+,28-,33?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033593
(CHEMBL356599 | Succinic acid mono-{2-[2-[(adamanta...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)C1C2CC3CC(C2)CC1C3)C(=O)N[C@H](COC(=O)CCC(O)=O)Cc1ccccc1 |wU:1.0,wD:1.13,28.32,TLB:13:15:17:20.21.19,THB:22:23:17:20.21.19,22:20:17:15.23.24,19:20:15:18.17.24,19:18:15:20.21.22,(4.56,-2.63,;5.88,-3.4,;5.9,-1.86,;6.38,-.4,;5.47,.83,;6.38,2.09,;7.84,1.6,;9.18,2.4,;10.5,1.63,;10.5,.06,;9.18,-.71,;7.84,.06,;4.79,-4.47,;3.3,-4.07,;2.92,-2.58,;1.9,-4.75,;.46,-3.99,;-1.03,-4.3,;-1.34,-5.79,;-.6,-7.01,;.87,-6.71,;1.09,-5.3,;2.35,-7.61,;1.65,-6.23,;.08,-6.56,;7.22,-4.17,;8.53,-3.4,;7.2,-5.71,;8.53,-6.48,;9.86,-5.69,;11.2,-6.45,;12.52,-5.68,;12.51,-4.14,;13.86,-6.42,;15.19,-5.65,;16.53,-6.4,;17.87,-5.61,;16.56,-7.94,;8.53,-8.02,;9.86,-8.79,;11.23,-7.99,;12.51,-8.79,;12.51,-10.33,;11.18,-11.08,;9.85,-10.33,)| Show InChI InChI=1S/C36H43N3O6/c1-36(19-27-20-37-30-10-6-5-9-29(27)30,39-34(43)33-25-14-23-13-24(16-25)17-26(33)15-23)35(44)38-28(18-22-7-3-2-4-8-22)21-45-32(42)12-11-31(40)41/h2-10,20,23-26,28,33,37H,11-19,21H2,1H3,(H,38,44)(H,39,43)(H,40,41)/t23?,24?,25?,26?,28-,33?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(HAMSTER) | BDBM50079412

((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C55H79N13O14S2/c1-30(2)21-38(50(77)62-36(47(57)74)17-19-83-5)61-43(69)28-59-55(82)46(31(3)4)68-54(81)40(23-33-15-11-8-12-16-33)65-51(78)39(22-32-13-9-7-10-14-32)64-53(80)42(26-45(72)73)67-52(79)41(24-34-27-58-29-60-34)66-49(76)37(18-20-84-6)63-48(75)35(56)25-44(70)71/h7-16,27,29-31,35-42,46H,17-26,28,56H2,1-6H3,(H2,57,74)(H,58,60)(H,59,82)(H,61,69)(H,62,77)(H,63,75)(H,64,80)(H,65,78)(H,66,76)(H,67,79)(H,68,81)(H,70,71)(H,72,73)/t35-,36-,37-,38-,39-,40-,41-,42-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 2 in membranes prepared from hamster urinary bladder labeled with [125 I] NKA |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033596
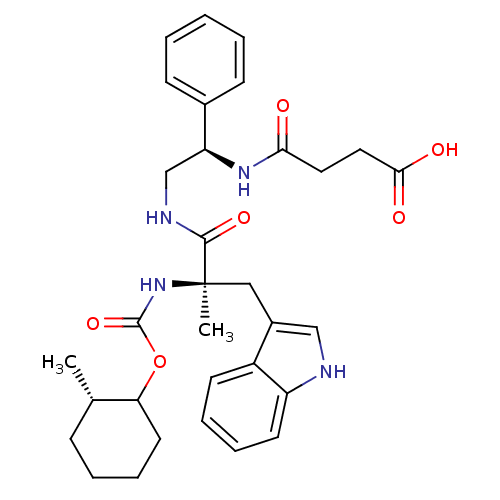
(CHEMBL153557 | N-{2-[3-(1H-Indol-3-yl)-2-methyl-2-...)Show SMILES C[C@H]1CCCCC1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1 Show InChI InChI=1S/C32H40N4O6/c1-21-10-6-9-15-27(21)42-31(41)36-32(2,18-23-19-33-25-14-8-7-13-24(23)25)30(40)34-20-26(22-11-4-3-5-12-22)35-28(37)16-17-29(38)39/h3-5,7-8,11-14,19,21,26-27,33H,6,9-10,15-18,20H2,1-2H3,(H,34,40)(H,35,37)(H,36,41)(H,38,39)/t21-,26-,27?,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033599
