

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59242 (Benzotriazole ester, 8 | acs.jmedchem.1c00409_ST.6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59240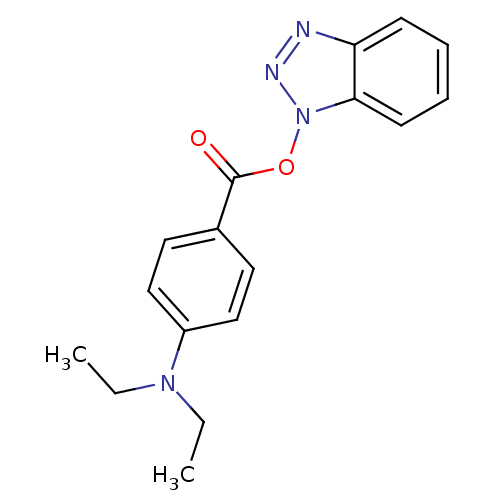 (Benzotriazole ester, 6 | acs.jmedchem.1c00409_ST.8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59239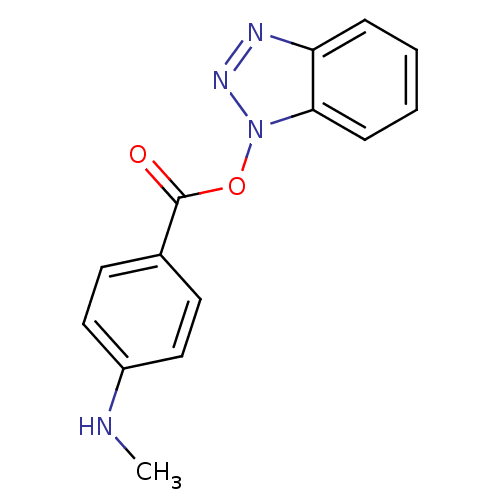 (Benzotriazole ester, 5 | acs.jmedchem.1c00409_ST.9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59243 (Benzotriazole ester, 9 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59244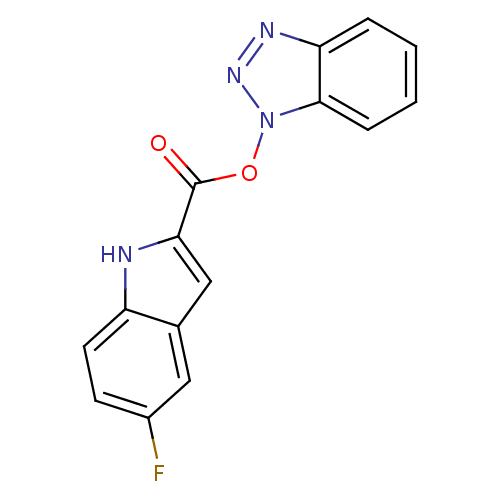 (Benzotriazole ester, 10 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59238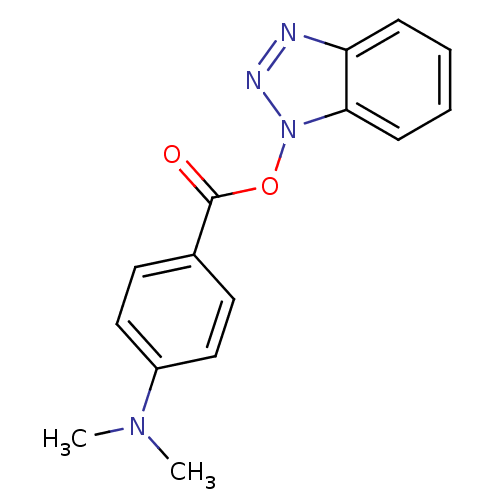 (Benzotriazole ester, 4 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59237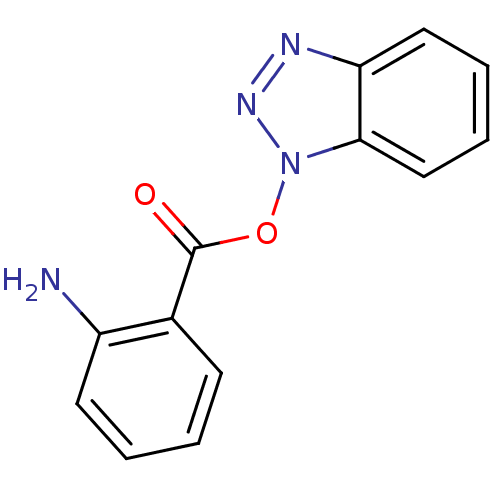 (Benzotriazole ester, 3 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39816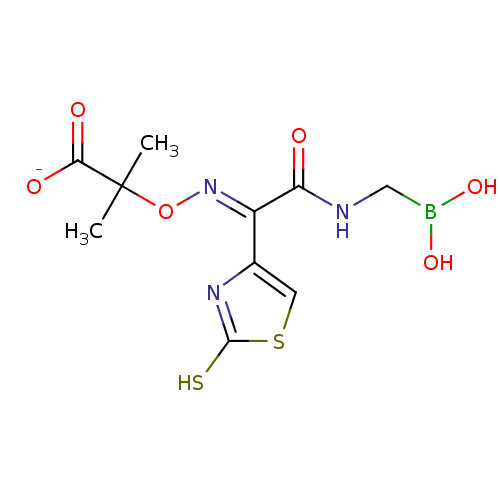 (Acylglycineboronic