 Found 4455 hits with Last Name = 'zhu' and Initial = 'b'
Found 4455 hits with Last Name = 'zhu' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50079482
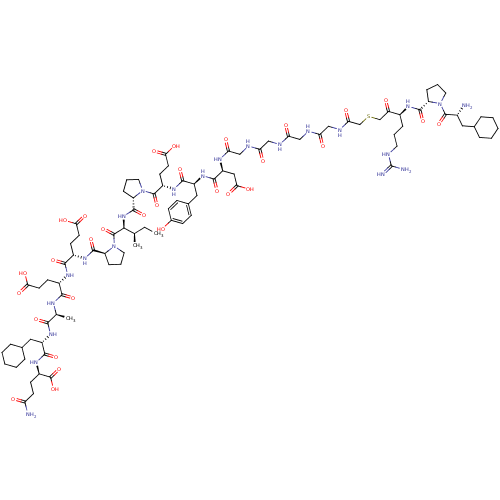
(Arginyl Ketomethylene analogue | CHEMBL410589)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CSCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C92H140N22O30S/c1-4-49(2)78(90(142)114-38-14-21-65(114)86(138)106-58(29-33-75(125)126)81(133)105-57(28-32-74(123)124)80(132)102-50(3)79(131)109-61(40-52-17-9-6-10-18-52)83(135)108-60(91(143)144)27-31-68(94)117)111-87(139)66-22-13-37-113(66)89(141)59(30-34-76(127)128)107-82(134)62(41-53-23-25-54(115)26-24-53)110-84(136)63(42-77(129)130)103-72(121)46-100-70(119)44-98-69(118)43-99-71(120)45-101-73(122)48-145-47-67(116)56(19-11-35-97-92(95)96)104-85(137)64-20-12-36-112(64)88(140)55(93)39-51-15-7-5-8-16-51/h23-26,49-52,55-66,78,115H,4-22,27-48,93H2,1-3H3,(H2,94,117)(H,98,118)(H,99,120)(H,100,119)(H,101,122)(H,102,132)(H,103,121)(H,104,137)(H,105,133)(H,106,138)(H,107,134)(H,108,135)(H,109,131)(H,110,136)(H,111,139)(H,123,124)(H,125,126)(H,127,128)(H,129,130)(H,143,144)(H4,95,96,97)/t49-,50+,55-,56+,57+,58+,59+,60-,61+,62+,63+,64+,65+,66+,78+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079489
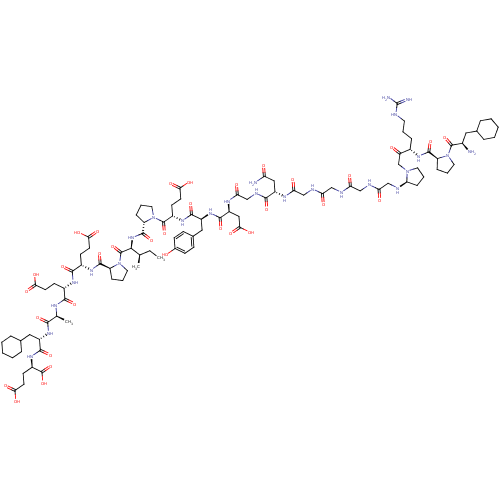
(Arginyl Ketomethylene analogue | CHEMBL428116)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CN[C@@H]1CCCN1CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C100H153N25O33/c1-4-53(2)85(98(156)125-41-14-22-70(125)94(152)116-62(30-34-81(136)137)89(147)115-61(29-33-80(134)135)88(146)111-54(3)86(144)119-65(43-56-18-9-6-10-19-56)91(149)118-64(99(157)158)32-36-83(140)141)121-95(153)71-23-13-40-124(71)97(155)63(31-35-82(138)139)117-90(148)66(44-57-25-27-58(126)28-26-57)120-92(150)68(46-84(142)143)113-79(133)51-110-87(145)67(45-73(102)128)112-78(132)50-109-77(131)49-108-76(130)48-107-75(129)47-106-74-24-15-38-122(74)52-72(127)60(20-11-37-105-100(103)104)114-93(151)69-21-12-39-123