 Found 165 hits with Last Name = 'wright' and Initial = 'je'
Found 165 hits with Last Name = 'wright' and Initial = 'je' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068808
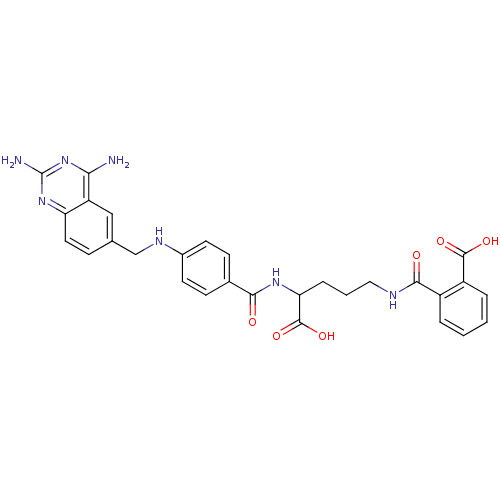
(CHEMBL297088 | N-(4-Carboxy-4-{4-[(2,4-diamino-qui...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H29N7O6/c30-24-21-14-16(7-12-22(21)35-29(31)36-24)15-33-18-10-8-17(9-11-18)25(37)34-23(28(41)42)6-3-13-32-26(38)19-4-1-2-5-20(19)27(39)40/h1-2,4-5,7-12,14,23,33H,3,6,13,15H2,(H,32,38)(H,34,37)(H,39,40)(H,41,42)(H4,30,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068813
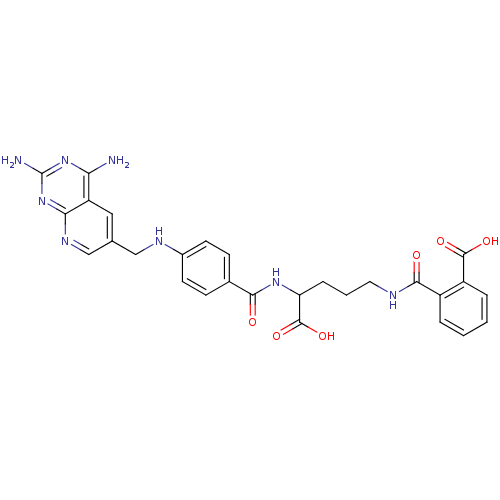
(CHEMBL149962 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C28H28N8O6/c29-22-20-12-15(14-33-23(20)36-28(30)35-22)13-32-17-9-7-16(8-10-17)24(37)34-21(27(41)42)6-3-11-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-10,12,14,21,32H,3,6,11,13H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011320
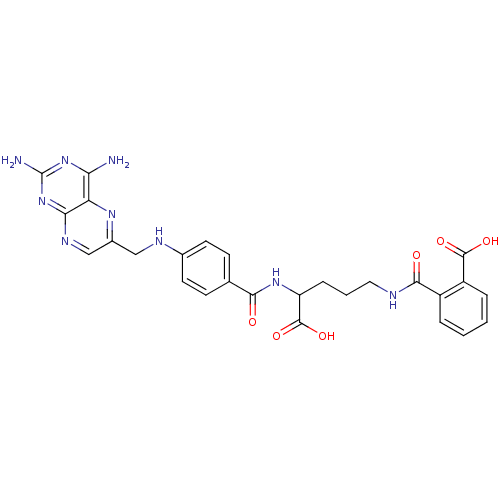
(CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C27H27N9O6/c28-21-20-22(36-27(29)35-21)32-13-16(33-20)12-31-15-9-7-14(8-10-15)23(37)34-19(26(41)42)6-3-11-30-24(38)17-4-1-2-5-18(17)25(39)40/h1-2,4-5,7-10,13,19,31H,3,6,11-12H2,(H,30,38)(H,34,37)(H,39,40)(H,41,42)(H4,28,29,32,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068810
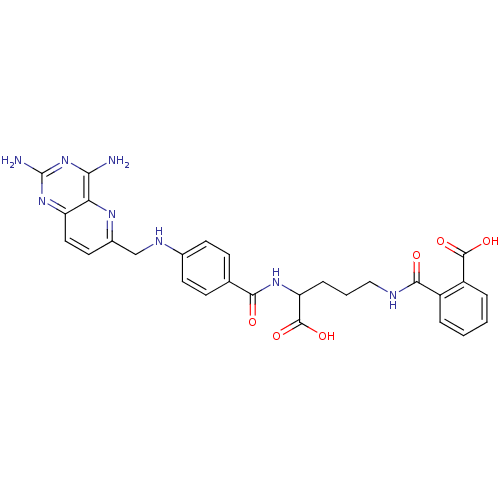
(CHEMBL149164 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C28H28N8O6/c29-23-22-20(35-28(30)36-23)12-11-17(33-22)14-32-16-9-7-15(8-10-16)24(37)34-21(27(41)42)6-3-13-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-12,21,32H,3,6,13-14H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068812
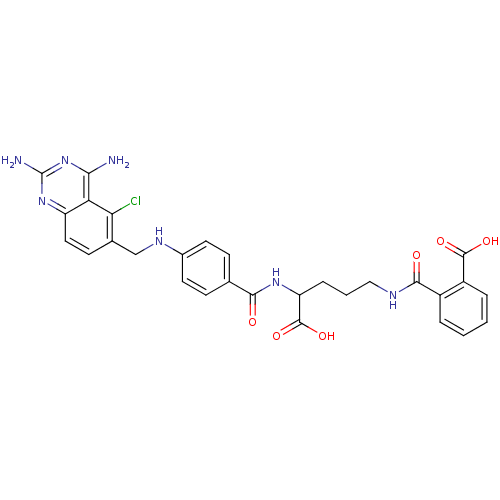
(CHEMBL146917 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-c...)Show SMILES Nc1nc(N)c2c(Cl)c(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H28ClN7O6/c30-23-16(9-12-20-22(23)24(31)37-29(32)36-20)14-34-17-10-7-15(8-11-17)25(38)35-21(28(42)43)6-3-13-33-26(39)18-4-1-2-5-19(18)27(40)41/h1-2,4-5,7-12,21,34H,3,6,13-14H2,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068811
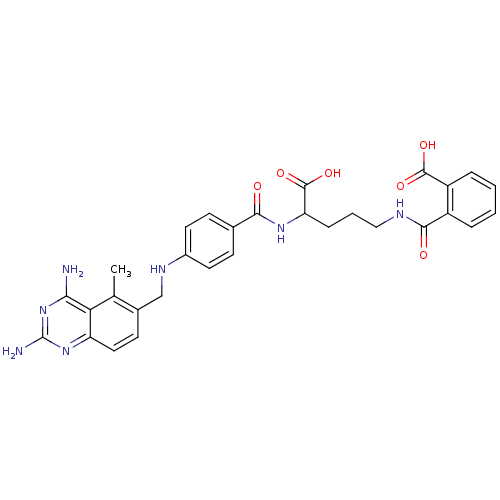