acid, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59241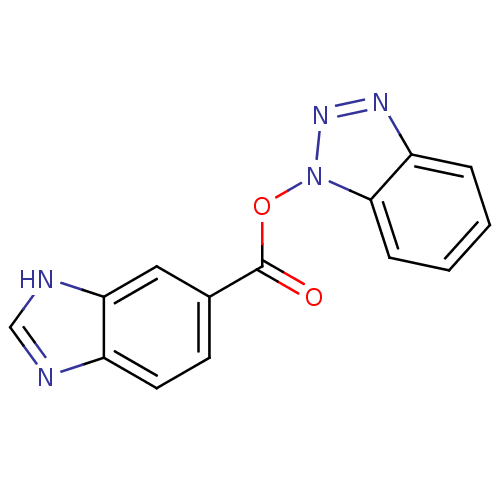 (Benzotriazole ester, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39812 (Acylglycineboronic acid, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39815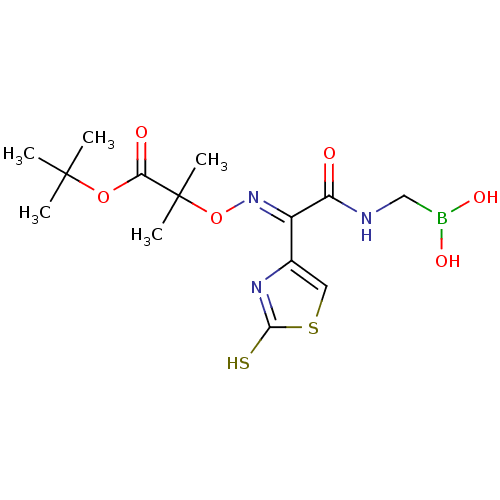 (Acylglycineboronic acid, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50115620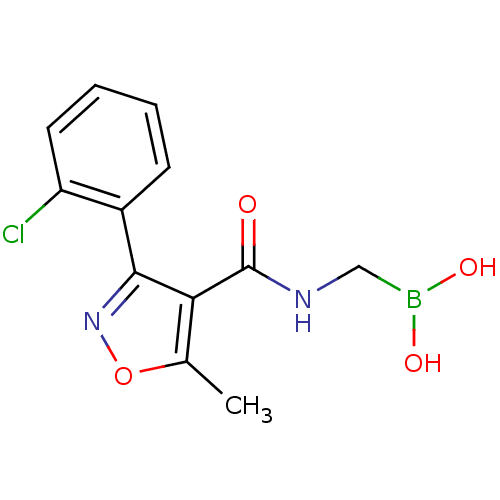 (3-(2-Chloro-phenyl)-5-methyl-2,3-dihydro-isoxazole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39814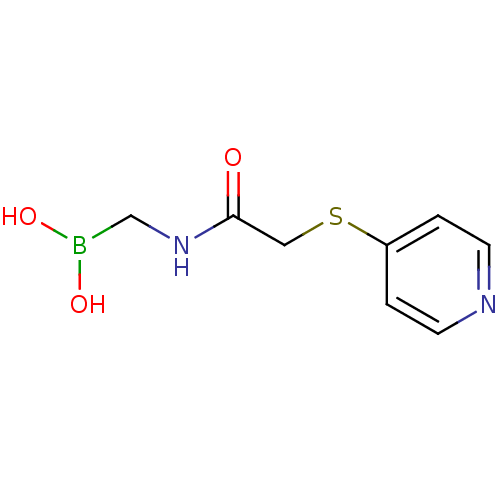 (Acylglycineboronic acid, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50283074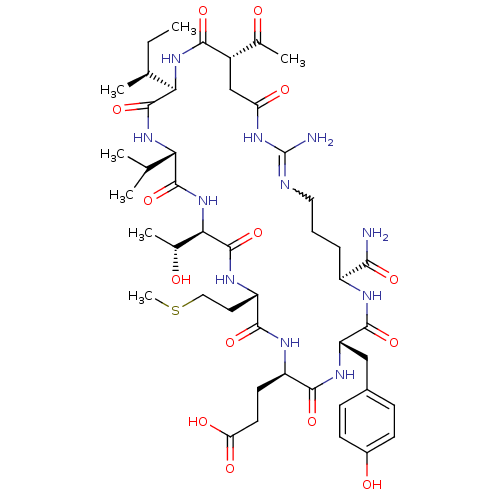 (CHEMBL413681 | Cyclic-Ac-Asp-Ile-Val-Thr-Met-Glu-T...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of chymotrypsin at 120 nM | Bioorg Med Chem Lett 4: 2123-2128 (1994) Article DOI: 10.1016/S0960-894X(01)80114-1 BindingDB Entry DOI: 10.7270/Q2QC03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50283074 (CHEMBL413681 | Cyclic-Ac-Asp-Ile-Val-Thr-Met-Glu-T...