(69)96(154)59(101)42-55-16-7-5-8-17-55/h25-28,53-56,59-71,74,85,106,126H,4-24,29-52,101H2,1-3H3,(H2,102,128)(H,107,129)(H,108,130)(H,109,131)(H,110,145)(H,111,146)(H,112,132)(H,113,133)(H,114,151)(H,115,147)(H,116,152)(H,117,148)(H,118,149)(H,119,144)(H,120,150)(H,121,153)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H,157,158)(H4,103,104,105)/t53-,54+,59-,60+,61+,62+,63+,64-,65+,66+,67+,68+,69+,70+,71+,74+,85+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079476
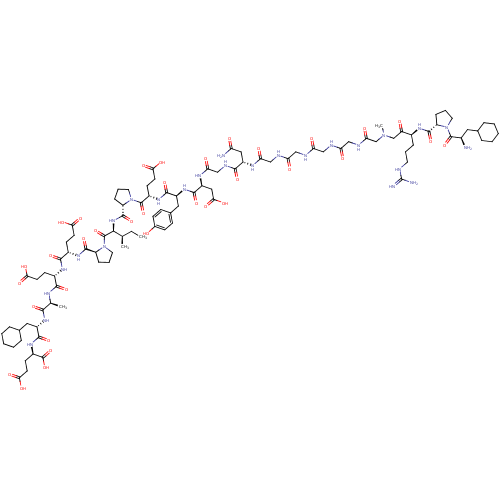
(Arginyl Ketomethylene analogue | CHEMBL437873)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(C)CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C99H151N25O34/c1-5-52(2)84(97(156)124-39-15-22-69(124)93(152)115-61(29-33-80(136)137)88(147)114-60(28-32-79(134)135)87(146)110-53(3)85(144)118-64(41-55-18-10-7-11-19-55)90(149)117-63(98(157)158)31-35-82(140)141)120-94(153)70-23-14-38-123(70)96(155)62(30-34-81(138)139)116-89(148)65(42-56-24-26-57(125)27-25-56)119-91(150)67(44-83(142)143)112-77(132)49-109-86(145)66(43-72(101)127)111-76(131)48-107-74(129)46-105-73(128)45-106-75(130)47-108-78(133)51-121(4)50-71(126)59(20-12-36-104-99(102)103)113-92(151)68-21-13-37-122(68)95(154)58(100)40-54-16-8-6-9-17-54/h24-27,52-55,58-70,84,125H,5-23,28-51,100H2,1-4H3,(H2,101,127)(H,105,128)(H,106,130)(H,107,129)(H,108,133)(H,109,145)(H,110,146)(H,111,131)(H,112,132)(H,113,151)(H,114,147)(H,115,152)(H,116,148)(H,117,149)(H,118,144)(H,119,150)(H,120,153)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H,157,158)(H4,102,103,104)/t52-,53+,58-,59+,60+,61+,62+,63-,64+,65+,66+,67+,68+,69+,70+,84+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079479
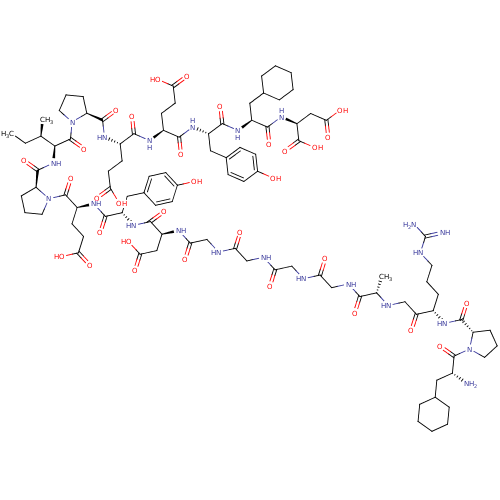