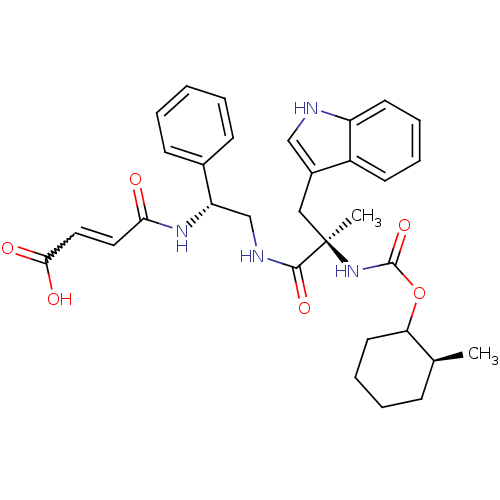
(3-{2-[3-(1H-Indol-3-yl)-2-methyl-2-(2-methyl-cyclo...)Show SMILES C[C@H]1CCCCC1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)NC[C@H](NC(=O)C=CC(O)=O)c1ccccc1 |w:32.35| Show InChI InChI=1S/C32H38N4O6/c1-21-10-6-9-15-27(21)42-31(41)36-32(2,18-23-19-33-25-14-8-7-13-24(23)25)30(40)34-20-26(22-11-4-3-5-12-22)35-28(37)16-17-29(38)39/h3-5,7-8,11-14,16-17,19,21,26-27,33H,6,9-10,15,18,20H2,1-2H3,(H,34,40)(H,35,37)(H,36,41)(H,38,39)/t21-,26-,27?,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50050641
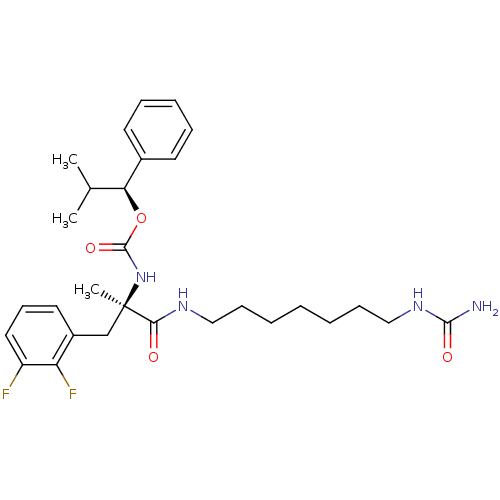
(CHEMBL45340 | PD-161182 | [(R)-2-(2,3-Difluoro-phe...)Show SMILES CC(C)[C@H](OC(=O)N[C@](C)(Cc1cccc(F)c1F)C(=O)NCCCCCCCNC(N)=O)c1ccccc1 Show InChI InChI=1S/C29H40F2N4O4/c1-20(2)25(21-13-8-7-9-14-21)39-28(38)35-29(3,19-22-15-12-16-23(30)24(22)31)26(36)33-17-10-5-4-6-11-18-34-27(32)37/h7-9,12-16,20,25H,4-6,10-11,17-19H2,1-3H3,(H,33,36)(H,35,38)(H3,32,34,37)/t25-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 3 in guinea pig cortical membranes labeled with [125 I]-[MePhe7] |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM50050641

(CHEMBL45340 | PD-161182 | [(R)-2-(2,3-Difluoro-phe...)Show SMILES CC(C)[C@H](OC(=O)N[C@](C)(Cc1cccc(F)c1F)C(=O)NCCCCCCCNC(N)=O)c1ccccc1 Show InChI InChI=1S/C29H40F2N4O4/c1-20(2)25(21-13-8-7-9-14-21)39-28(38)35-29(3,19-22-15-12-16-23(30)24(22)31)26(36)33-17-10-5-4-6-11-18-34-27(32)37/h7-9,12-16,20,25H,4-6,10-11,17-19H2,1-3H3,(H,33,36)(H,35,38)(H3,32,34,37)/t25-,29+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 3 in guinea pig cortical membranes labeled with [125 I]-[MePhe7] |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033602

(CHEMBL359361 | N-{2-[2-[(Adamantane-2-carbonyl)-am...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)C1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CNC(=O)CCC(O)=O)Cc1ccccc1 |wU:1.0,wD:1.13,28.32,TLB:24:23:21:18.17.19,THB:13:15:21:18.17.19,24:18:15.23.22:21,19:18:15:20.22.21,19:20:15:18.17.24,(4.56,-2.64,;5.89,-3.41,;5.91,-1.86,;6.4,-.4,;5.48,.84,;6.4,2.09,;7.85,1.61,;9.19,2.4,;10.52,1.63,;10.52,.06,;9.19,-.71,;7.85,.06,;4.8,-4.48,;3.31,-4.08,;2.92,-2.58,;2,-4.9,;.46,-4.32,;1.23,-5.56,;1.17,-6.98,;-.25,-7.44,;-1.11,-6.3,;-.99,-4.79,;.39,-6.91,;1.9,-6.41,;2.74,-7.73,;7.23,-4.18,;8.55,-3.41,;7.21,-5.72,;8.55,-6.49,;9.88,-5.7,;11.22,-6.46,;12.55,-5.69,;12.54,-4.15,;13.89,-6.43,;15.21,-5.66,;16.56,-6.41,;17.9,-5.62,;16.59,-7.95,;8.55,-8.04,;9.88,-8.81,;11.25,-8,;12.54,-8.81,;12.54,-10.35,;11.2,-11.1,;9.87,-10.35,)| Show InChI InChI=1S/C36H44N4O5/c1-36(19-27-20-37-30-10-6-5-9-29(27)30,40-34(44)33-25-14-23-13-24(16-25)17-26(33)15-23)35(45)39-28(18-22-7-3-2-4-8-22)21-38-31(41)11-12-32(42)43/h2-10,20,23-26,28,33,37H,11-19,21H2,1H3,(H,38,41)(H,39,45)(H,40,44)(H,42,43)/t23?,24?,25?,26?,28-,33?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007446
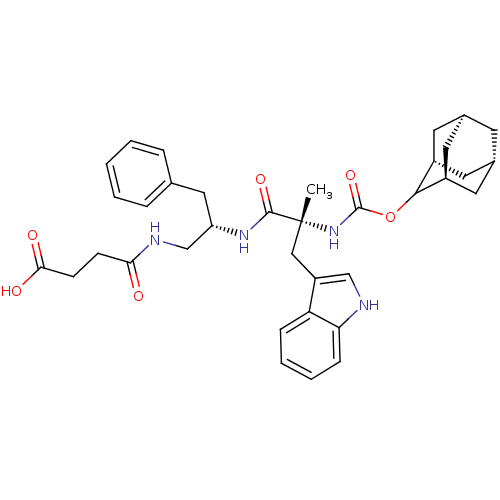
(CHEMBL335914 | N-{2-[2-(Adamantan-2-yloxycarbonyla...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N[C@H](CNC(=O)CCC(O)=O)Cc1ccccc1 |wU:21.28,17.19,29.33,1.0,wD:1.13,19.20,23.24,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,(5.95,-4.92,;5.95,-3.38,;5.95,-1.84,;7.06,-.78,;6.14,.47,;7.06,1.72,;8.51,1.24,;9.85,2.01,;11.18,1.24,;11.18,-.3,;9.85,-1.07,;8.51,-.3,;4.65,-4.18,;3.3,-3.41,;3.28,-1.87,;1.97,-4.18,;.43,-4.18,;-.28,-5.53,;-1.76,-6.2,;-2.76,-5.05,;-4.29,-4.73,;-2.63,-4.15,;-1.96,-2.83,;-.55,-3.03,;-2.04,-3.64,;-1.69,-5.21,;7.29,-4.15,;7.29,-5.69,;8.63,-3.38,;9.96,-4.15,;9.96,-5.69,;8.63,-6.46,;9.14,-7.9,;10.65,-8.19,;8.15,-9.06,;6.64,-8.8,;5.63,-9.96,;6.14,-11.4,;4.11,-9.67,;11.29,-3.35,;12.62,-4.12,;13.96,-3.35,;15.28,-4.12,;15.3,-5.66,;13.96,-6.43,;12.62,-5.66,)| Show InChI InChI=1S/C36H44N4O6/c1-36(19-27-20-37-30-10-6-5-9-29(27)30,40-35(45)46-33-25-14-23-13-24(16-25)17-26(33)15-23)34(44)39-28(18-22-7-3-2-4-8-22)21-38-31(41)11-12-32(42)43/h2-10,20,23-26,28,33,37H,11-19,21H2,1H3,(H,38,41)(H,39,44)(H,40,45)(H,42,43)/t23-,24+,25-,26+,28-,33?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex Cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007443

(4-{2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-ind...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N[C@H](CNC(=O)CCCC(O)=O)Cc1ccccc1 |wU:21.28,23.26,17.19,29.33,1.0,wD:1.13,19.20,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,20:19:16:21.22.25,(11.41,-8.37,;11.31,-6.86,;11.29,-5.31,;12.38,-4.24,;11.48,-3,;12.38,-1.75,;13.86,-2.23,;15.18,-1.45,;16.53,-2.22,;16.53,-3.77,;15.18,-4.54,;13.86,-3.77,;9.97,-7.65,;8.62,-6.89,;8.62,-5.35,;7.31,-7.66,;5.75,-7.65,;5.06,-9.01,;3.57,-9.67,;2.57,-8.53,;1.04,-8.21,;2.68,-7.63,;3.35,-6.3,;4.77,-6.49,;3.29,-7.11,;3.64,-8.68,;12.64,-7.62,;13.98,-6.82,;12.66,-9.16,;14.01,-9.91,;14.02,-11.45,;12.7,-12.25,;12.71,-13.79,;14.05,-14.54,;11.38,-14.57,;10.04,-13.82,;8.71,-14.6,;7.36,-13.83,;6.02,-14.62,;7.34,-12.29,;15.34,-9.14,;16.68,-9.9,;16.68,-11.42,;18.03,-12.19,;19.36,-11.42,;19.33,-9.87,;18,-9.11,)| Show InChI InChI=1S/C37H46N4O6/c1-37(20-28-21-38-31-11-6-5-10-30(28)31,41-36(46)47-34-26-15-24-14-25(17-26)18-27(34)16-24)35(45)40-29(19-23-8-3-2-4-9-23)22-39-32(42)12-7-13-33(43)44/h2-6,8-11,21,24-27,29,34,38H,7,12-20,22H2,1H3,(H,39,42)(H,40,45)(H,41,46)(H,43,44)/t24-,25+,26-,27+,29-,34?,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex Cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033607

(Adamantane-2-carboxylic acid [1-(1-hydroxymethyl-2...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)C1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CO)Cc1ccccc1 |wU:1.0,wD:1.13,28.32,TLB:13:15:17:20.21.19,THB:22:23:17:20.21.19,22:20:17:15.23.24,19:20:15:18.17.24,19:18:15:20.21.22,(7.54,-2.58,;8.87,-3.35,;8.89,-1.81,;9.37,-.36,;8.45,.89,;9.37,2.14,;10.82,1.66,;12.16,2.43,;13.49,1.66,;13.49,.12,;12.16,-.65,;10.82,.12,;7.77,-4.44,;6.27,-4.02,;5.9,-2.55,;4.88,-4.65,;3.44,-3.86,;1.92,-4.09,;1.57,-5.56,;2.26,-6.81,;3.73,-6.58,;4.01,-5.18,;5.2,-7.55,;4.53,-6.14,;2.95,-6.4,;10.2,-4.12,;11.52,-3.35,;10.18,-5.66,;11.52,-6.43,;12.84,-5.66,;14.18,-6.4,;11.52,-7.97,;12.84,-8.74,;12.83,-10.28,;14.16,-11.05,;15.5,-10.28,;15.5,-8.74,;14.21,-7.94,)| Show InChI InChI=1S/C32H39N3O3/c1-32(17-25-18-33-28-10-6-5-9-27(25)28,31(38)34-26(19-36)16-20-7-3-2-4-8-20)35-30(37)29-23-12-21-11-22(14-23)15-24(29)13-21/h2-10,18,21-24,26,29,33,36H,11-17,19H2,1H3,(H,34,38)(H,35,37)/t21?,22?,23?,24?,26-,29?,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007437
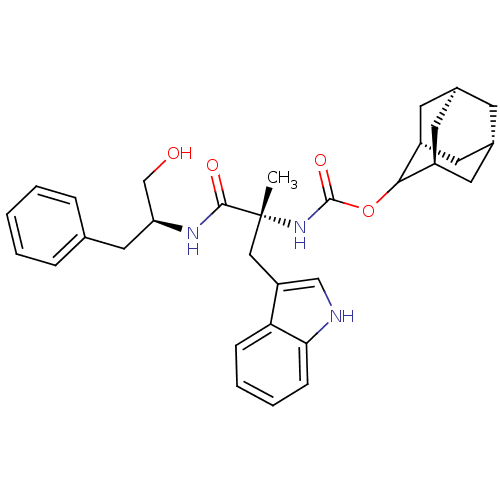
(CHEMBL423329 | [1-(1-Hydroxymethyl-2-phenyl-ethylc...