

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930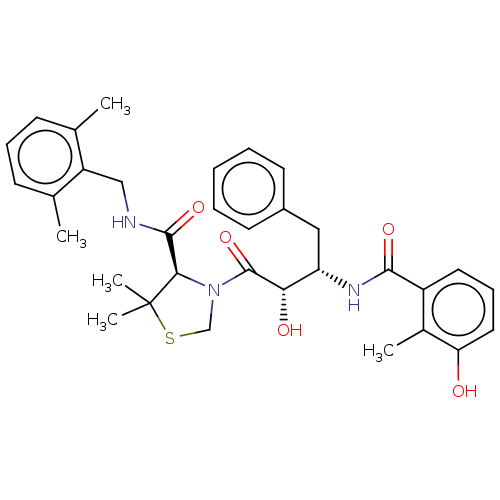 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256265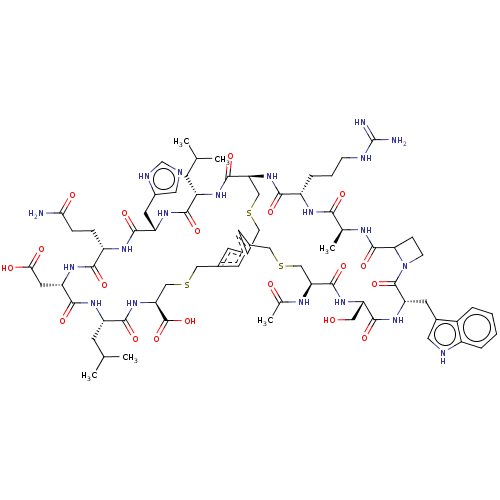 (CHEMBL4089486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256283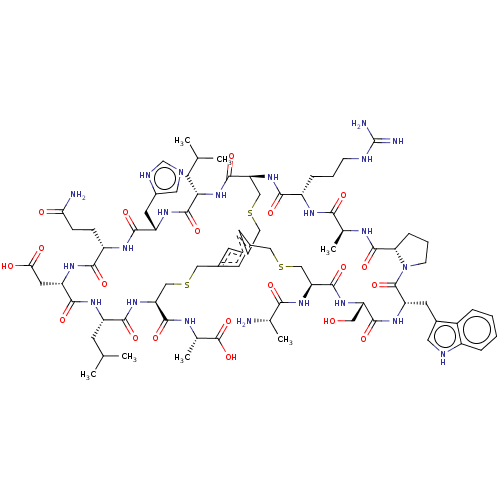 (CHEMBL4079711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256264 (CHEMBL4099333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256277 (CHEMBL4093698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480931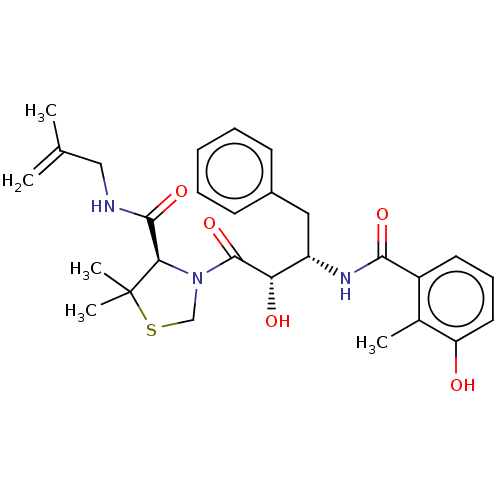 (CHEMBL575512 | KNI-1614) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18617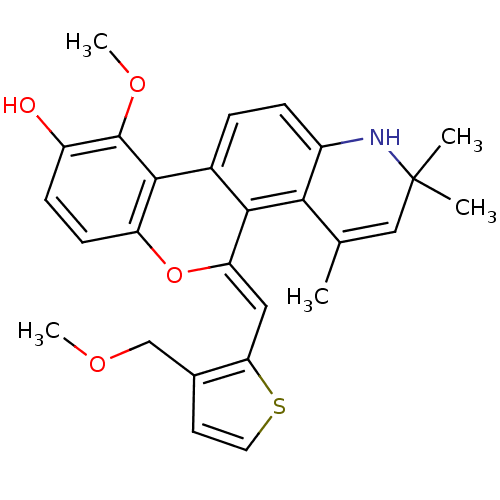 ((18Z)-12-methoxy-18-{[3-(methoxymethyl)thiophen-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -51.5 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256286 (CHEMBL4076199) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256262 (CHEMBL4070056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM263346 (US9546162, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | 11 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description Cells were seeded at a density of 5×104 cells per well in Biocoat® Poly-D-lysine-coated black-wall, clear-bottom 96-well plates (Becton-Dickinson) an... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256260 (CHEMBL4094403) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256276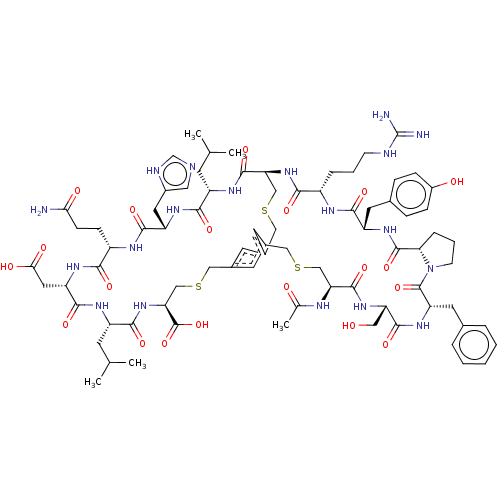 (CHEMBL4079260) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM50256284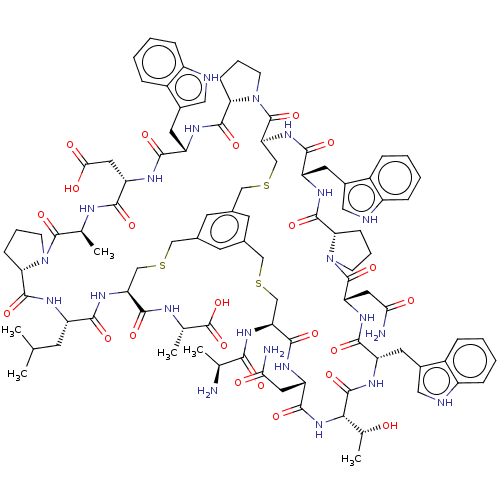 (CHEMBL4075544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM263361 (US9546162, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | 2 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, or EP4 receptors were washed with TME buffer, scraped from the bottom o... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256288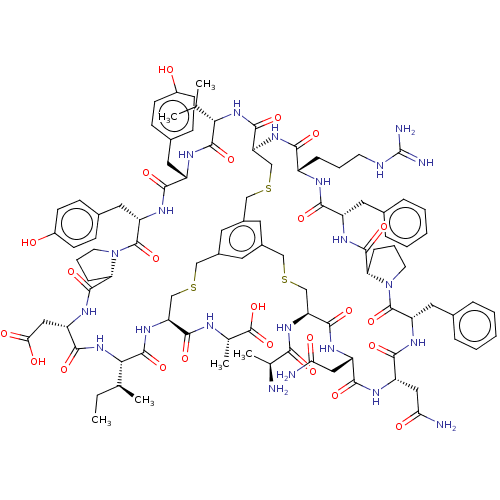 (CHEMBL4085408) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18622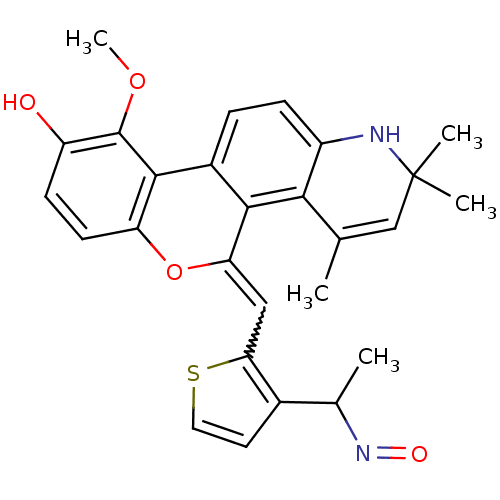 ((18Z)-18-({3-[(1E)-1-(hydroxyimino)ethyl]thiophen-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -48.9 | 1.10 | n/a | 5 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18616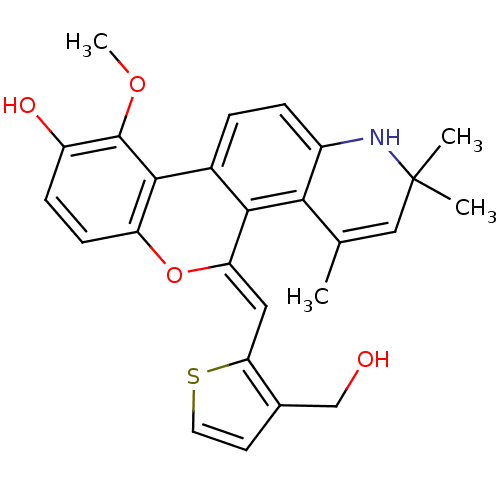 ((18Z)-18-{[3-(hydroxymethyl)thiophen-2-yl]methylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -48.6 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000655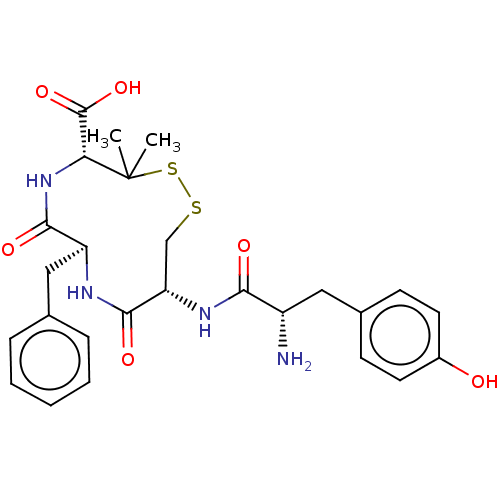 ((4R,7S,10R)-10-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 in guinea pig brain membranes | Bioorg Med Chem Lett 8: 2685-8 (1999) BindingDB Entry DOI: 10.7270/Q2154HJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM263357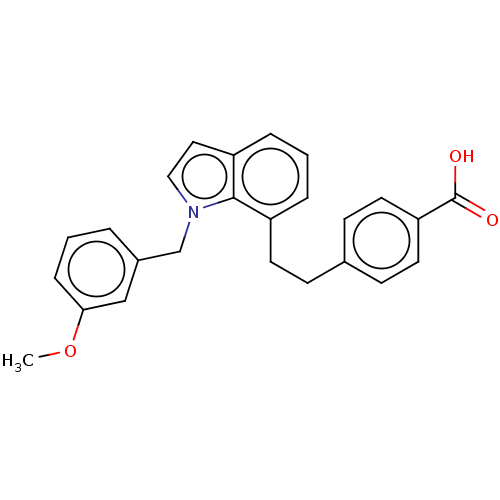 (US9546162, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | 1 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, or EP4 receptors were washed with TME buffer, scraped from the bottom o... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238312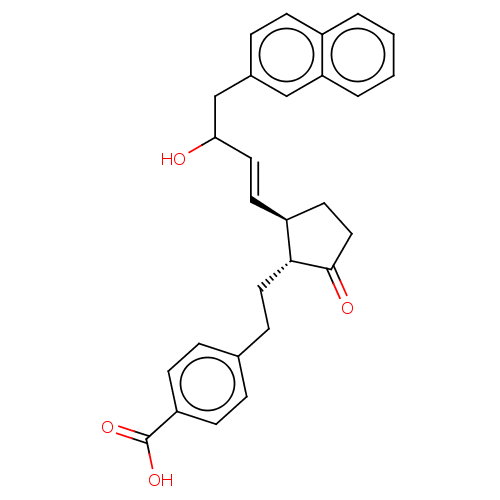 (US9394273, 1 | US9394273, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480929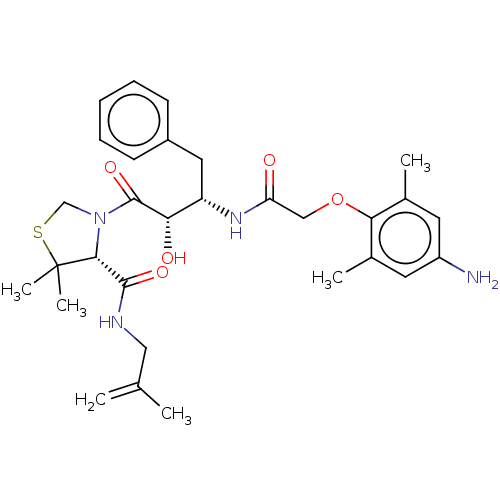 (CHEMBL573975 | KNI-1689) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM50256283 (CHEMBL4079711) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238319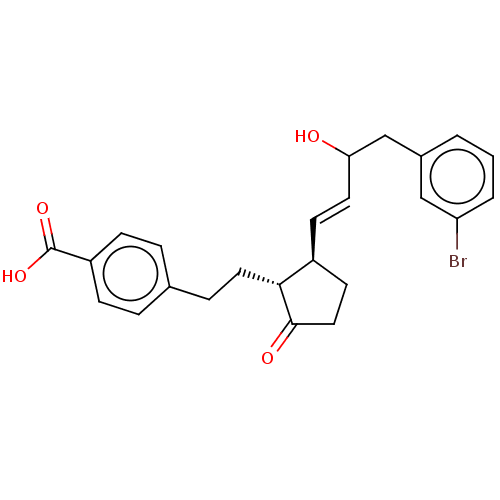 (US9394273, 10 | US9394273, 8 | US9394273, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238322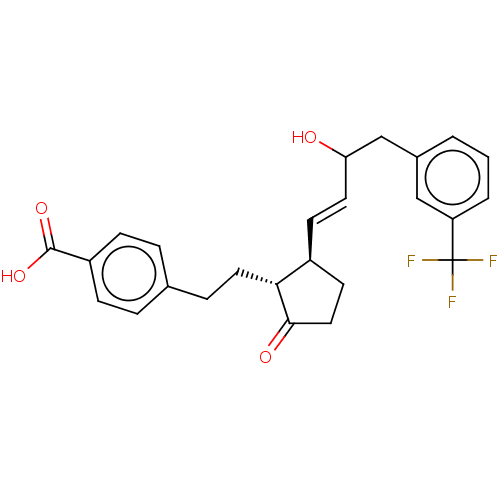 (US9394273, 11 | US9394273, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Synaptic vesicular amine transporter (Bos taurus) | BDBM50017712 ((-)-reserpine | (3beta,16beta,17alpha,18beta,20alp...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wichita State University Curated by ChEMBL | Assay Description Inhibition of dopamine uptake at VMAT in bovine chromaffin granule ghosts | J Med Chem 51: 760-8 (2008) Article DOI: 10.1021/jm070875p BindingDB Entry DOI: 10.7270/Q2M909J6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238319 (US9394273, 10 | US9394273, 8 | US9394273, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18623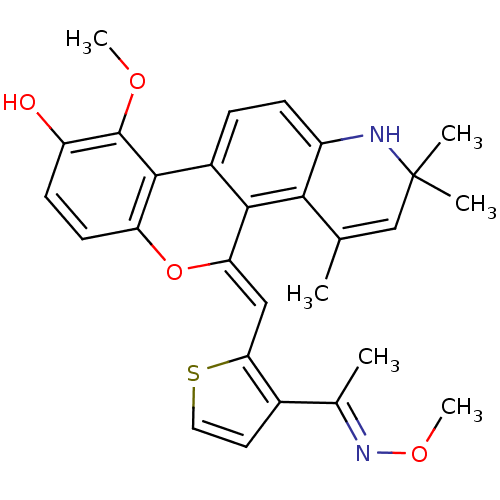 ((18Z)-12-methoxy-18-({3-[(1E)-1-(methoxyimino)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | -47.5 | 0.200 | n/a | 0.100 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18632 ((18Z)-18-{[3-(1-hydroxyethyl)thiophen-2-yl]methyli...