(CHEMBL149218 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)ccc2nc(N)nc(N)c12 Show InChI InChI=1S/C30H31N7O6/c1-16-18(10-13-22-24(16)25(31)37-30(32)36-22)15-34-19-11-8-17(9-12-19)26(38)35-23(29(42)43)7-4-14-33-27(39)20-5-2-3-6-21(20)28(40)41/h2-3,5-6,8-13,23,34H,4,7,14-15H2,1H3,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068809
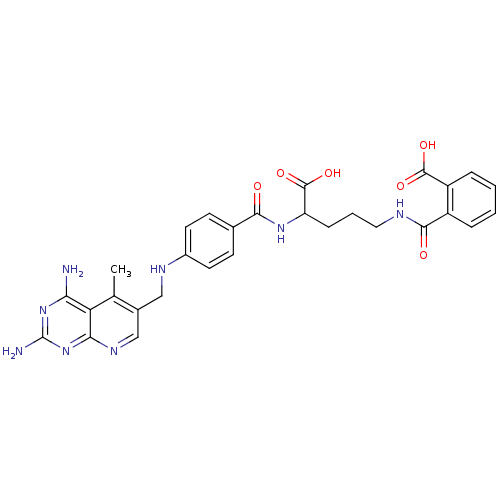
(CHEMBL150607 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C29H30N8O6/c1-15-17(14-34-24-22(15)23(30)36-29(31)37-24)13-33-18-10-8-16(9-11-18)25(38)35-21(28(42)43)7-4-12-32-26(39)19-5-2-3-6-20(19)27(40)41/h2-3,5-6,8-11,14,21,33H,4,7,12-13H2,1H3,(H,32,39)(H,35,38)(H,40,41)(H,42,43)(H4,30,31,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068808

(CHEMBL297088 | N-(4-Carboxy-4-{4-[(2,4-diamino-qui...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H29N7O6/c30-24-21-14-16(7-12-22(21)35-29(31)36-24)15-33-18-10-8-17(9-11-18)25(37)34-23(28(41)42)6-3-13-32-26(38)19-4-1-2-5-20(19)27(39)40/h1-2,4-5,7-12,14,23,33H,3,6,13,15H2,(H,32,38)(H,34,37)(H,39,40)(H,41,42)(H4,30,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0000140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111552
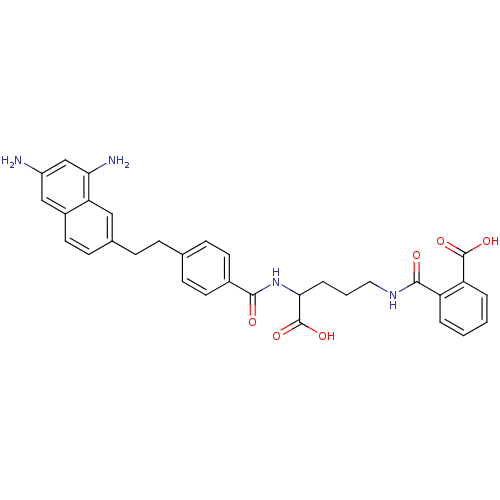
(CHEMBL297558 | N-(4-Carboxy-4-{4-[2-(6,8-diamino-n...)Show SMILES Nc1cc(N)c2cc(CCc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2c1 Show InChI InChI=1S/C32H32N4O6/c33-23-17-22-14-11-20(16-26(22)27(34)18-23)8-7-19-9-12-21(13-10-19)29(37)36-28(32(41)42)6-3-15-35-30(38)24-4-1-2-5-25(24)31(39)40/h1-2,4-5,9-14,16-18,28H,3,6-8,15,33-34H2,(H,35,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.000210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111550
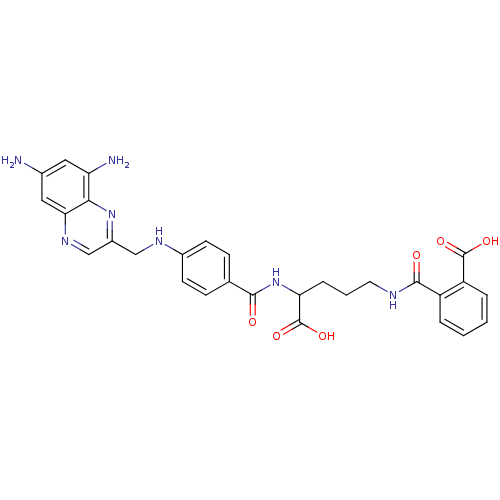
(CHEMBL296545 | N-(4-Carboxy-4-{4-[(6,8-diamino-qui...)Show SMILES Nc1cc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C29H29N7O6/c30-17-12-22(31)25-24(13-17)34-15-19(35-25)14-33-18-9-7-16(8-10-18)26(37)36-23(29(41)42)6-3-11-32-27(38)20-4-1-2-5-21(20)28(39)40/h1-2,4-5,7-10,12-13,15,23,33H,3,6,11,14,30-31H2,(H,32,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.000330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111549
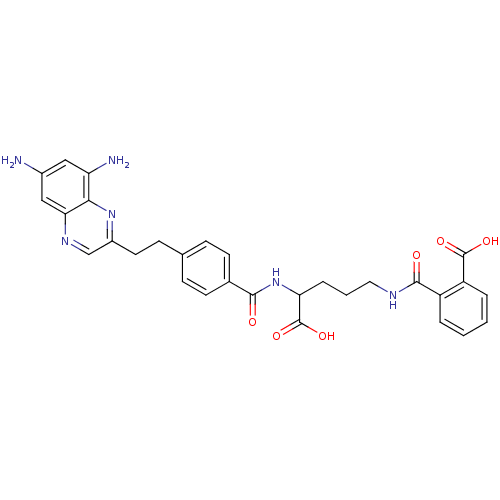
(CHEMBL47689 | N-(4-Carboxy-4-{4-[2-(6,8-diamino-qu...)Show SMILES Nc1cc(N)c2nc(CCc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C30H30N6O6/c31-19-14-23(32)26-25(15-19)34-16-20(35-26)12-9-17-7-10-18(11-8-17)27(37)36-24(30(41)42)6-3-13-33-28(38)21-4-1-2-5-22(21)29(39)40/h1-2,4-5,7-8,10-11,14-16,24H,3,6,9,12-13,31-32H2,(H,33,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111548