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N[C@H](CO)Cc1ccccc1 |wU:21.28,23.26,17.19,29.33,1.0,wD:1.13,19.20,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,20:19:16:21.22.25,(14.92,-9.95,;14.82,-8.44,;14.8,-6.9,;15.89,-5.82,;14.99,-4.57,;15.89,-3.32,;17.37,-3.8,;18.68,-3.03,;20.03,-3.8,;20.03,-5.34,;18.68,-6.11,;17.37,-5.34,;13.49,-9.22,;12.14,-8.45,;12.14,-6.91,;10.82,-9.24,;9.27,-9.22,;8.58,-10.57,;7.09,-11.24,;6.1,-10.08,;4.57,-9.79,;6.2,-9.19,;6.88,-7.87,;8.29,-8.06,;6.81,-8.67,;7.17,-10.25,;16.15,-9.19,;17.49,-8.41,;16.17,-10.73,;17.52,-11.49,;17.53,-13.03,;16.2,-13.81,;18.84,-10.69,;20.18,-11.46,;20.18,-13,;21.53,-13.76,;22.85,-12.97,;22.82,-11.43,;21.5,-10.68,)| Show InChI InChI=1S/C32H39N3O4/c1-32(17-25-18-33-28-10-6-5-9-27(25)28,30(37)34-26(19-36)16-20-7-3-2-4-8-20)35-31(38)39-29-23-12-21-11-22(14-23)15-24(29)13-21/h2-10,18,21-24,26,29,33,36H,11-17,19H2,1H3,(H,34,37)(H,35,38)/t21-,22+,23-,24+,26-,29?,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007437

(CHEMBL423329 | [1-(1-Hydroxymethyl-2-phenyl-ethylc...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N[C@H](CO)Cc1ccccc1 |wU:21.28,23.26,17.19,29.33,1.0,wD:1.13,19.20,TLB:15:16:22:19.24.20,THB:18:17:22:19.24.20,18:19:22:16.17.25,20:19:16:21.22.25,(14.92,-9.95,;14.82,-8.44,;14.8,-6.9,;15.89,-5.82,;14.99,-4.57,;15.89,-3.32,;17.37,-3.8,;18.68,-3.03,;20.03,-3.8,;20.03,-5.34,;18.68,-6.11,;17.37,-5.34,;13.49,-9.22,;12.14,-8.45,;12.14,-6.91,;10.82,-9.24,;9.27,-9.22,;8.58,-10.57,;7.09,-11.24,;6.1,-10.08,;4.57,-9.79,;6.2,-9.19,;6.88,-7.87,;8.29,-8.06,;6.81,-8.67,;7.17,-10.25,;16.15,-9.19,;17.49,-8.41,;16.17,-10.73,;17.52,-11.49,;17.53,-13.03,;16.2,-13.81,;18.84,-10.69,;20.18,-11.46,;20.18,-13,;21.53,-13.76,;22.85,-12.97,;22.82,-11.43,;21.5,-10.68,)| Show InChI InChI=1S/C32H39N3O4/c1-32(17-25-18-33-28-10-6-5-9-27(25)28,30(37)34-26(19-36)16-20-7-3-2-4-8-20)35-31(38)39-29-23-12-21-11-22(14-23)15-24(29)13-21/h2-10,18,21-24,26,29,33,36H,11-17,19H2,1H3,(H,34,37)(H,35,38)/t21-,22+,23-,24+,26-,29?,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033603
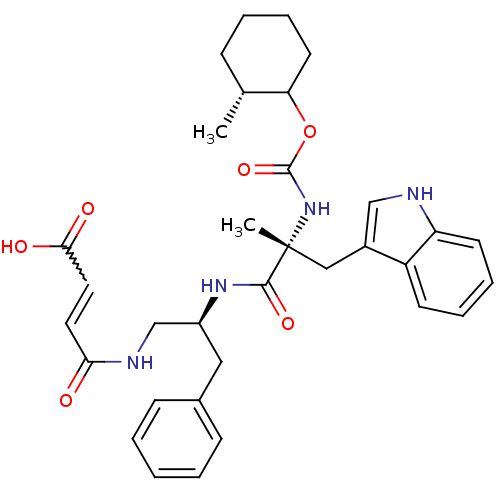
(3-{2-[3-(1H-Indol-3-yl)-2-methyl-2-(2-methyl-cyclo...)Show SMILES C[C@@H]1CCCCC1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)N[C@H](CNC(=O)C=CC(O)=O)Cc1ccccc1 |w:32.35| Show InChI InChI=1S/C33H40N4O6/c1-22-10-6-9-15-28(22)43-32(42)37-33(2,19-24-20-34-27-14-8-7-13-26(24)27)31(41)36-25(18-23-11-4-3-5-12-23)21-35-29(38)16-17-30(39)40/h3-5,7-8,11-14,16-17,20,22,25,28,34H,6,9-10,15,18-19,21H2,1-2H3,(H,35,38)(H,36,41)(H,37,42)(H,39,40)/t22-,25+,28?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50453000