(Arginyl Ketomethylene analogue | CHEMBL407043)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H](C)NCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C98H144N22O32/c1-4-52(2)83(96(150)120-40-14-21-71(120)92(146)111-63(32-35-79(130)131)85(139)110-62(31-34-78(128)129)86(140)113-67(44-57-25-29-59(122)30-26-57)88(142)114-65(42-55-17-9-6-10-18-55)89(143)116-69(97(151)152)46-82(136)137)117-93(147)72-22-13-39-119(72)95(149)64(33-36-80(132)133)112-87(141)66(43-56-23-27-58(121)28-24-56)115-90(144)68(45-81(134)135)108-77(127)51-106-75(125)49-104-74(124)48-105-76(126)50-107-84(138)53(3)103-47-73(123)61(19-11-37-102-98(100)101)109-91(145)70-20-12-38-118(70)94(148)60(99)41-54-15-7-5-8-16-54/h23-30,52-55,60-72,83,103,121-122H,4-22,31-51,99H2,1-3H3,(H,104,124)(H,105,126)(H,106,125)(H,107,138)(H,108,127)(H,109,145)(H,110,139)(H,111,146)(H,112,141)(H,113,140)(H,114,142)(H,115,144)(H,116,143)(H,117,147)(H,128,129)(H,130,131)(H,132,133)(H,134,135)(H,136,137)(H,151,152)(H4,100,101,102)/t52-,53+,60-,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,83+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079485
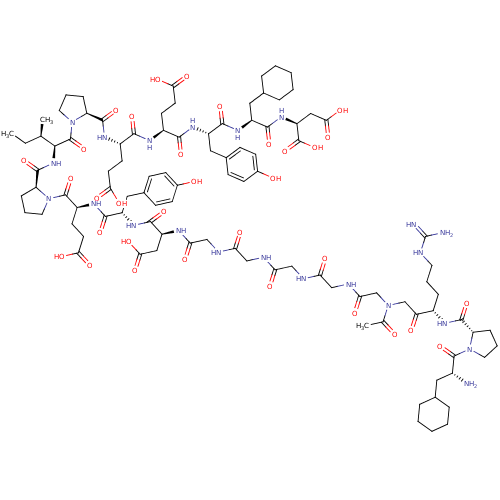
(Arginyl Ketomethylene analogue | CHEMBL414489)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C99H144N22O33/c1-4-53(2)85(97(152)121-40-14-21-72(121)93(148)111-64(32-35-81(133)134)86(141)110-63(31-34-80(131)132)87(142)113-68(44-58-25-29-60(124)30-26-58)89(144)114-66(42-56-17-9-6-10-18-56)90(145)116-70(98(153)154)46-84(139)140)117-94(149)73-22-13-39-120(73)96(151)65(33-36-82(135)136)112-88(143)67(43-57-23-27-59(123)28-24-57)115-91(146)69(45-83(137)138)108-78(129)50-106-76(127)48-104-75(126)47-105-77(128)49-107-79(130)52-118(54(3)122)51-74(125)62(19-11-37-103-99(101)102)109-92(147)71-20-12-38-119(71)95(150)61(100)41-55-15-7-5-8-16-55/h23-30,53,55-56,61-73,85,123-124H,4-22,31-52,100H2,1-3H3,(H,104,126)(H,105,128)(H,106,127)(H,107,130)(H,108,129)(H,109,147)(H,110,141)(H,111,148)(H,112,143)(H,113,142)(H,114,144)(H,115,146)(H,116,145)(H,117,149)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,153,154)(H4,101,102,103)/t53-,61-,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,85+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079478
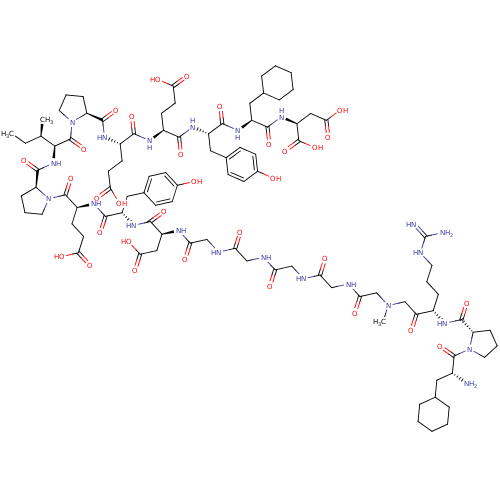