(CHEMBL47919 | N-(4-Carboxy-4-{4-[1-(6,8-diamino-qu...)Show SMILES Nc1cc(N)c2nc(CC(CC#C)c3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C33H32N6O6/c1-2-6-21(15-23-18-37-28-17-22(34)16-26(35)29(28)38-23)19-10-12-20(13-11-19)30(40)39-27(33(44)45)9-5-14-36-31(41)24-7-3-4-8-25(24)32(42)43/h1,3-4,7-8,10-13,16-18,21,27H,5-6,9,14-15,34-35H2,(H,36,41)(H,39,40)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111551

(CHEMBL295859 | N-(4-Carboxy-4-{4-[1-(6,8-diamino-q...)Show SMILES CCC(Cc1cnc2cc(N)cc(N)c2n1)c1ccc(cc1)C(=O)NC(CCCNC(=O)c1ccccc1C(O)=O)C(O)=O Show InChI InChI=1S/C32H34N6O6/c1-2-18(14-22-17-36-27-16-21(33)15-25(34)28(27)37-22)19-9-11-20(12-10-19)29(39)38-26(32(43)44)8-5-13-35-30(40)23-6-3-4-7-24(23)31(41)42/h3-4,6-7,9-12,15-18,26H,2,5,8,13-14,33-34H2,1H3,(H,35,40)(H,38,39)(H,41,42)(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50158569
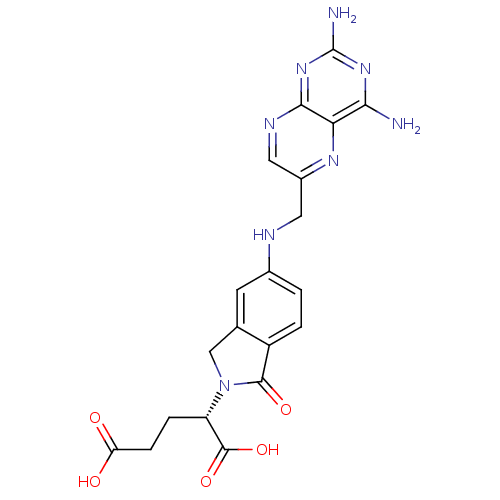
(2-S-[5-[2,4-diaminopteridin-6-yl)methyamino]-2,3-d...)Show SMILES Nc1nc(N)c2nc(CNc3ccc4C(=O)N(Cc4c3)[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C20H20N8O5/c21-16-15-17(27-20(22)26-16)24-7-11(25-15)6-23-10-1-2-12-9(5-10)8-28(18(12)31)13(19(32)33)3-4-14(29)30/h1-2,5,7,13,23H,3-4,6,8H2,(H,29,30)(H,32,33)(H4,21,22,24,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR |
J Med Chem 47: 6958-63 (2004)
Article DOI: 10.1021/jm040122s
BindingDB Entry DOI: 10.7270/Q2NZ873F |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50158570
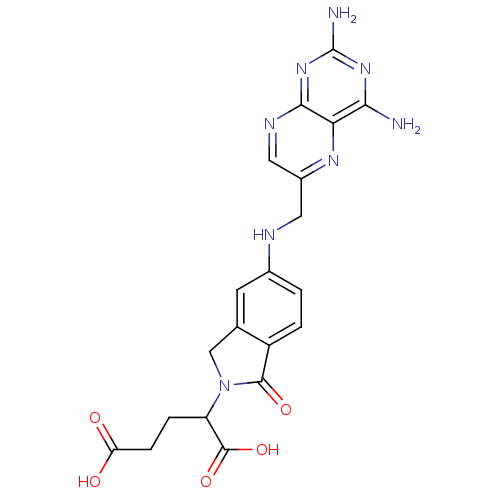
(2-R,S-[5-[(2,4-diaminopteridin-6-yl)methylamino]-2...)Show SMILES Nc1nc(N)c2nc(CNc3ccc4C(=O)N(Cc4c3)C(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H20N8O5/c21-16-15-17(27-20(22)26-16)24-7-11(25-15)6-23-10-1-2-12-9(5-10)8-28(18(12)31)13(19(32)33)3-4-14(29)30/h1-2,5,7,13,23H,3-4,6,8H2,(H,29,30)(H,32,33)(H4,21,22,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR |
J Med Chem 47: 6958-63 (2004)
Article DOI: 10.1021/jm040122s
BindingDB Entry DOI: 10.7270/Q2NZ873F |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50158571
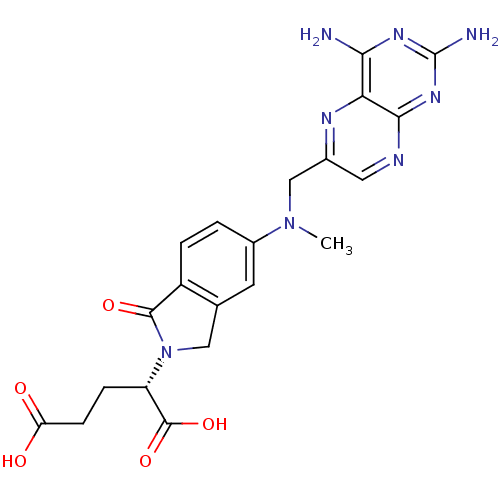
