

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274048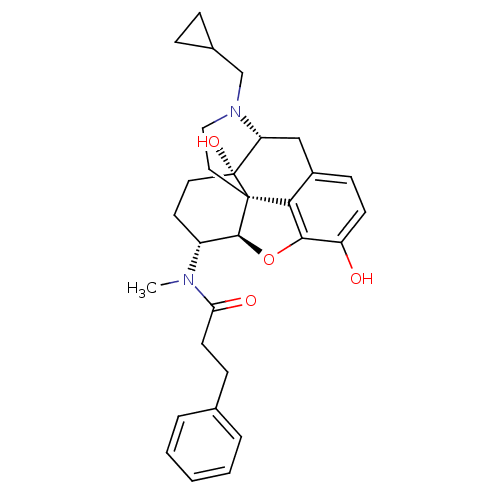 (CHEMBL489866 | N-[(5R,6R)-17-(Cyclopropylmethyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274519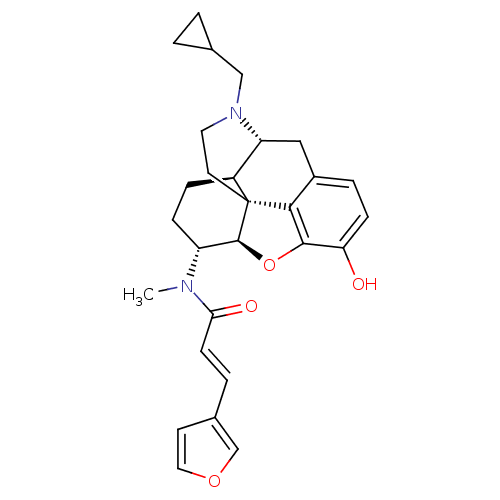 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274401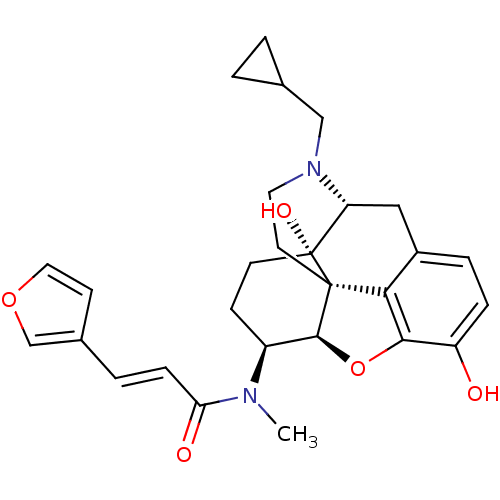 ((2E)-N-[(5R,6S)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274178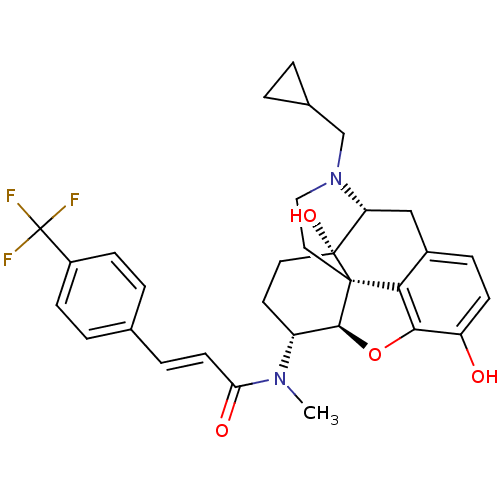 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274255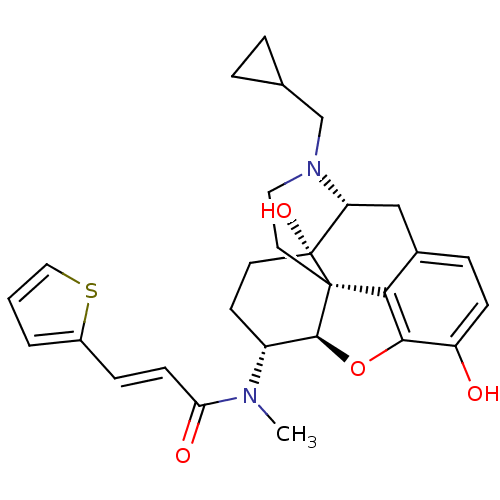 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274256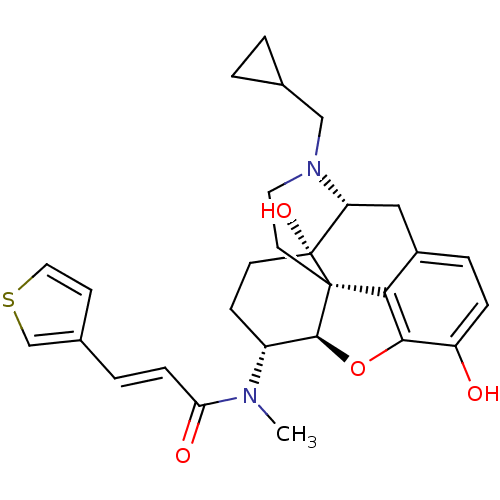 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274175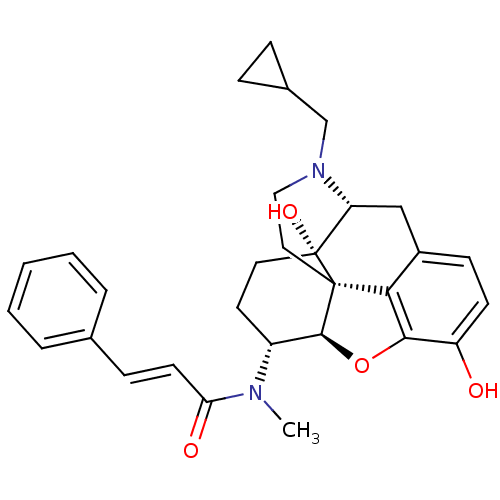 