(CHEMBL2112695)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)NCC(OC(=O)CCC(O)=O)c1ccccc1)[C@@]([H])(C2)C3 |wU:14.15,1.0,6.6,wD:3.3,45.49,TLB:10:9:5:1.8.2,THB:47:45:5:1.8.2,47:1:5:9.45.48,2:3:9:1.8.47,2:1:9:3.5.48,(-2.04,-16.44,;-1.27,-15.12,;-2.79,-14.8,;-1.14,-14.22,;-2.15,-13.07,;-.47,-12.9,;.94,-13.09,;1.67,-11.75,;-.55,-13.71,;1.92,-14.26,;3.46,-14.26,;4.79,-13.49,;4.78,-11.95,;6.14,-14.24,;7.45,-13.45,;7.55,-14.96,;7.44,-11.91,;8.54,-10.84,;7.64,-9.6,;8.54,-8.35,;10.01,-8.83,;11.34,-8.05,;12.67,-8.82,;12.67,-10.37,;11.34,-11.14,;10.01,-10.37,;8.8,-14.21,;10.12,-13.42,;8.8,-15.75,;10.15,-16.5,;11.21,-15.35,;12.8,-14.95,;14.13,-14.16,;14.12,-12.62,;15.47,-14.92,;16.79,-14.13,;18.14,-14.89,;19.45,-14.1,;18.16,-16.43,;12.81,-16.49,;14.13,-15.7,;15.47,-16.46,;15.5,-18.01,;14.16,-18.78,;12.81,-18.03,;1.22,-15.6,;2.26,-16.73,;-.28,-16.27,;-.19,-15.27,)| Show InChI InChI=1S/C35H41N3O7/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,38-34(43)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(42)37-20-29(23-7-3-2-4-8-23)44-31(41)12-11-30(39)40/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,42)(H,38,43)(H,39,40)/t21-,22+,24-,25+,29?,32?,35-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Research Unit
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of specific binding of [125I]bolton hunter CCK-8 to mouse cerebral cortex cholecystokinin type B receptor |
J Med Chem 34: 404-14 (1991)
BindingDB Entry DOI: 10.7270/Q2RJ4K3V |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033585
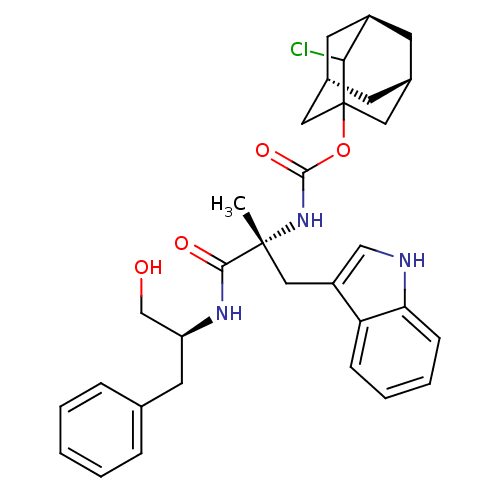
(CHEMBL153760 | [1-(1-Hydroxymethyl-2-phenyl-ethylc...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC12C[C@H]3C[C@H](C[C@H](C3)C1Cl)C2)C(=O)N[C@H](CO)Cc1ccccc1 |TLB:19:20:24:17.18.23,25:24:26.20.21:17.18.23,THB:19:18:24:26.20.21| Show InChI InChI=1S/C32H38ClN3O4/c1-31(17-24-18-34-27-10-6-5-9-26(24)27,29(38)35-25(19-37)14-20-7-3-2-4-8-20)36-30(39)40-32-15-21-11-22(16-32)13-23(12-21)28(32)33/h2-10,18,21-23,25,28,34,37H,11-17,19H2,1H3,(H,35,38)(H,36,39)/t21-,22+,23-,25-,28?,31+,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50033587