(2-S-[5-[N-(2,4--diaminopteridin-6-yl)methyl)-N-met...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc2C(=O)N(Cc2c1)[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N8O5/c1-28(9-11-7-24-18-16(25-11)17(22)26-21(23)27-18)12-2-3-13-10(6-12)8-29(19(13)32)14(20(33)34)4-5-15(30)31/h2-3,6-7,14H,4-5,8-9H2,1H3,(H,30,31)(H,33,34)(H4,22,23,24,26,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR |
J Med Chem 47: 6958-63 (2004)
Article DOI: 10.1021/jm040122s
BindingDB Entry DOI: 10.7270/Q2NZ873F |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM50011885
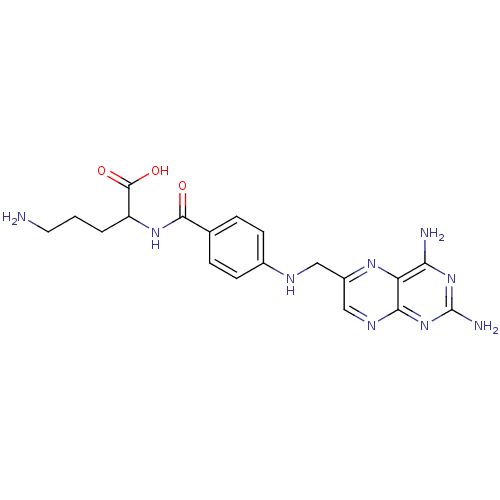
(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...)Show SMILES NCCCC(NC(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1)C(O)=O Show InChI InChI=1S/C19H23N9O3/c20-7-1-2-13(18(30)31)26-17(29)10-3-5-11(6-4-10)23-8-12-9-24-16-14(25-12)15(21)27-19(22)28-16/h3-6,9,13,23H,1-2,7-8,20H2,(H,26,29)(H,30,31)(H4,21,22,24,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver |
J Med Chem 29: 655-60 (1986)
BindingDB Entry DOI: 10.7270/Q2RR1ZTR |
More data for this
Ligand-Target Pair | |
Reduced folate transporter
(Homo sapiens (Human)) | BDBM50158569

(2-S-[5-[2,4-diaminopteridin-6-yl)methyamino]-2,3-d...)Show SMILES Nc1nc(N)c2nc(CNc3ccc4C(=O)N(Cc4c3)[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C20H20N8O5/c21-16-15-17(27-20(22)26-16)24-7-11(25-15)6-23-10-1-2-12-9(5-10)8-28(18(12)31)13(19(32)33)3-4-14(29)30/h1-2,5,7,13,23H,3-4,6,8H2,(H,29,30)(H,32,33)(H4,21,22,24,26,27)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of RFC-mediated [3H]MTX influx into human CCRF-CEM cells |
J Med Chem 47: 6958-63 (2004)
Article DOI: 10.1021/jm040122s
BindingDB Entry DOI: 10.7270/Q2NZ873F |
More data for this
Ligand-Target Pair | |
Reduced folate transporter
(Homo sapiens (Human)) | BDBM50158570

(2-R,S-[5-[(2,4-diaminopteridin-6-yl)methylamino]-2...)Show SMILES Nc1nc(N)c2nc(CNc3ccc4C(=O)N(Cc4c3)C(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H20N8O5/c21-16-15-17(27-20(22)26-16)24-7-11(25-15)6-23-10-1-2-12-9(5-10)8-28(18(12)31)13(19(32)33)3-4-14(29)30/h1-2,5,7,13,23H,3-4,6,8H2,(H,29,30)(H,32,33)(H4,21,22,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of RFC-mediated [3H]MTX influx into human CCRF-CEM cells |
J Med Chem 47: 6958-63 (2004)
Article DOI: 10.1021/jm040122s
BindingDB Entry DOI: 10.7270/Q2NZ873F |
More data for this
Ligand-Target Pair | |
Reduced folate transporter
(Homo sapiens (Human)) | BDBM50158571