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.166 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274214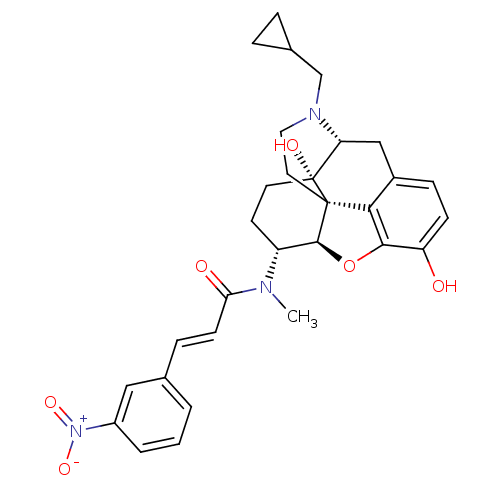 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274212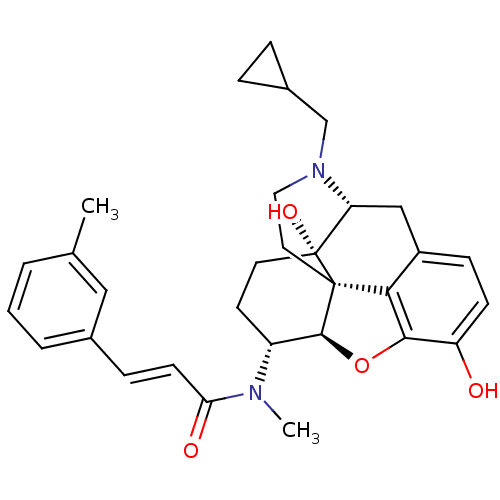 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274349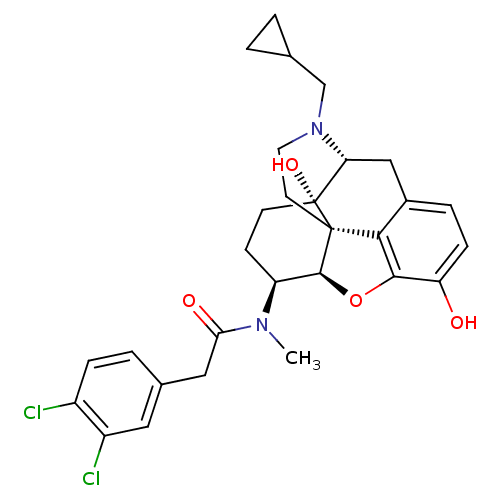 (CHEMBL491075 | N-[(5R,6S)-17-(Cyclopropylmethyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274348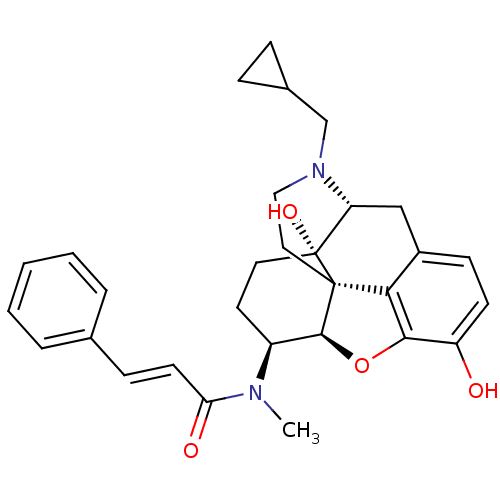 ((2E)-N-[(5R,6S)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274017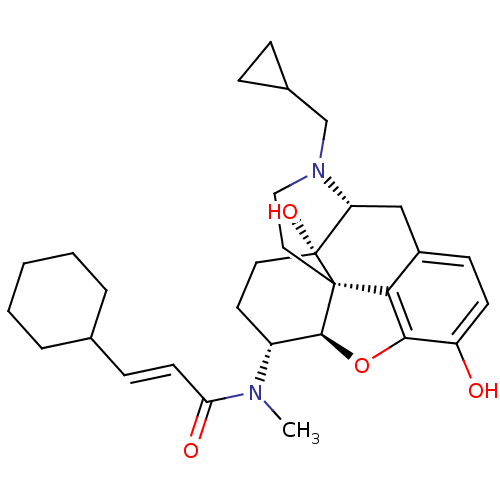 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.457 