(Arginyl Ketomethylene analogue | CHEMBL414760)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(C)CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C98H144N22O32/c1-4-53(2)84(96(150)120-40-14-21-71(120)92(146)110-63(32-35-80(131)132)85(139)109-62(31-34-79(129)130)86(140)112-67(44-57-25-29-59(122)30-26-57)88(142)113-65(42-55-17-9-6-10-18-55)89(143)115-69(97(151)152)46-83(137)138)116-93(147)72-22-13-39-119(72)95(149)64(33-36-81(133)134)111-87(141)66(43-56-23-27-58(121)28-24-56)114-90(144)68(45-82(135)136)107-77(127)50-105-75(125)48-103-74(124)47-104-76(126)49-106-78(128)52-117(3)51-73(123)61(19-11-37-102-98(100)101)108-91(145)70-20-12-38-118(70)94(148)60(99)41-54-15-7-5-8-16-54/h23-30,53-55,60-72,84,121-122H,4-22,31-52,99H2,1-3H3,(H,103,124)(H,104,126)(H,105,125)(H,106,128)(H,107,127)(H,108,145)(H,109,139)(H,110,146)(H,111,141)(H,112,140)(H,113,142)(H,114,144)(H,115,143)(H,116,147)(H,129,130)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,151,152)(H4,100,101,102)/t53-,60-,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,84+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079491
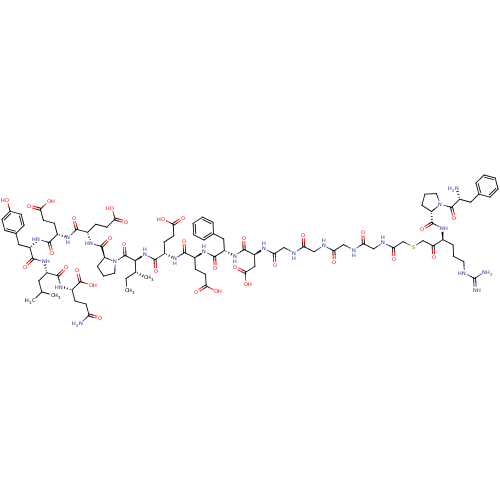
(Arginyl Ketomethylene analogue | CHEMBL412457)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CSCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C95H134N22O32S/c1-5-51(4)81(93(147)117-38-14-21-68(117)91(145)110-60(29-34-78(130)131)83(137)107-59(28-33-77(128)129)84(138)113-65(42-54-22-24-55(118)25-23-54)88(142)112-63(39-50(2)3)86(140)111-62(94(148)149)26-31-70(97)120)115-85(139)61(30-35-79(132)133)108-82(136)58(27-32-76(126)127)109-87(141)64(41-53-17-10-7-11-18-53)114-89(143)66(43-80(134)135)105-74(124)47-103-72(122)45-101-71(121)44-102-73(123)46-104-75(125)49-150-48-69(119)57(19-12-36-100-95(98)99)106-90(144)67-20-13-37-116(67)92(146)56(96)40-52-15-8-6-9-16-52/h6-11,15-18,22-25,50-51,56-68,81,118H,5,12-14,19-21,26-49,96H2,1-4H3,(H2,97,120)(H,101,121)(H,102,123)(H,103,122)(H,104,125)(H,105,124)(H,106,144)(H,107,137)(H,108,136)(H,109,141)(H,110,145)(H,111,140)(H,112,142)(H,113,138)(H,114,143)(H,115,139)(H,126,127)(H,128,129)(H,130,131)(H,132,133)(H,134,135)(H,148,149)(H4,98,99,100)/t51-,56-,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,81+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142111
