

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029090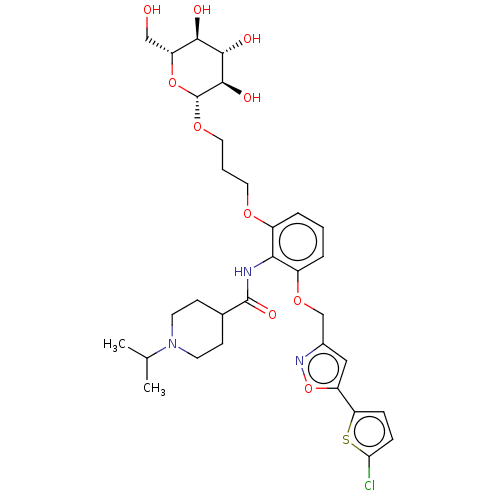 (CHEMBL3343301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029090 (CHEMBL3343301) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029091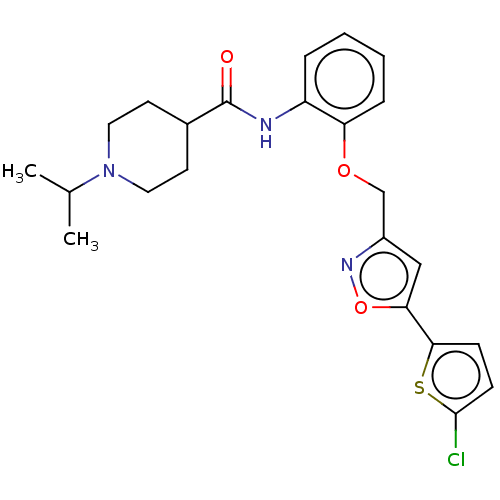 (CHEMBL3343299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029095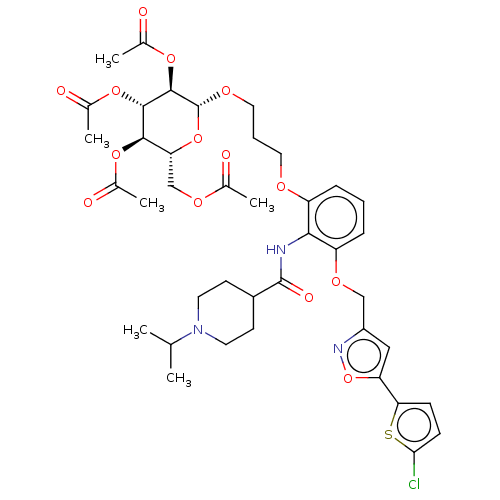 (CHEMBL3343300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029091 (CHEMBL3343299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210854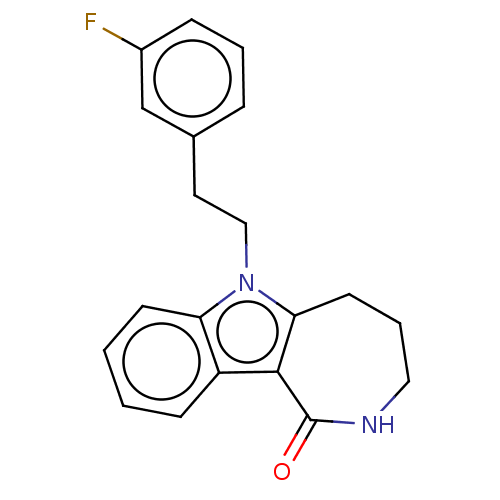 (CHEMBL3960040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Competitive inhibition of horse serum BChE in presence of varying levels of butyrylthiocholine iodide substrate by Lineweaver-burk plot method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029090 (CHEMBL3343301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029091 (CHEMBL3343299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029090 (CHEMBL3343301) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029095 (CHEMBL3343300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029092 (CHEMBL3343304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029091 (CHEMBL3343299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029094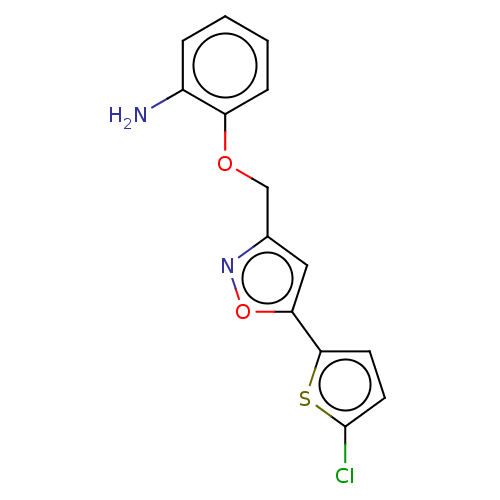 (CHEMBL3343302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029093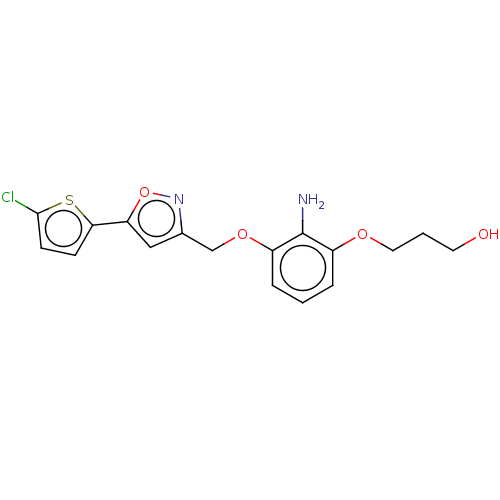 (CHEMBL3343303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029093 (CHEMBL3343303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029094 (CHEMBL3343302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029092 (CHEMBL3343304) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210854 (CHEMBL3960040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50210854 (CHEMBL3960040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of human BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50210852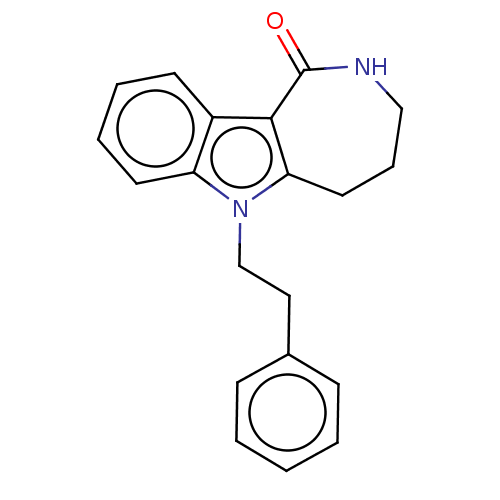 (CHEMBL3971112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of human BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210852 (CHEMBL3971112) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210848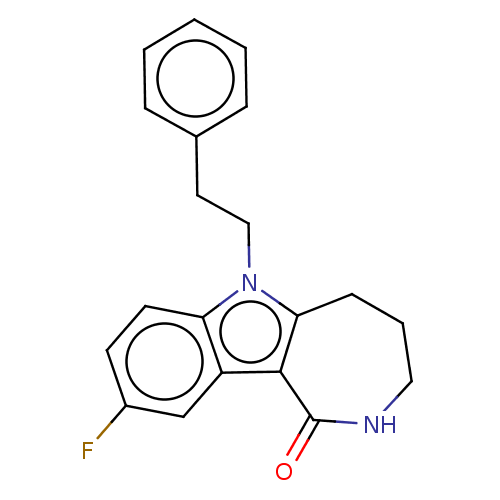 (CHEMBL3907854) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210847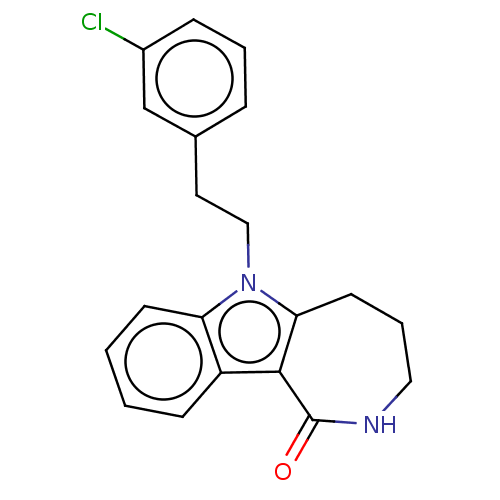 (CHEMBL3889911) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210842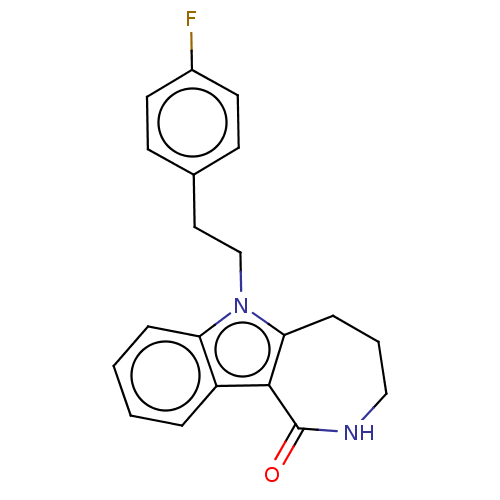 (CHEMBL3919289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210768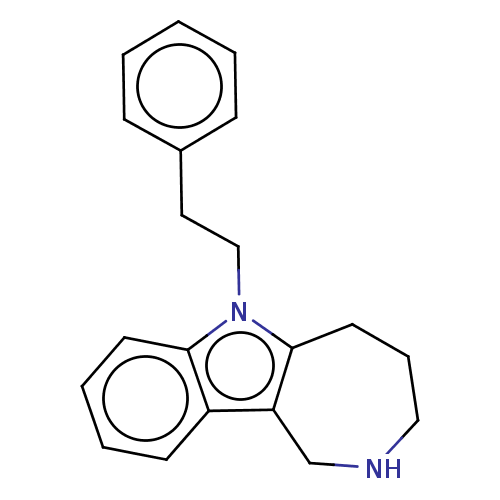 (CHEMBL3914751) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210849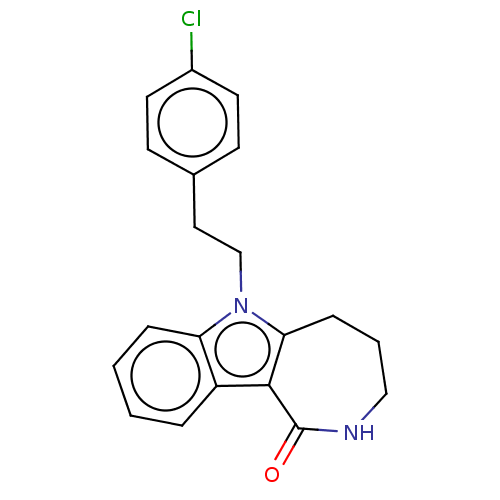 (CHEMBL3962431) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50210842 (CHEMBL3919289) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of human BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50210842 (CHEMBL3919289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of human AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210851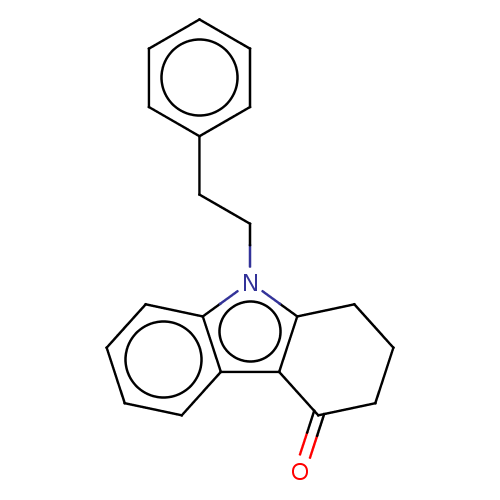 (CHEMBL3962358) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210851 (CHEMBL3962358) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210855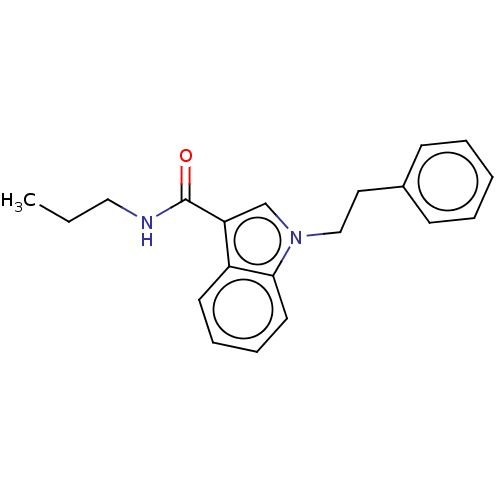 (CHEMBL3935090) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210844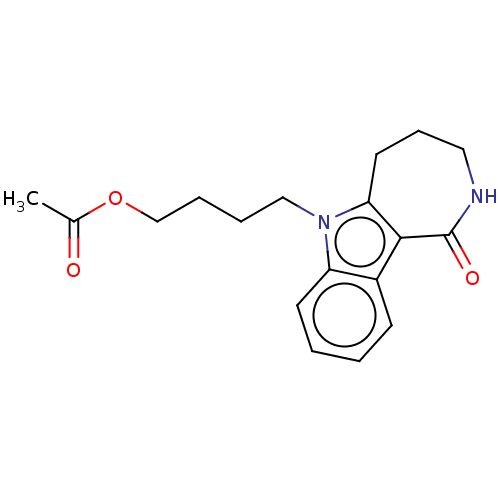 (CHEMBL3937926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210855 (CHEMBL3935090) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50210852 (CHEMBL3971112) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of human AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210843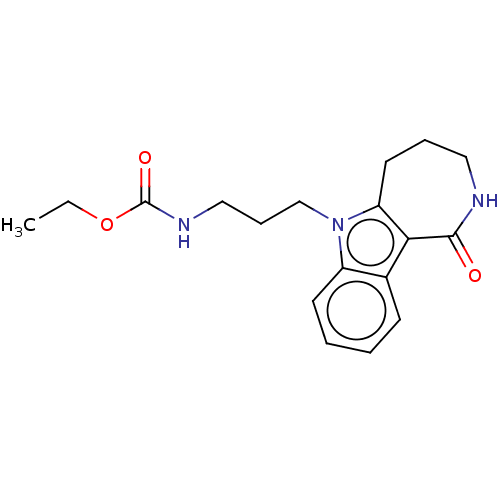 (CHEMBL3971444) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210852 (CHEMBL3971112) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210846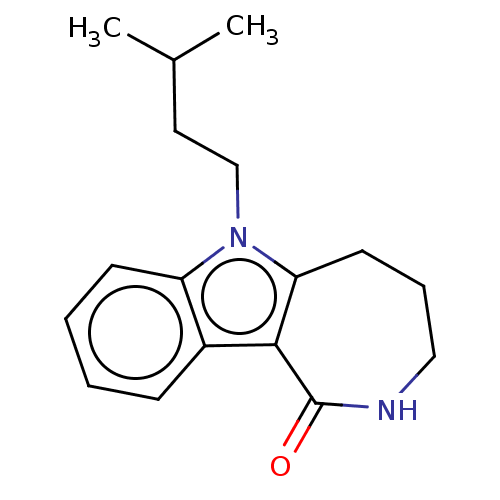 (CHEMBL3910836) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210853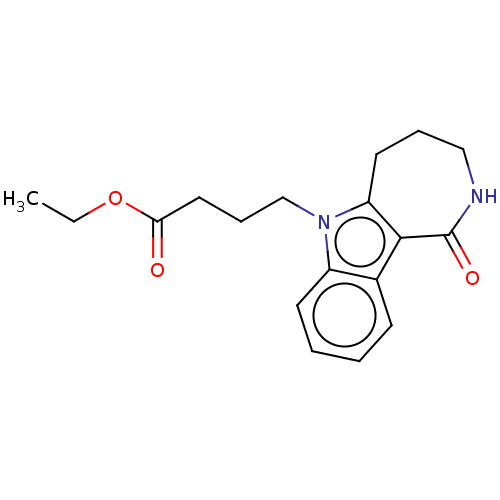 (CHEMBL3900327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210845 (CHEMBL3892498) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210853 (CHEMBL3900327) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210850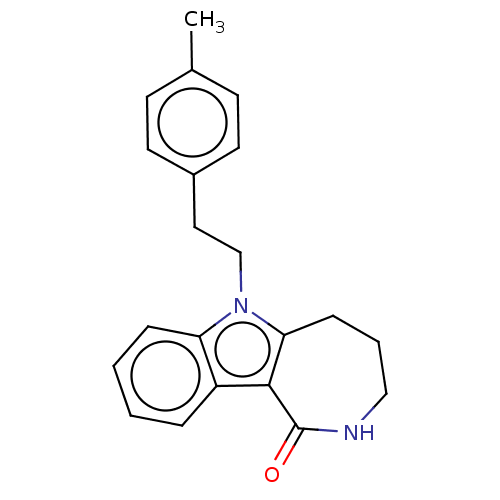 (CHEMBL3979295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of horse serum BChE pretreated for 20 mins followed by butyrylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210846 (CHEMBL3910836) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210843 (CHEMBL3971444) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari"A. Moro" Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pretreated for 20 mins followed by acetylthiocholine iodide substrate addition measured for 5 mins by Ellman's method | Eur J Med Chem 125: 288-298 (2017) Article DOI: 10.1016/j.ejmech.2016.09.037 BindingDB Entry DOI: 10.7270/Q2G44S9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||