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274177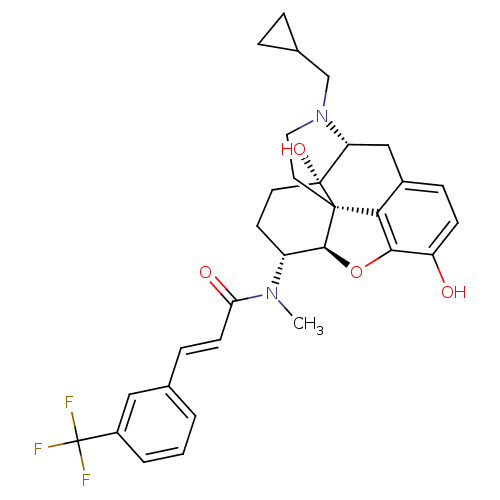 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274257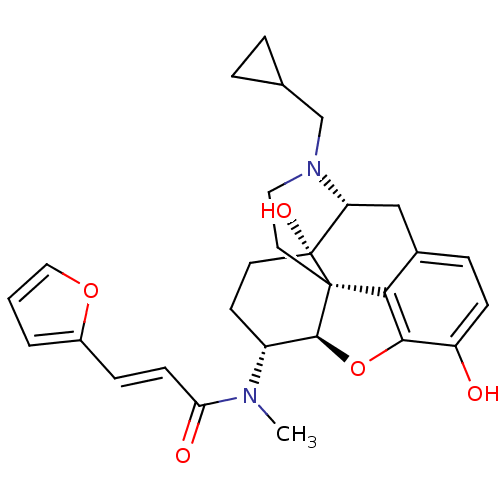 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274016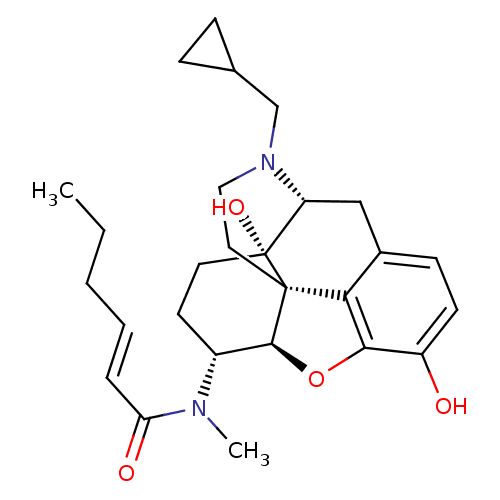 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.647 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274179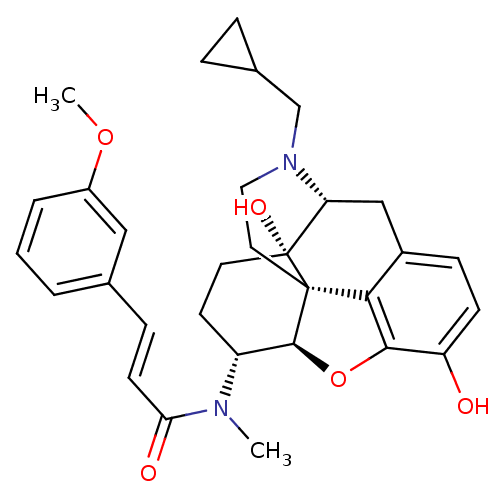 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274520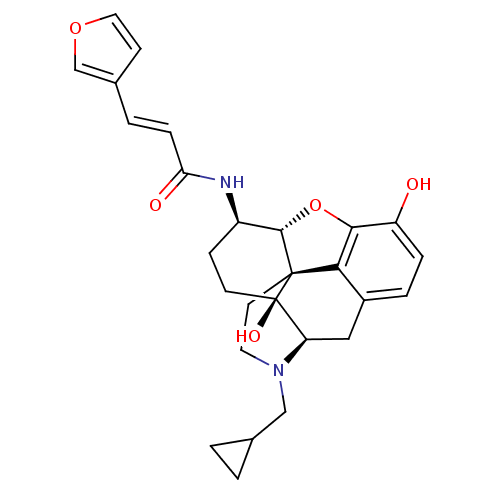 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.869 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in guinea pig ileum assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274213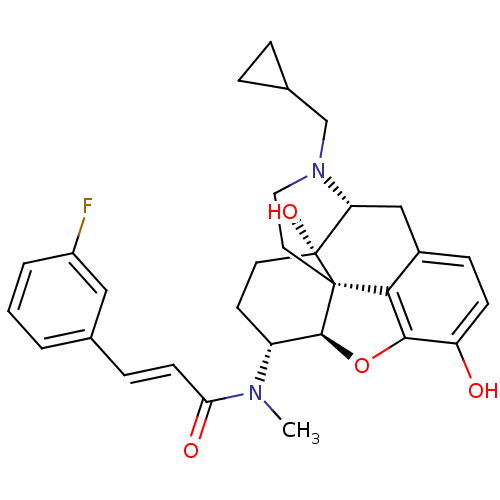 