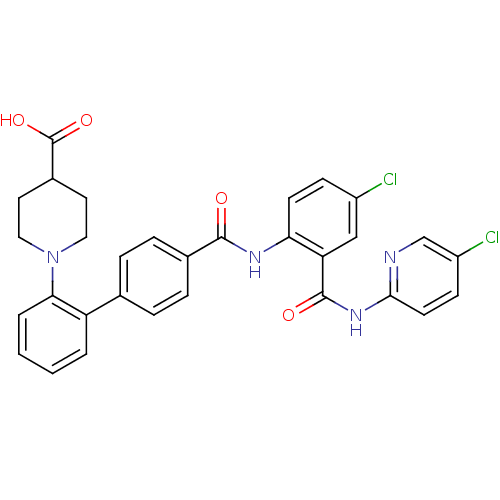
(1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H26Cl2N4O4/c32-22-9-11-26(25(17-22)30(39)36-28-12-10-23(33)18-34-28)35-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)37-15-13-21(14-16-37)31(40)41/h1-12,17-18,21H,13-16H2,(H,35,38)(H,40,41)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124984
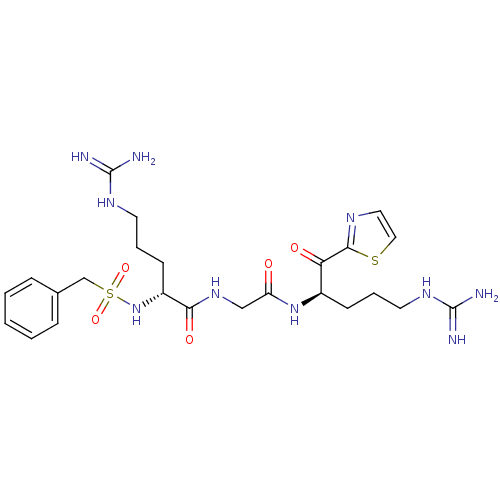
((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C24H36N10O5S2/c25-23(26)30-10-4-8-17(20(36)22-29-12-13-40-22)33-19(35)14-32-21(37)18(9-5-11-31-24(27)28)34-41(38,39)15-16-6-2-1-3-7-16/h1-3,6-7,12-13,17-18,34H,4-5,8-11,14-15H2,(H,32,37)(H,33,35)(H4,25,26,30)(H4,27,28,31)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Tested for binding affinity against human Coagulation factor Xa (trypsin-like serine protease) |
Bioorg Med Chem Lett 12: 1651-5 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RFZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079480
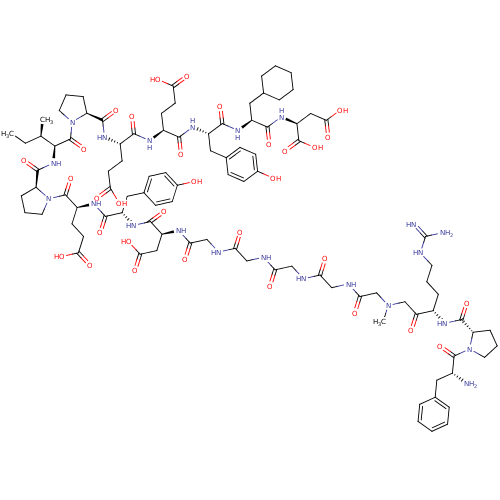
(Arginyl Ketomethylene analogue | CHEMBL415375)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(C)CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C98H138N22O32/c1-4-53(2)84(96(150)120-40-14-21-71(120)92(146)110-63(32-35-80(131)132)85(139)109-62(31-34-79(129)130)86(140)112-67(44-57-25-29-59(122)30-26-57)88(142)113-65(42-55-17-9-6-10-18-55)89(143)115-69(97(151)152)46-83(137)138)116-93(147)72-22-13-39-119(72)95(149)64(33-36-81(133)134)111-87(141)66(43-56-23-27-58(121)28-24-56)114-90(144)68(45-82(135)136)107-77(127)50-105-75(125)48-103-74(124)47-104-76(126)49-106-78(128)52-117(3)51-73(123)61(19-11-37-102-98(100)101)108-91(145)70-20-12-38-118(70)94(148)60(99)41-54-15-7-5-