(2-S-[5-[N-(2,4--diaminopteridin-6-yl)methyl)-N-met...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc2C(=O)N(Cc2c1)[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N8O5/c1-28(9-11-7-24-18-16(25-11)17(22)26-21(23)27-18)12-2-3-13-10(6-12)8-29(19(13)32)14(20(33)34)4-5-15(30)31/h2-3,6-7,14H,4-5,8-9H2,1H3,(H,30,31)(H,33,34)(H4,22,23,24,26,27)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of RFC-mediated [3H]MTX influx into human CCRF-CEM cells |
J Med Chem 47: 6958-63 (2004)
Article DOI: 10.1021/jm040122s
BindingDB Entry DOI: 10.7270/Q2NZ873F |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50039121
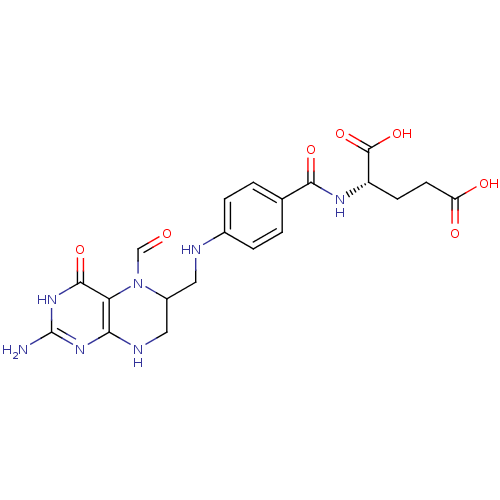
((S)-2-{4-[(2-Amino-5-formyl-4-oxo-1,4,5,6,7,8-hexa...)Show SMILES Nc1nc2NCC(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)N(C=O)c2c(=O)[nH]1 Show InChI InChI=1S/C20H23N7O7/c21-20-25-16-15(18(32)26-20)27(9-28)12(8-23-16)7-22-11-3-1-10(2-4-11)17(31)24-13(19(33)34)5-6-14(29)30/h1-4,9,12-13,22H,5-8H2,(H,24,31)(H,29,30)(H,33,34)(H4,21,23,25,26,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested for the inhibition of [14C]-DDATHF influx in CCRF-CEM cells of human leukemic lymphoblast |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver |
J Med Chem 29: 655-60 (1986)
BindingDB Entry DOI: 10.7270/Q2RR1ZTR |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM50011886
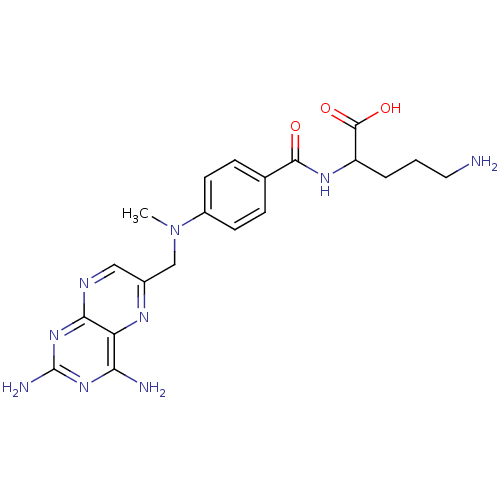
(5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-me...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCCN)C(O)=O Show InChI InChI=1S/C20H25N9O3/c1-29(10-12-9-24-17-15(25-12)16(22)27-20(23)28-17)13-6-4-11(5-7-13)18(30)26-14(19(31)32)3-2-8-21/h4-7,9,14H,2-3,8,10,21H2,1H3,(H,26,30)(H,31,32)(H4,22,23,24,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver |
J Med Chem 29: 655-60 (1986)
BindingDB Entry DOI: 10.7270/Q2RR1ZTR |
More data for this
Ligand-Target Pair | |
Folylpolyglutamate synthase, mitochondrial
(Mus musculus) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver |
J Med Chem 29: 655-60 (1986)
BindingDB Entry DOI: 10.7270/Q2RR1ZTR |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011320

(CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C27H27N9O6/c28-21-20-22(36-27(29)35-21)32-13-16(33-20)12-31-15-9-7-14(8-10-15)23(37)34-19(26(41)42)6-3-11-30-24(38)17-4-1-2-5-18(17)25(39)40/h1-2,4-5,7-10,13,19,31H,3,6,11-12H2,(H,30,38)(H,34,37)(H,39,40)(H,41,42)(H4,28,29,32,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibitory concentration against CCRF-CEM human Leukemic lymphoblast by using DHFR as primary target |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against dihydrofolate reductase in human |
J Med Chem 36: 3103-12 (1993)
BindingDB Entry DOI: 10.7270/Q23X85PX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of rat liver dihydrofolate reductase. |
J Med Chem 36: 3103-12 (1993)
BindingDB Entry DOI: 10.7270/Q23X85PX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase (DHFR) of in rat liver |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50016460
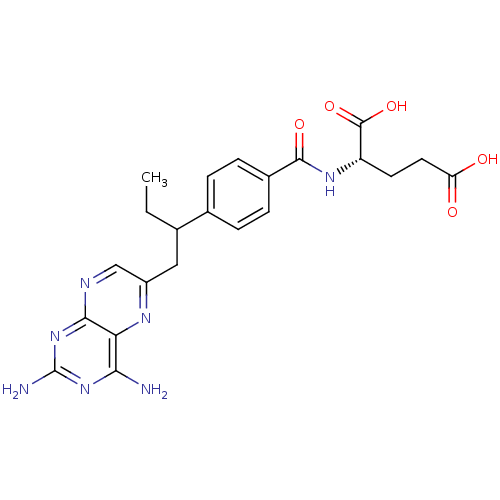
((S)-2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-prop...)Show SMILES CCC(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-2-11(9-14-10-25-19-17(26-14)18(23)28-22(24)29-19)12-3-5-13(6-4-12)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,10-11,15H,2,7-9H2,1H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,25,28,29)/t11?,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibitory concentration against CCRF-CEM human Leukemic lymphoblast by using DHFR as primary target |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of rat liver dihydrofolate reductase. |
J Med Chem 36: 3103-12 (1993)
BindingDB Entry DOI: 10.7270/Q23X85PX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase (DHFR) of in rat liver |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50031871
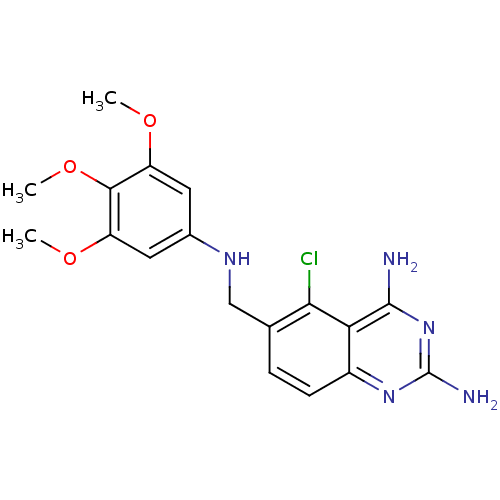
(5-Chloro-6-[(3,4,5-trimethoxy-phenylamino)-methyl]...)Show InChI InChI=1S/C18H20ClN5O3/c1-25-12-6-10(7-13(26-2)16(12)27-3)22-8-9-4-5-11-14(15(9)19)17(20)24-18(21)23-11/h4-7,22H,8H2,1-3H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase of Toxoplasma gondii |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50031871

(5-Chloro-6-[(3,4,5-trimethoxy-phenylamino)-methyl]...)Show InChI InChI=1S/C18H20ClN5O3/c1-25-12-6-10(7-13(26-2)16(12)27-3)22-8-9-4-5-11-14(15(9)19)17(20)24-18(21)23-11/h4-7,22H,8H2,1-3H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase (DHFR) of in rat liver |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50035481