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274775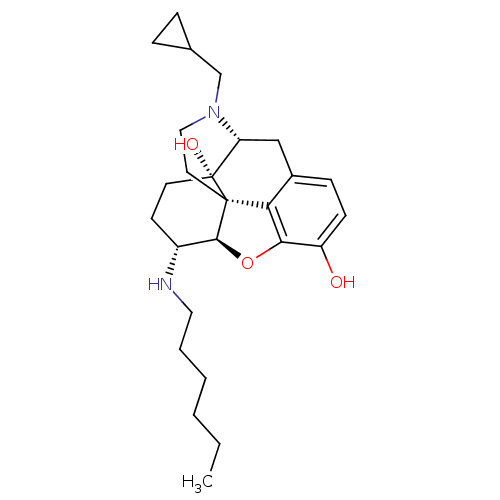 (CHEMBL457735 | N-hexyl-[(5R,6R)-17-(cyclopropylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274015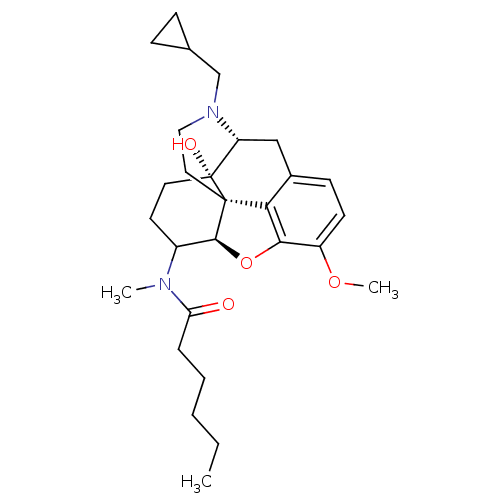 (CHEMBL459978 | N-[(5R,6R)-17-(Cyclopropylmethyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274721 ((5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-6-hexylox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274176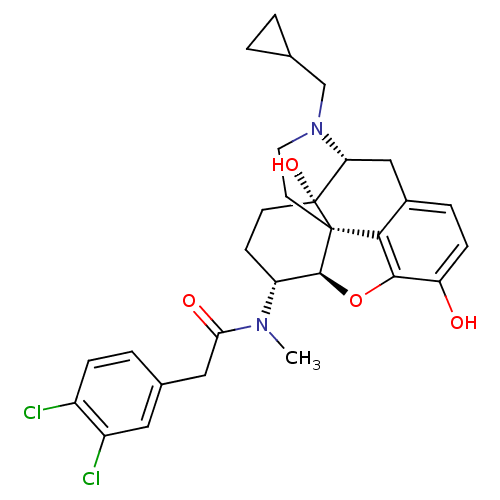 (CHEMBL523151 | N-[(5R,6R)-17-(Cyclopropylmethyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274774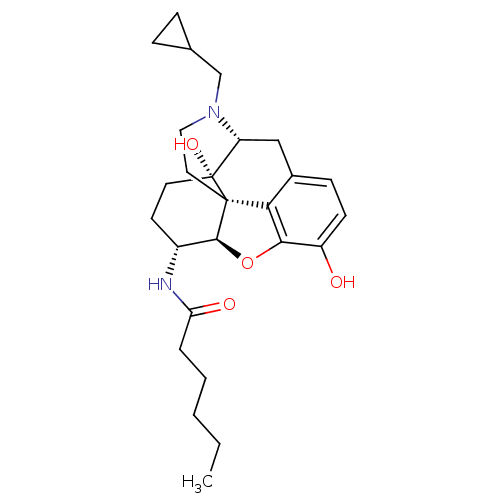 (CHEMBL456659 | N-[(5R,6R)-17-(Cyclopropylmethyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274482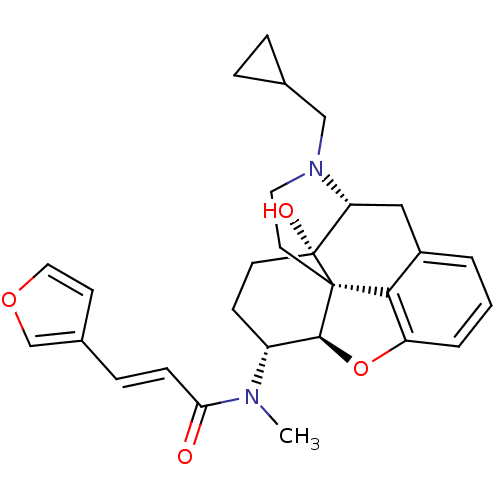 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274402 ((2E)-N-[(5R,6R)-4,5-Epoxy-3,14-dihydroxy-17-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156472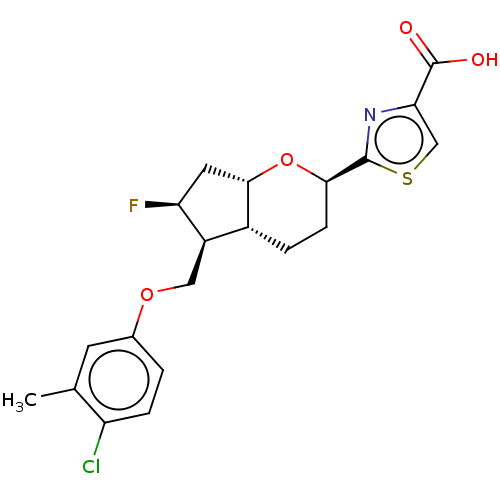 (CHEMBL3793167) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156473 (CHEMBL3793724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156474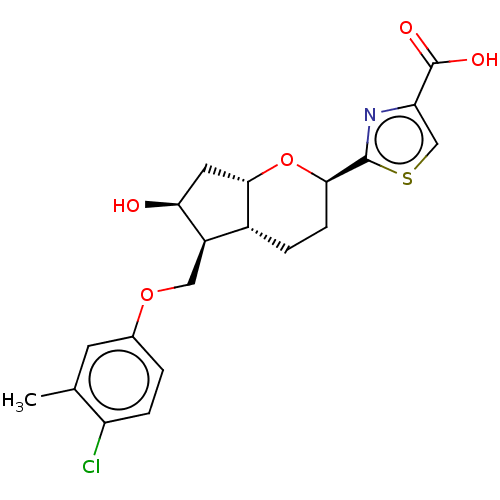 (CHEMBL3793515) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156491 (CHEMBL3792668) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156528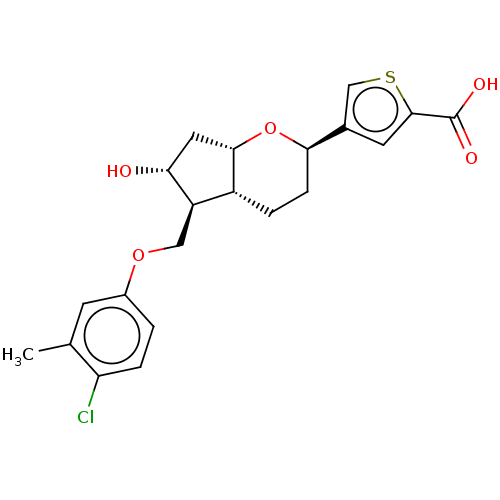 (CHEMBL3794185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 