((1R,2R)-[1-(1-Hydroxymethyl-2-phenyl-ethylcarbamoy...)Show SMILES CC1CCCCC1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)N[C@H](CO)Cc1ccccc1 Show InChI InChI=1S/C29H37N3O4/c1-20-10-6-9-15-26(20)36-28(35)32-29(2,17-22-18-30-25-14-8-7-13-24(22)25)27(34)31-23(19-33)16-21-11-4-3-5-12-21/h3-5,7-8,11-14,18,20,23,26,30,33H,6,9-10,15-17,19H2,1-2H3,(H,31,34)(H,32,35)/t20?,23-,26?,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type A receptor in the rat pancreas. |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM50050649
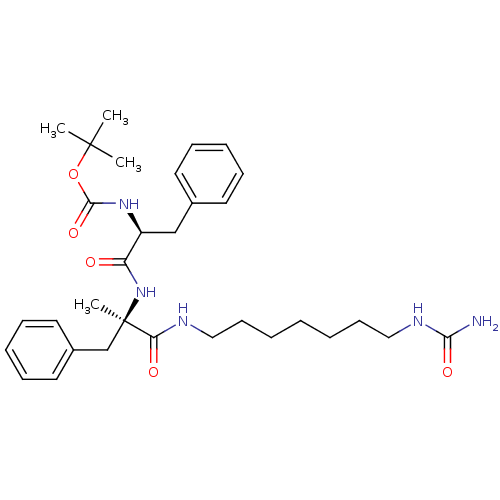
(CHEMBL444832 | {(S)-1-[(R)-1-Methyl-2-phenyl-1-(7-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@](C)(Cc1ccccc1)C(=O)NCCCCCCCNC(N)=O Show InChI InChI=1S/C32H47N5O5/c1-31(2,3)42-30(41)36-26(22-24-16-10-8-11-17-24)27(38)37-32(4,23-25-18-12-9-13-19-25)28(39)34-20-14-6-5-7-15-21-35-29(33)40/h8-13,16-19,26H,5-7,14-15,20-23H2,1-4H3,(H,34,39)(H,36,41)(H,37,38)(H3,33,35,40)/t26-,32+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 3 in guinea pig cortical membranes labeled with [125 I]-[MePhe7] |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281277
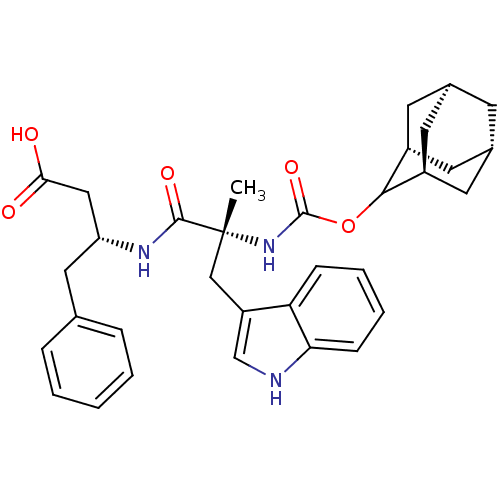
((R)-3-[(S)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1 |wU:1.13,29.33,21.23,23.26,wD:19.27,17.19,1.0,THB:22:21:16.23.24:18,20:21:16:19.24.18,20:19:16:21.22.25,(9.25,-3.09,;9.25,-4.63,;9.25,-6.16,;8.78,-7.64,;9.69,-8.88,;8.78,-10.13,;7.31,-9.64,;5.97,-10.43,;4.65,-9.67,;4.65,-8.13,;5.97,-7.34,;7.31,-8.11,;7.75,-5.02,;6.69,-3.88,;7.18,-2.41,;5.18,-4.19,;4.19,-3.04,;2.69,-3.3,;1.84,-1.95,;2.1,-.36,;1.19,-1.45,;1.98,-2.72,;3.37,-2.45,;4.4,-1.42,;3.6,-.12,;1.61,-4.44,;10.73,-4.23,;11.13,-2.74,;11.82,-5.3,;13.3,-4.91,;13.7,-3.44,;15.19,-3.02,;15.58,-1.54,;16.28,-4.12,;14.4,-6,;15.86,-5.6,;15.86,-7.15,;17.2,-7.92,;18.53,-7.16,;18.53,-5.6,;17.2,-4.86,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21-,22+,23-,24+,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Rattus norvegicus) | BDBM50052524

((S)-N-((S)-1-{[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-m...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(N)=O Show InChI InChI=1S/C40H55N7O11S/c1-24(2)19-28(37(55)45-27(36(41)54)17-18-59-4)44-33(49)23-42-39(57)31(21-26-13-9-6-10-14-26)47(3)40(58)30(20-25-11-7-5-8-12-25)46-38(56)29(22-35(52)53)43-32(48)15-16-34(50)51/h5-14,24,27-31H,15-23H2,1-4H3,(H2,41,54)(H,42,57)(H,43,48)(H,44,49)(H,45,55)(H,46,56)(H,50,51)(H,52,53)/t27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against cloned human Tachykinin receptor 3 in CHO cells labeled with [125I][MePhe7]-NKB |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50052524