(5-Chloro-N*6*-(3,4,5-trimethoxy-benzyl)-quinazolin...)Show InChI InChI=1S/C18H20ClN5O3/c1-25-12-6-9(7-13(26-2)16(12)27-3)8-22-11-5-4-10-14(15(11)19)17(20)24-18(21)23-10/h4-7,22H,8H2,1-3H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase (DHFR) of in rat liver |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50031865
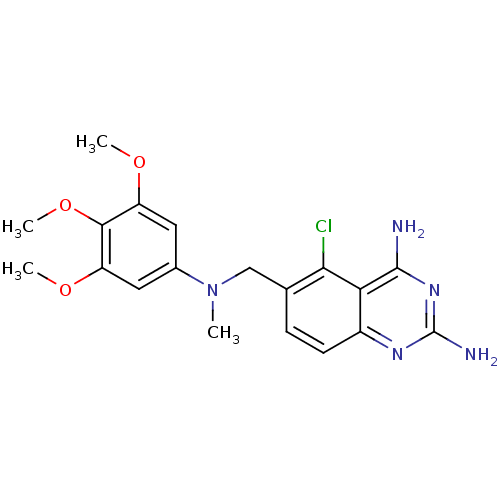
(5-Chloro-6-{[methyl-(3,4,5-trimethoxy-phenyl)-amin...)Show SMILES COc1cc(cc(OC)c1OC)N(C)Cc1ccc2nc(N)nc(N)c2c1Cl Show InChI InChI=1S/C19H22ClN5O3/c1-25(11-7-13(26-2)17(28-4)14(8-11)27-3)9-10-5-6-12-15(16(10)20)18(21)24-19(22)23-12/h5-8H,9H2,1-4H3,(H4,21,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase of Toxoplasma gondii |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035481

(5-Chloro-N*6*-(3,4,5-trimethoxy-benzyl)-quinazolin...)Show InChI InChI=1S/C18H20ClN5O3/c1-25-12-6-9(7-13(26-2)16(12)27-3)8-22-11-5-4-10-14(15(11)19)17(20)24-18(21)23-10/h4-7,22H,8H2,1-3H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase of Toxoplasma gondii |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase of Toxoplasma gondii |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50039117
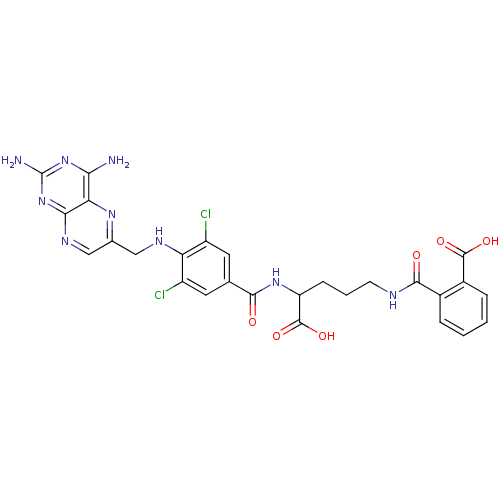
(CHEMBL309160 | N-(4-Carboxy-4-{3,5-dichloro-4-[(2,...)Show SMILES Nc1nc(N)c2nc(CNc3c(Cl)cc(cc3Cl)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C27H25Cl2N9O6/c28-16-8-12(9-17(29)19(16)33-10-13-11-34-22-20(35-13)21(30)37-27(31)38-22)23(39)36-18(26(43)44)6-3-7-32-24(40)14-4-1-2-5-15(14)25(41)42/h1-2,4-5,8-9,11,18,33H,3,6-7,10H2,(H,32,40)(H,36,39)(H,41,42)(H,43,44)(H4,30,31,34,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against human dihydrofolate reductase(DHFR) |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase in Toxoplasma gondii. |
J Med Chem 36: 3103-12 (1993)
BindingDB Entry DOI: 10.7270/Q23X85PX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of dihydrofolate reductase in pneumocystis carinii. |
J Med Chem 36: 3103-12 (1993)
BindingDB Entry DOI: 10.7270/Q23X85PX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50039119
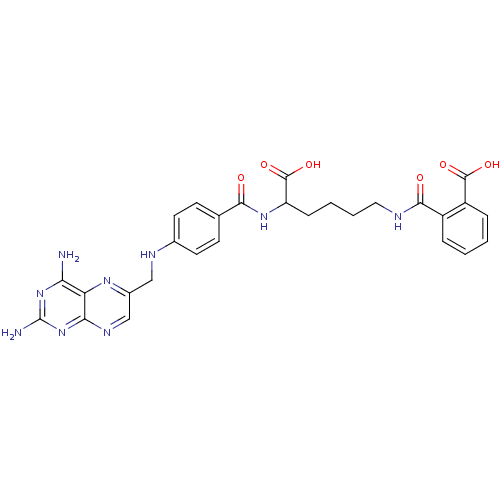
(CHEMBL44919 | N-(5-Carboxy-5-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C28H29N9O6/c29-22-21-23(37-28(30)36-22)33-14-17(34-21)13-32-16-10-8-15(9-11-16)24(38)35-20(27(42)43)7-3-4-12-31-25(39)18-5-1-2-6-19(18)26(40)41/h1-2,5-6,8-11,14,20,32H,3-4,7,12-13H2,(H,31,39)(H,35,38)(H,40,41)(H,42,43)(H4,29,30,33,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