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of chymotrypsin at 69 nM | Bioorg Med Chem Lett 4: 2123-2128 (1994) Article DOI: 10.1016/S0960-894X(01)80114-1 BindingDB Entry DOI: 10.7270/Q2QC03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39813 (Acylglycineboronic acid, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39811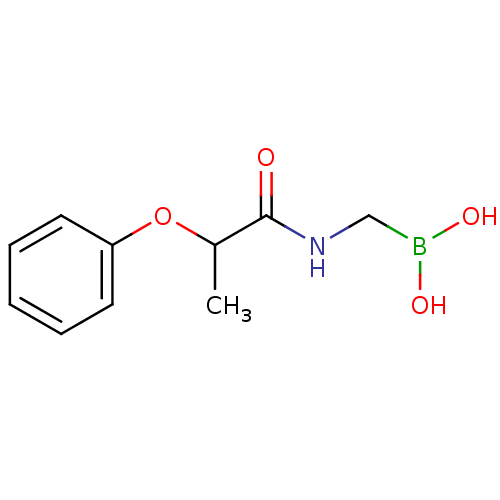 (Acylglycineboronic acid, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50115621 ((2-(thiophen-2-yl)acetamido)methylboronic acid | A...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem | PDB Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM39816 (Acylglycineboronic acid, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39809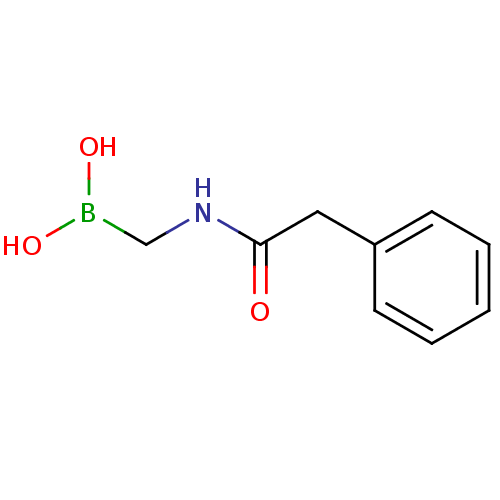 (Acylglycineboronic acid, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50283075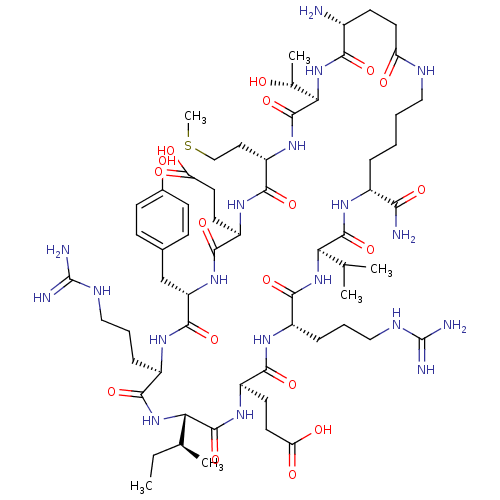 (CHEMBL269484 | Cyclic-NH2-Glu-Thr -Met-Glu-Tyr-Arg...