8-16-54/h5,7-8,15-16,23-30,53,55,60-72,84,121-122H,4,6,9-14,17-22,31-52,99H2,1-3H3,(H,103,124)(H,104,126)(H,105,125)(H,106,128)(H,107,127)(H,108,145)(H,109,139)(H,110,146)(H,111,141)(H,112,140)(H,113,142)(H,114,144)(H,115,143)(H,116,147)(H,129,130)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,151,152)(H4,100,101,102)/t53-,60-,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,84+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
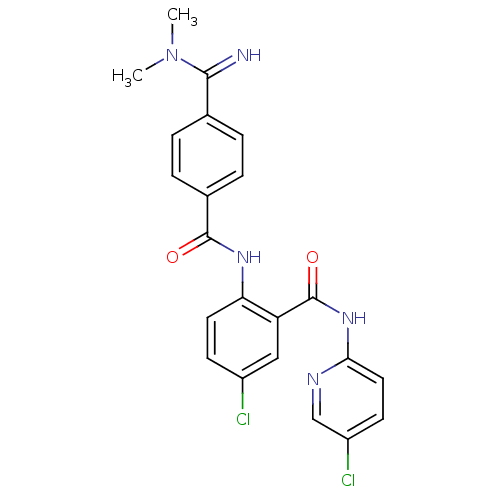
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
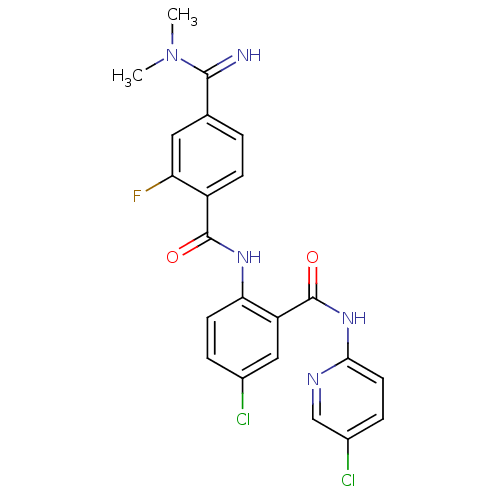
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50079490
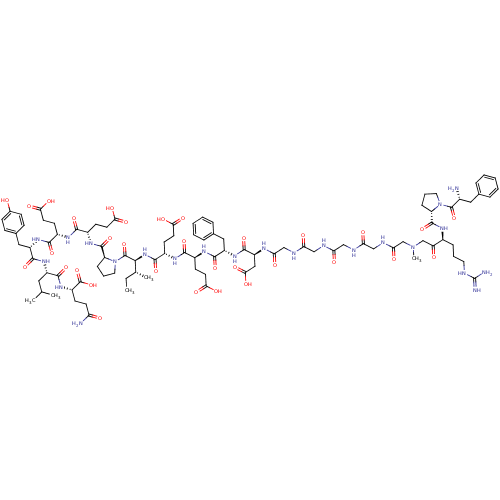
(Arginyl Ketomethylene analogue | CHEMBL437999)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(C)CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C96H137N23O32/c1-6-52(4)82(94(149)119-39-15-22-69(119)92(147)111-61(30-35-79(132)133)84(139)108-60(29-34-78(130)131)85(140)114-66(43-55-23-25-56(120)26-24-55)89(144)113-64(40-51(2)3)87(142)112-63(95(150)151)27-32-71(98)122)116-86(141)62(31-36-80(134)135)109-83(138)59(28-33-77(128)129)110-88(143)65(42-54-18-11-8-12-19-54)115-90(145)67(44-81(136)137)106-75(126)48-104-73(124)46-102-72(123)45-103-74(125)47-105-76(127)50-117(5)49-70(121)58(20-13-37-101-96(99)100)107-91(146)68-21-14-38-118(68)93(148)57(97)41-53-16-9-7-10-17-53/h7-12,16-19,23-26,51-52,57-69,82,120H,6,13-15,20-22,27-50,97H2,1-5H3,(H2,98,122)(H,102,123)(H,103,125)(H,104,124)(H,105,127)(H,106,126)(H,107,146)(H,108,139)(H,109,138)(H,110,143)(H,111,147)(H,112,142)(H,113,144)(H,114,140)(H,115,145)(H,116,141)(H,128,129)(H,130,131)(H,132,133)(H,134,135)(H,136,137)(H,150,151)(H4,99,100,101)/t52-,57-,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,82+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079488
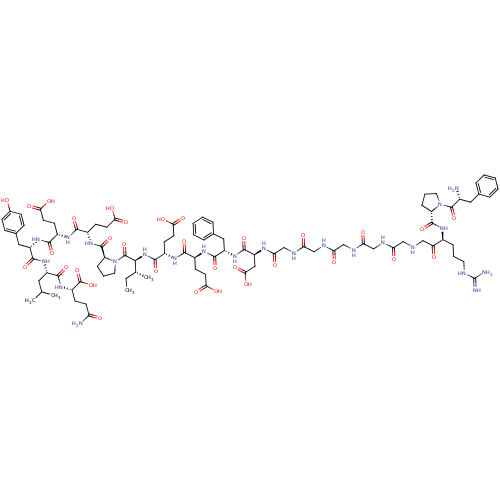