rested for inhibitory concentration against human dihydrofolate reductase(DHFR) |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against human dihydrofolate reductase(DHFR) |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50031865

(5-Chloro-6-{[methyl-(3,4,5-trimethoxy-phenyl)-amin...)Show SMILES COc1cc(cc(OC)c1OC)N(C)Cc1ccc2nc(N)nc(N)c2c1Cl Show InChI InChI=1S/C19H22ClN5O3/c1-25(11-7-13(26-2)17(28-4)14(8-11)27-3)9-10-5-6-12-15(16(10)20)18(21)24-19(22)23-12/h5-8H,9H2,1-4H3,(H4,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase of Pneumocystis carinii |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against human dihydrofolate reductase(DHFR) |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50031865

(5-Chloro-6-{[methyl-(3,4,5-trimethoxy-phenyl)-amin...)Show SMILES COc1cc(cc(OC)c1OC)N(C)Cc1ccc2nc(N)nc(N)c2c1Cl Show InChI InChI=1S/C19H22ClN5O3/c1-25(11-7-13(26-2)17(28-4)14(8-11)27-3)9-10-5-6-12-15(16(10)20)18(21)24-19(22)23-12/h5-8H,9H2,1-4H3,(H4,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase (DHFR) of in rat liver |
J Med Chem 37: 4522-8 (1995)
BindingDB Entry DOI: 10.7270/Q2DF6RVD |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011320

(CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C27H27N9O6/c28-21-20-22(36-27(29)35-21)32-13-16(33-20)12-31-15-9-7-14(8-10-15)23(37)34-19(26(41)42)6-3-11-30-24(38)17-4-1-2-5-18(17)25(39)40/h1-2,4-5,7-10,13,19,31H,3,6,11-12H2,(H,30,38)(H,34,37)(H,39,40)(H,41,42)(H4,28,29,32,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against human dihydrofolate reductase(DHFR) |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50039118

(CHEMBL42819 | N-(3-Carboxy-3-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C26H25N9O6/c27-20-19-21(35-26(28)34-20)31-12-15(32-19)11-30-14-7-5-13(6-8-14)22(36)33-18(25(40)41)9-10-29-23(37)16-3-1-2-4-17(16)24(38)39/h1-8,12,18,30H,9-11H2,(H,29,37)(H,33,36)(H,38,39)(H,40,41)(H4,27,28,31,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against human dihydrofolate reductase(DHFR) |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against human dihydrofolate reductase(DHFR) |
J Med Chem 37: 2167-74 (1994)
BindingDB Entry DOI: 10.7270/Q2Q240W0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data