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 656 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of chymotrypsin at 276 nM | Bioorg Med Chem Lett 4: 2123-2128 (1994) Article DOI: 10.1016/S0960-894X(01)80114-1 BindingDB Entry DOI: 10.7270/Q2QC03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50283075 (CHEMBL269484 | Cyclic-NH2-Glu-Thr -Met-Glu-Tyr-Arg...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 668 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of chymotrypsin at 138 nM | Bioorg Med Chem Lett 4: 2123-2128 (1994) Article DOI: 10.1016/S0960-894X(01)80114-1 BindingDB Entry DOI: 10.7270/Q2QC03F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39810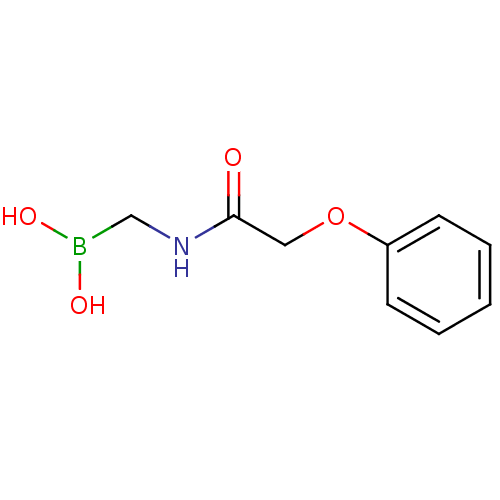 (Acylglycineboronic acid, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59246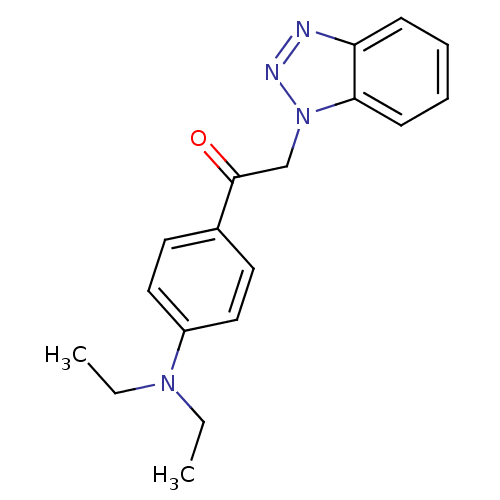 (Benzotriazole ester, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59249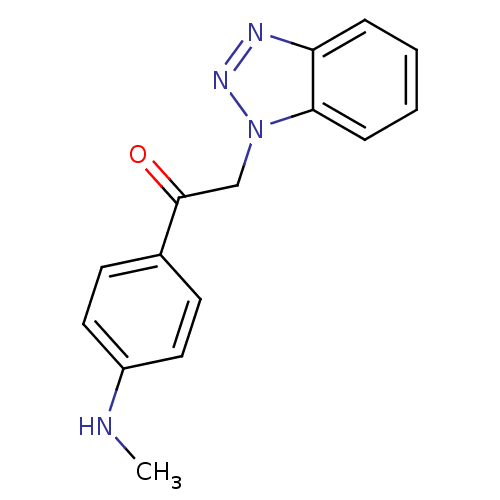 (Benzotriazole ester, 17 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39808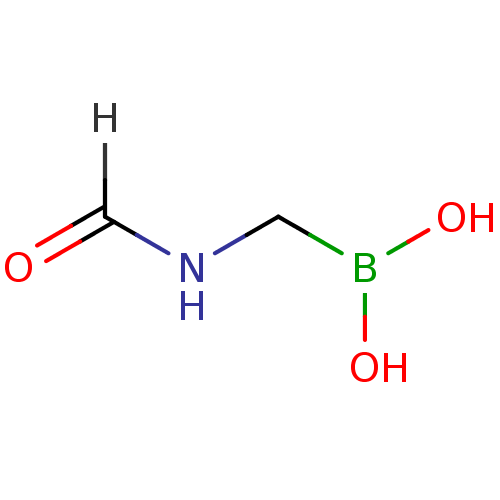 (Acylglycineboronic acid, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50115621 ((2-(thiophen-2-yl)acetamido)methylboronic acid | A...