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | -47.3 | 1.60 | n/a | 1.30 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256263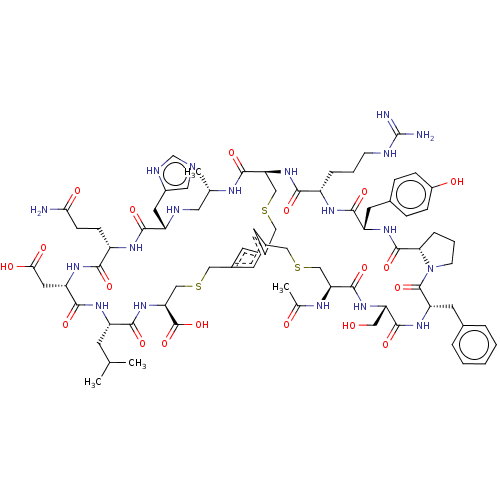 (CHEMBL4088185) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of platelet derived growth factor receptor beta phosphorylation in MG63 cells in the presence of human plasma | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18628 ((18Z)-12-methoxy-3,5,5-trimethyl-18-{[3-(2,2,2-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -47.0 | 0.400 | n/a | 0.300 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238316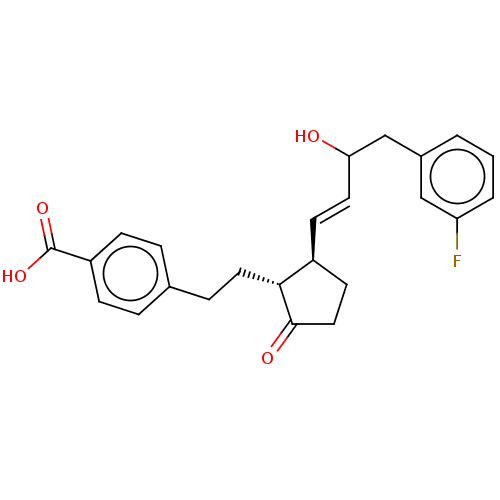 (US9394273, 5 | US9394273, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM263347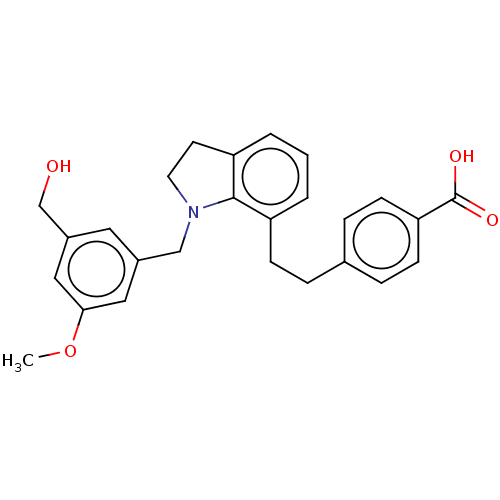 (US9546162, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | 11 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, or EP4 receptors were washed with TME buffer, scraped from the bottom o... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238336 (US9394273, 25 | US9394273, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM50256277 (CHEMBL4093698) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM263366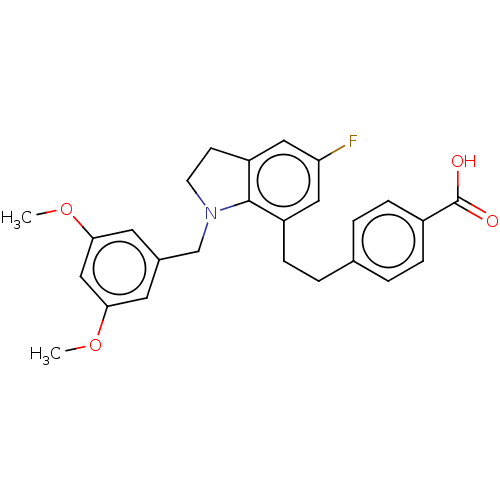 (US9546162, 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | 2 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description Cells were seeded at a density of 5×104 cells per well in Biocoat® Poly-D-lysine-coated black-wall, clear-bottom 96-well plates (Becton-Dickinson) an... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM263343 (US9546162, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, or EP4 receptors were washed with TME buffer, scraped from the bottom o... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238318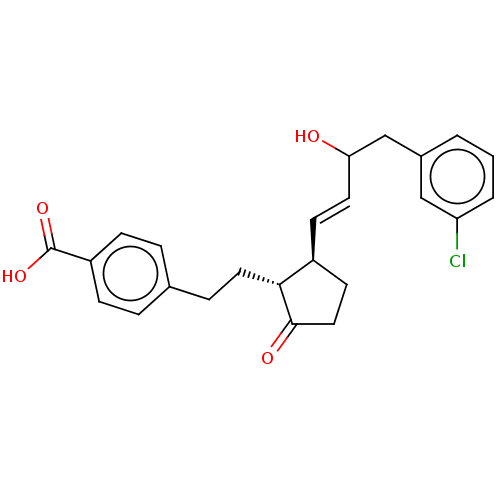 (US9394273, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.10 | -46.0 | 1.40 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256287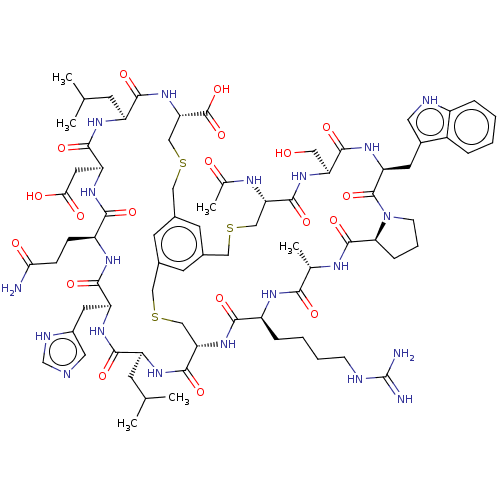 (CHEMBL4105318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256284 (CHEMBL4075544) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50071865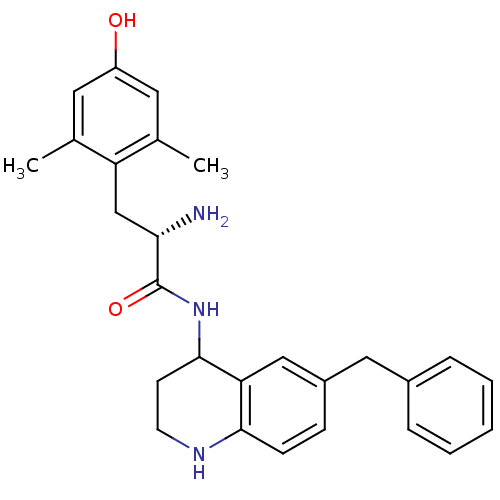 ((S)-2-Amino-N-(6-benzyl-1,2,3,4-tetrahydro-quinoli...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor in guinea pig brain membranes | Bioorg Med Chem Lett 8: 2685-8 (1999) BindingDB Entry DOI: 10.7270/Q2154HJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50071865 ((S)-2-Amino-N-(6-benzyl-1,2,3,4-tetrahydro-quinoli...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 in guinea pig brain membranes | Bioorg Med Chem Lett 8: 2685-8 (1999) BindingDB Entry DOI: 10.7270/Q2154HJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50256261 (CHEMBL4059991) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate additio... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238330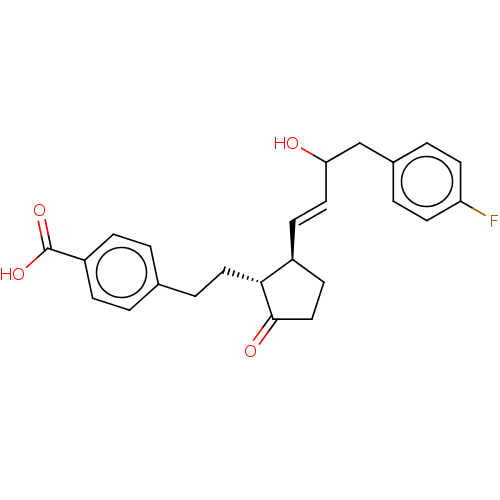 (US9394273, 19 | US9394273, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM263357 (US9546162, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | 11 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, or EP4 receptors were washed with TME buffer, scraped from the bottom o... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM238334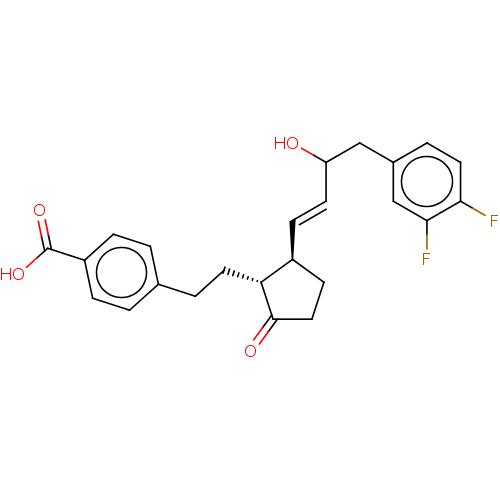 (US9394273, 23 | US9394273, 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALLERGAN, INC. US Patent | Assay Description The cell homogenate was centrifuged at 19000 r.p.m. for 20 min at 4° C. using a Beckman Ti-60 rotor. The resultant pellet was resuspended in TME buf... | US Patent US9394273 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM263343 (US9546162, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | 7.20 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, or EP4 receptors were washed with TME buffer, scraped from the bottom o... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Rattus norvegicus) | BDBM50256261 (CHEMBL4059991) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of recombinant Sprague-Dawley rat plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins foll... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein (Sus scrofa) | BDBM50256277 (CHEMBL4093698) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bicycle Therapeutics Limited, Building 900, Babraham Research Campus , Cambridge CB22 3AT , U.K. Curated by ChEMBL | Assay Description Inhibition of pig plasma kallikrein using fluorogenic H-Pro-Phe-Arg-AMC peptide as substrate preincubated for 15 mins followed by substrate addition ... | J Med Chem 61: 2823-2836 (2018) Article DOI: 10.1021/acs.jmedchem.7b01625 BindingDB Entry DOI: 10.7270/Q2862JW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3117 total ) | Next | Last >> |