((S)-N-((S)-1-{[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-m...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(N)=O Show InChI InChI=1S/C40H55N7O11S/c1-24(2)19-28(37(55)45-27(36(41)54)17-18-59-4)44-33(49)23-42-39(57)31(21-26-13-9-6-10-14-26)47(3)40(58)30(20-25-11-7-5-8-12-25)46-38(56)29(22-35(52)53)43-32(48)15-16-34(50)51/h5-14,24,27-31H,15-23H2,1-4H3,(H2,41,54)(H,42,57)(H,43,48)(H,44,49)(H,45,55)(H,46,56)(H,50,51)(H,52,53)/t27-,28-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 3 in guinea pig cortical membranes labeled with [125I]-[MePhe7] |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM50052524

((S)-N-((S)-1-{[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-m...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(N)=O Show InChI InChI=1S/C40H55N7O11S/c1-24(2)19-28(37(55)45-27(36(41)54)17-18-59-4)44-33(49)23-42-39(57)31(21-26-13-9-6-10-14-26)47(3)40(58)30(20-25-11-7-5-8-12-25)46-38(56)29(22-35(52)53)43-32(48)15-16-34(50)51/h5-14,24,27-31H,15-23H2,1-4H3,(H2,41,54)(H,42,57)(H,43,48)(H,44,49)(H,45,55)(H,46,56)(H,50,51)(H,52,53)/t27-,28-,29-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge University Forvie Site
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 3 in guinea pig cortical membranes labeled with [125I]-[MePhe7] |
J Med Chem 39: 1664-75 (1996)
Article DOI: 10.1021/jm950892r
BindingDB Entry DOI: 10.7270/Q2CJ8F4R |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50033605
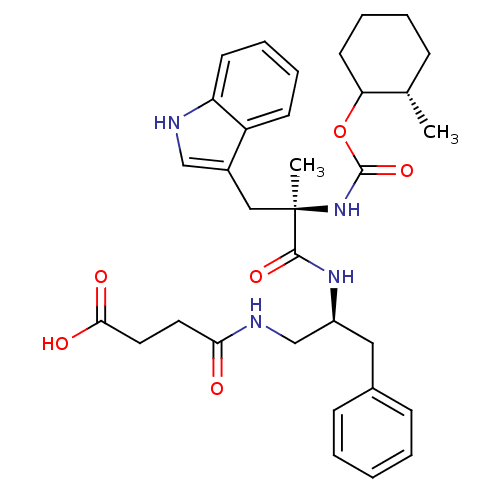
(CHEMBL436065 | N-{2-[3-(1H-Indol-3-yl)-2-methyl-2-...)Show SMILES C[C@H]1CCCCC1OC(=O)N[C@](C)(Cc1c[nH]c2ccccc12)C(=O)N[C@H](CNC(=O)CCC(O)=O)Cc1ccccc1 Show InChI InChI=1S/C33H42N4O6/c1-22-10-6-9-15-28(22)43-32(42)37-33(2,19-24-20-34-27-14-8-7-13-26(24)27)31(41)36-25(18-23-11-4-3-5-12-23)21-35-29(38)16-17-30(39)40/h3-5,7-8,11-14,20,22,25,28,34H,6,9-10,15-19,21H2,1-2H3,(H,35,38)(H,36,41)(H,37,42)(H,39,40)/t22-,25-,28?,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of binding of [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 36: 552-65 (1993)
BindingDB Entry DOI: 10.7270/Q2JM2B88 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