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59250 (Benzotriazole ester, 18 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50115620 (3-(2-Chloro-phenyl)-5-methyl-2,3-dihydro-isoxazole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM39809 (Acylglycineboronic acid, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50202233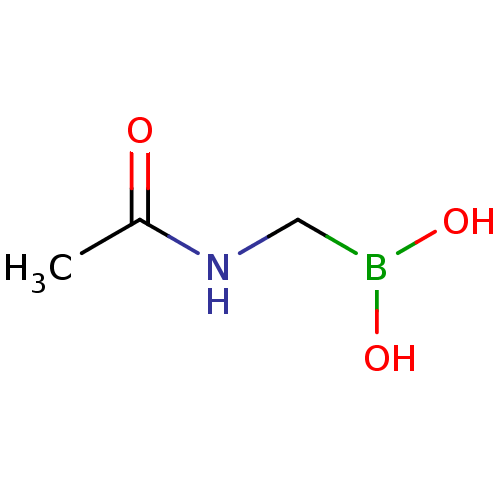 (Acylglycineboronic acid, 5 | CHEMBL227673 | acetam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM39808 (Acylglycineboronic acid, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59251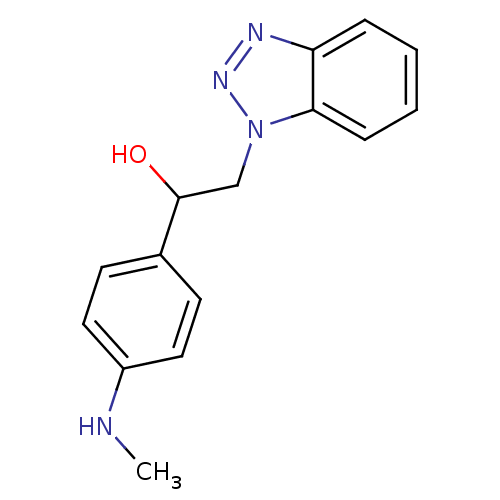 (Benzotriazole ester, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59252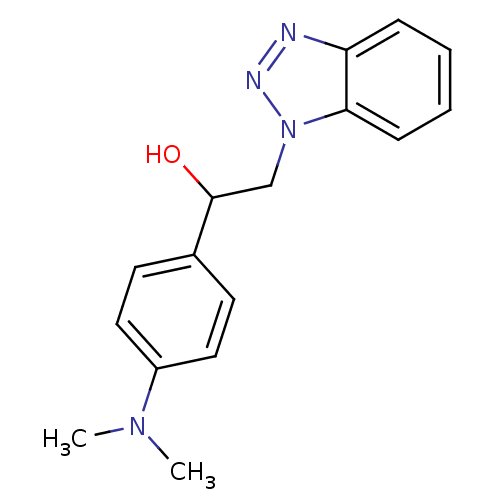 (Benzotriazole ester, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50202233 (Acylglycineboronic acid, 5 | CHEMBL227673 | acetam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39818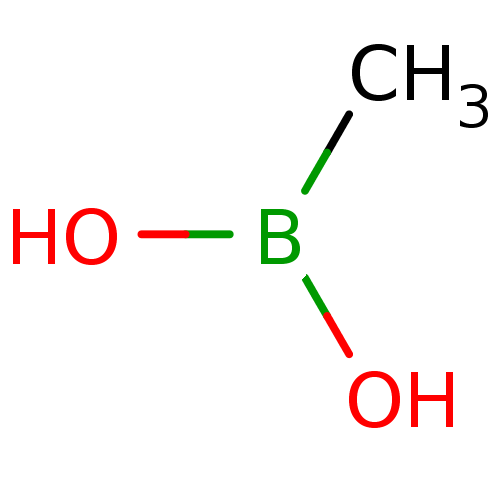 (Methylboronic Acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM39817 (Boric acid | Boronic Acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM39818 (Methylboronic Acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39817 (Boric acid | Boronic Acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem | Article PubMed | 2.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232913 (CHEMBL4103350) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232926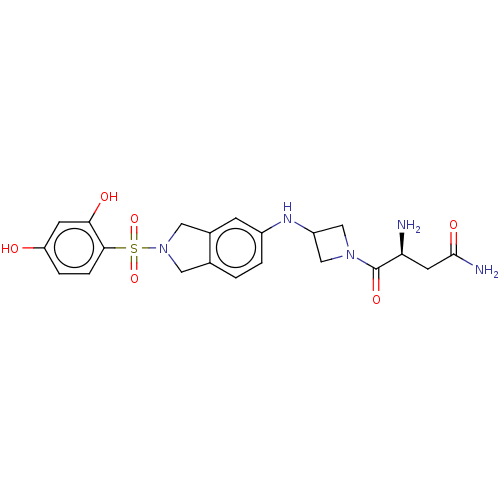 (CHEMBL4080017) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232920 (CHEMBL4078432) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232912 (CHEMBL4104843) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232924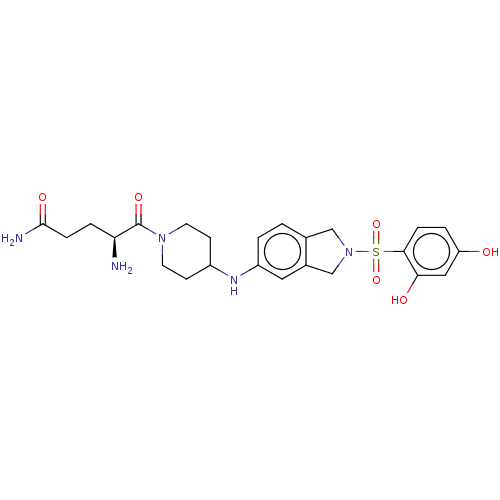 (CHEMBL4085424) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232913 (CHEMBL4103350) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 in presence of E1 protein and PDC core E2/E3BP incubated for 10 mins by titration as... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232911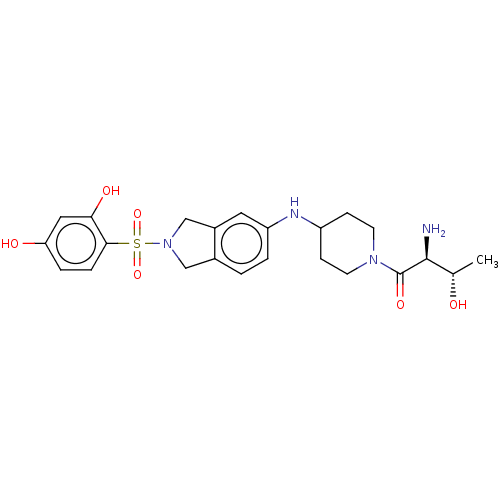 (CHEMBL4080613) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232915 (CHEMBL4066580) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232916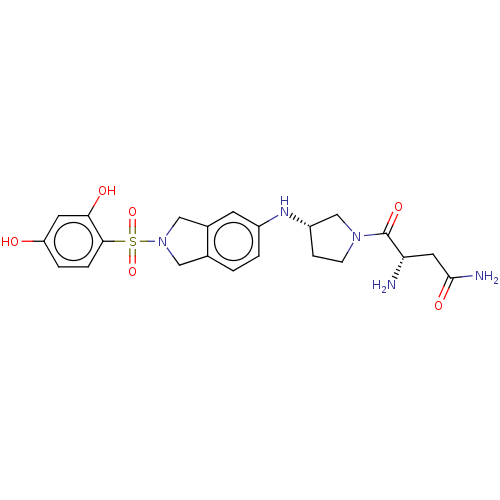 (CHEMBL4087901) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232905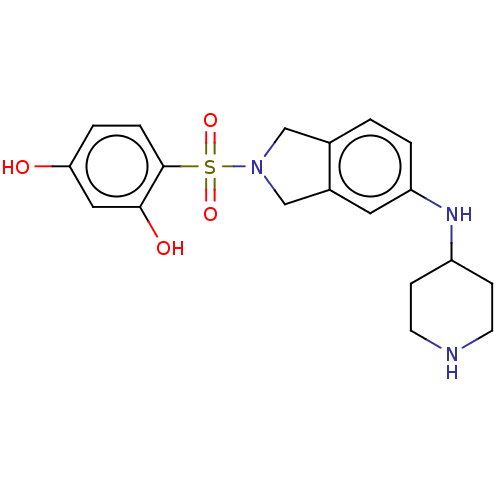 (CHEMBL4091824) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM50232917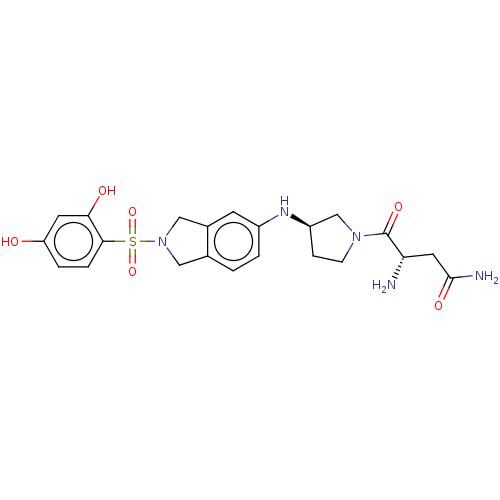 (CHEMBL4104250) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Science Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-SUMO tagged recombinant human PDK2 using [32P]-gamma-ATP assessed as decrease in incorporation of radioactivity into E1... | J Med Chem 60: 1142-1150 (2017) Article DOI: 10.1021/acs.jmedchem.6b01540 BindingDB Entry DOI: 10.7270/Q2BP0514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 126 total ) | Next | Last >> |