114 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156529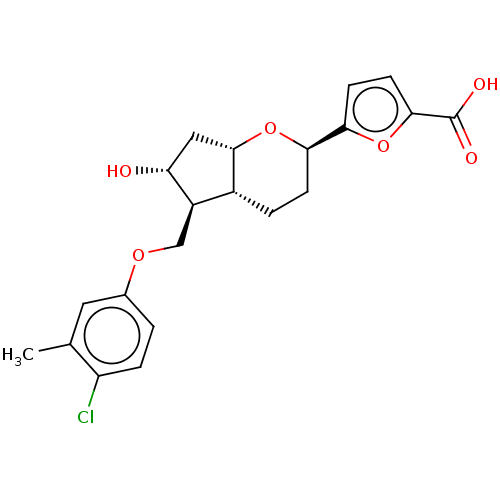 (CHEMBL3794226) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156530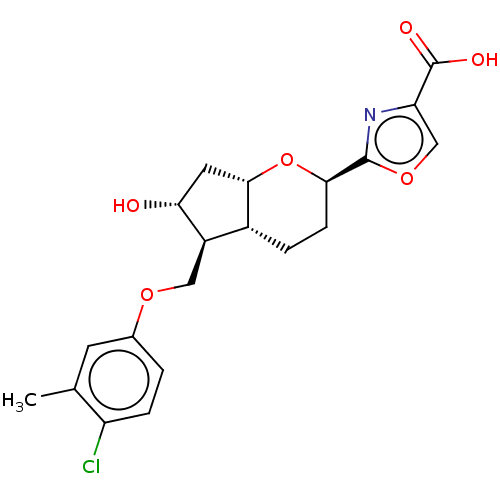 (CHEMBL3793009) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156531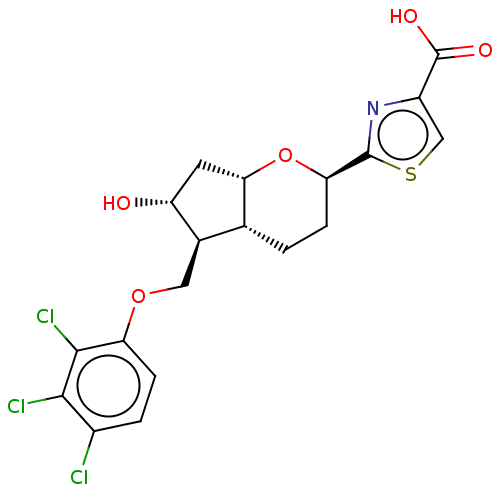 (CHEMBL3792632) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156532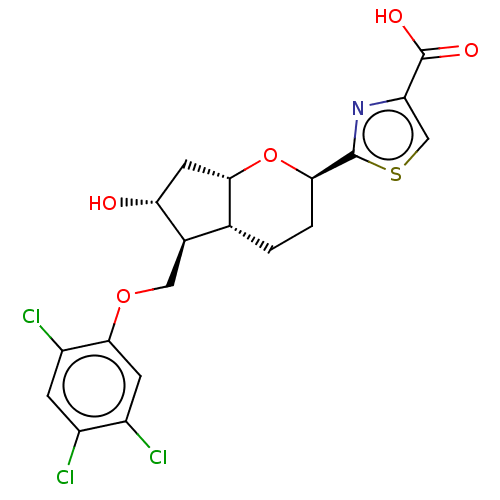 (CHEMBL3793862) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156533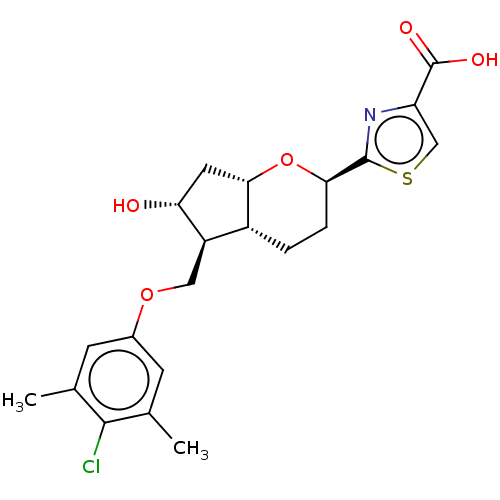 (CHEMBL3794016) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156534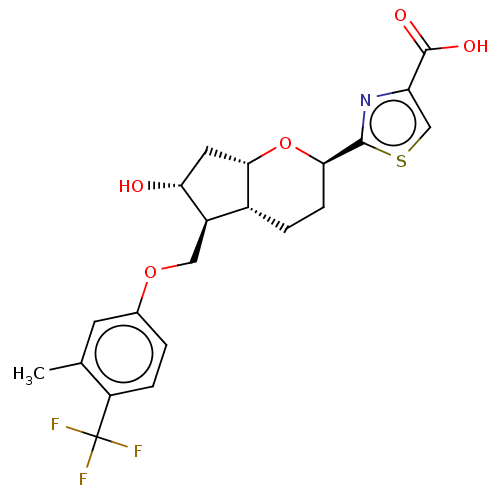 (CHEMBL3793809) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156535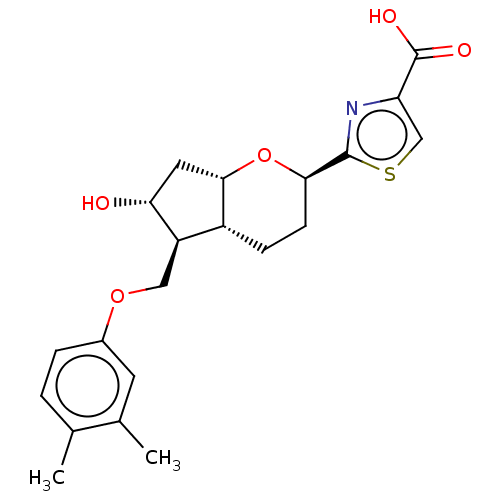 (CHEMBL3793797) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156536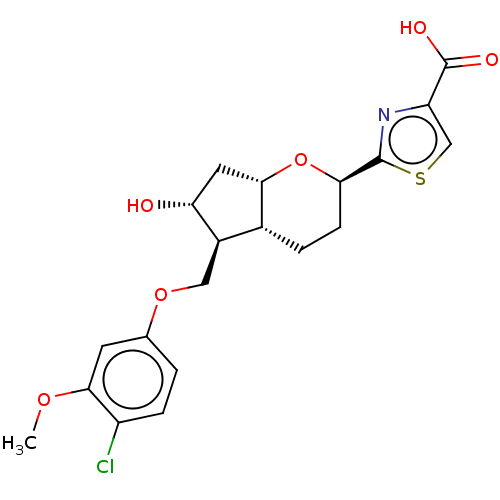 (CHEMBL3794585) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156537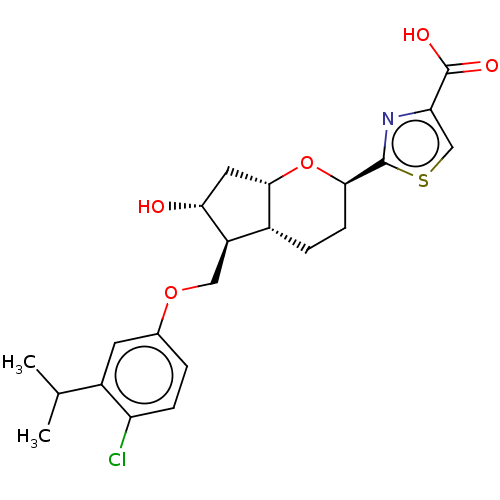 (CHEMBL3793020) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156538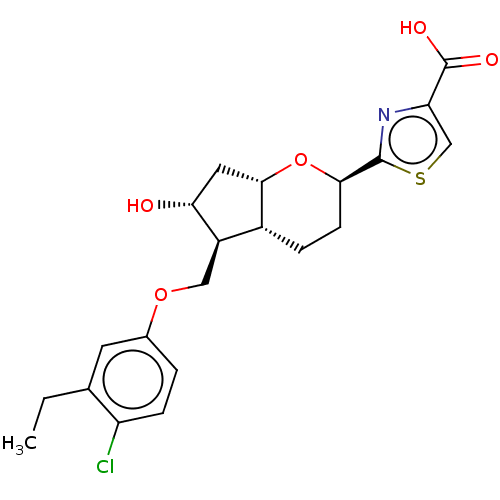 (CHEMBL3794466) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156539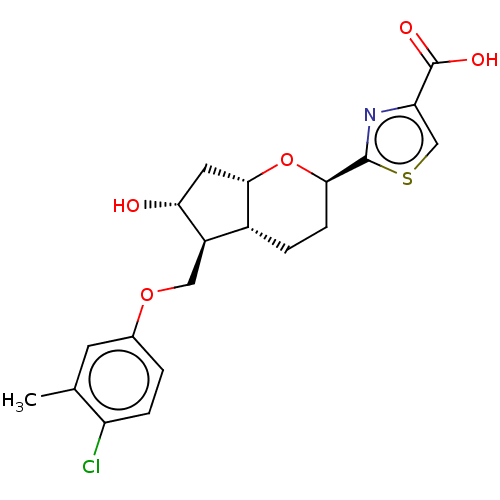 (CHEMBL3793892) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156540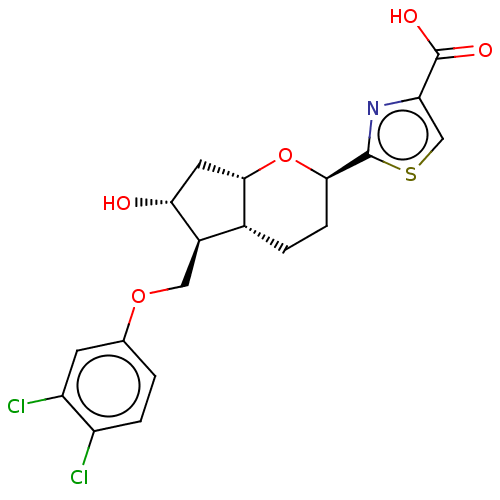 (CHEMBL3793802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156541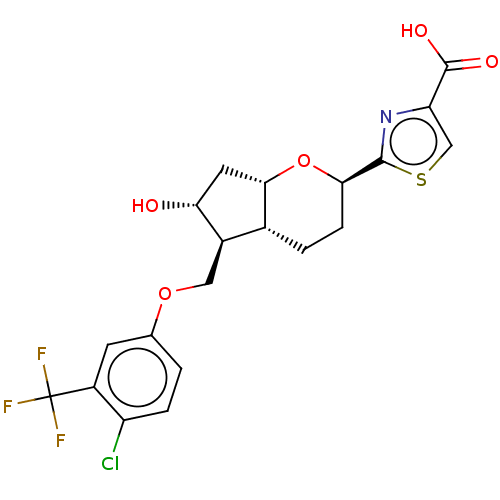 (CHEMBL3794302) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156542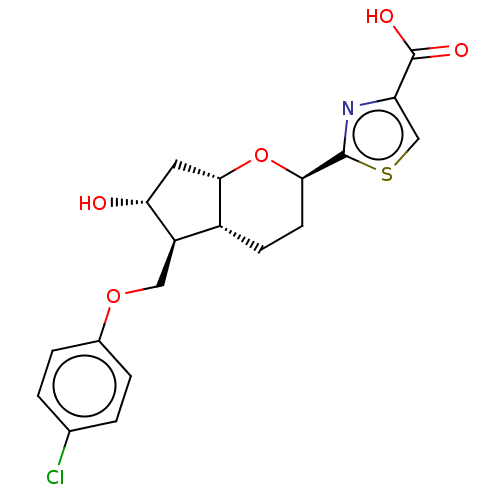 (CHEMBL3793786) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156543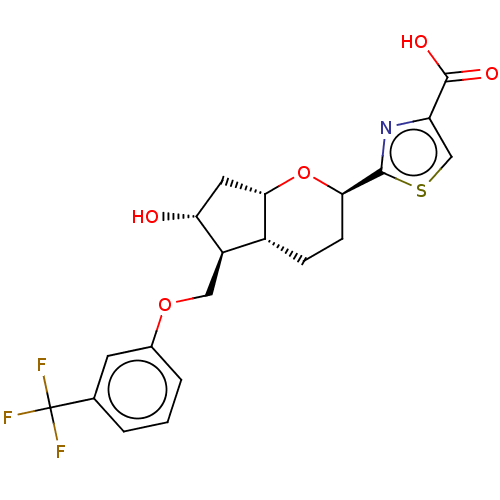 (CHEMBL3793045) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156544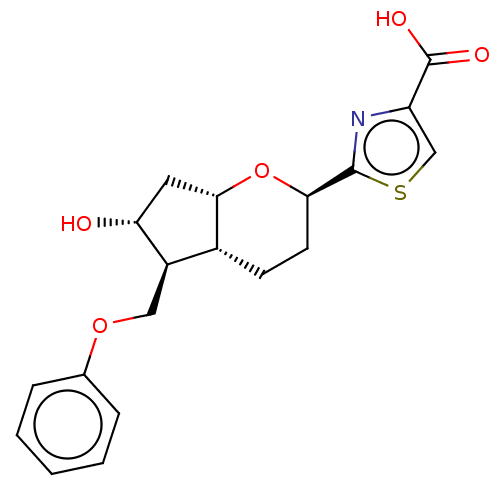 (CHEMBL3793209) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in CHO cells assessed as cAMP level by HTRF assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50156544 (CHEMBL3793209) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in HEK293 cells after 24 hrs beta-arrestin recruitment assay | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 254 total ) | Next | Last >> |