(Arginyl Ketomethylene analogue | CHEMBL414974)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CNCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C95H135N23O32/c1-5-51(4)81(93(148)118-38-14-21-68(118)91(146)111-60(29-34-78(131)132)83(138)108-59(28-33-77(129)130)84(139)114-65(42-54-22-24-55(119)25-23-54)88(143)113-63(39-50(2)3)86(141)112-62(94(149)150)26-31-70(97)121)116-85(140)61(30-35-79(133)134)109-82(137)58(27-32-76(127)128)110-87(142)64(41-53-17-10-7-11-18-53)115-89(144)66(43-80(135)136)106-75(126)49-105-74(125)48-104-73(124)47-103-72(123)46-102-71(122)45-100-44-69(120)57(19-12-36-101-95(98)99)107-90(145)67-20-13-37-117(67)92(147)56(96)40-52-15-8-6-9-16-52/h6-11,15-18,22-25,50-51,56-68,81,100,119H,5,12-14,19-21,26-49,96H2,1-4H3,(H2,97,121)(H,102,122)(H,103,123)(H,104,124)(H,105,125)(H,106,126)(H,107,145)(H,108,138)(H,109,137)(H,110,142)(H,111,146)(H,112,141)(H,113,143)(H,114,139)(H,115,144)(H,116,140)(H,127,128)(H,129,130)(H,131,132)(H,133,134)(H,135,136)(H,149,150)(H4,98,99,101)/t51-,56-,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,81+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142090
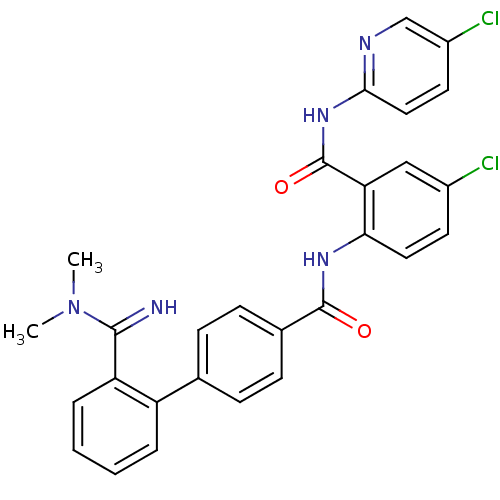
(2'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)22-6-4-3-5-21(22)17-7-9-18(10-8-17)27(36)33-24-13-11-19(29)15-23(24)28(37)34-25-14-12-20(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142125
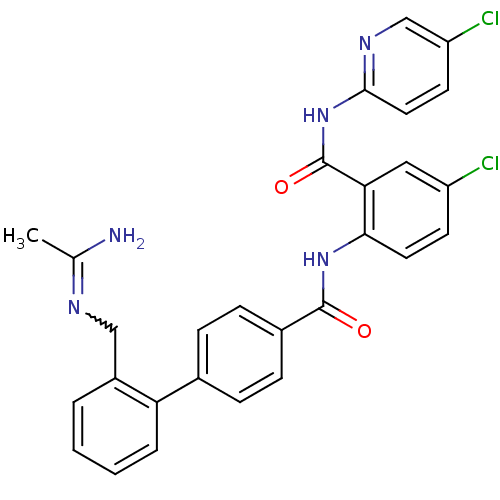
(2'-(Acetimidoylamino-methyl)-biphenyl-4-carboxylic...)Show SMILES CC(N)=NCc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C28H23Cl2N5O2/c1-17(31)32-15-20-4-2-3-5-23(20)18-6-8-19(9-7-18)27(36)34-25-12-10-21(29)14-24(25)28(37)35-26-13-11-22(30)16-33-26/h2-14,16H,15H2,1H3,(H2,31,32)(H,34,36)(H,33,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (828-1132) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM116315

(US8637526, 248)Show SMILES Nc1ccn2ncc(C(=O)Nc3c[nH]nc3-c3cc(Cl)ccc3Cl)c2n1 Show InChI InChI=1S/C16H11Cl2N7O/c17-8-1-2-11(18)9(5-8)14-12(7-20-24-14)22-16(26)10-6-21-25-4-3-13(19)23-15(10)25/h1-7H,(H2,19,23)(H,20,24)(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc.
US Patent
| Assay Description
To determine the inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 kinase reactions containing 0.2 nM purified JAK2 ... |
US Patent US8637526 (2014)
BindingDB Entry DOI: 10.7270/Q25719Q3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142139
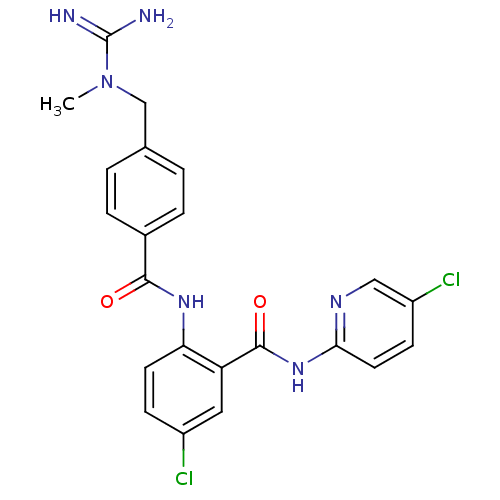
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-{4-[(N-methyl...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(N)=N Show InChI InChI=1S/C22H20Cl2N6O2/c1-30(22(25)26)12-13-2-4-14(5-3-13)20(31)28-18-8-6-15(23)10-17(18)21(32)29-19-9-7-16(24)11-27-19/h2-11H,12H2,1H3,(H3,25,26)(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142112
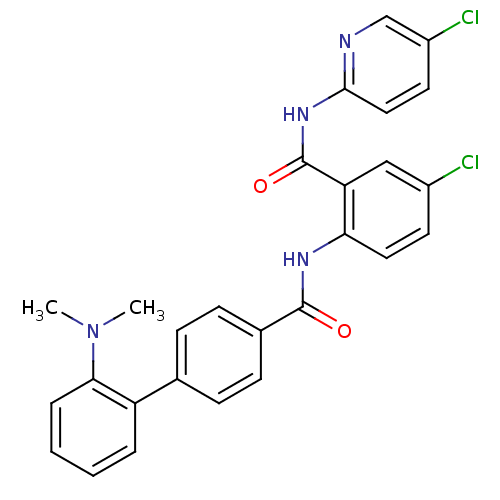
(2'-Dimethylamino-biphenyl-4-carboxylic acid [4-chl...)Show SMILES CN(C)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N4O2/c1-33(2)24-6-4-3-5-21(24)17-7-9-18(10-8-17)26(34)31-23-13-11-19(28)15-22(23)27(35)32-25-14-12-20(29)16-30-25/h3-16H,1-2H3,(H,31,34)(H,30,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
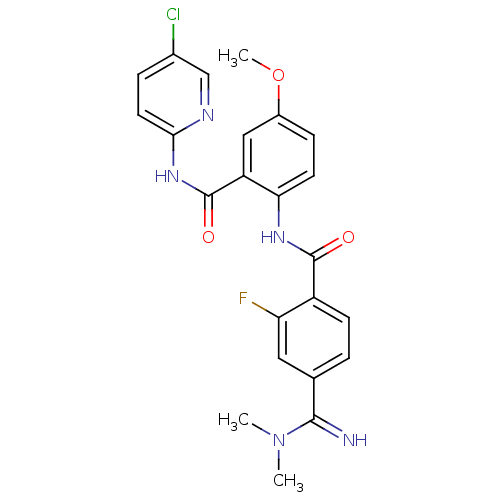
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
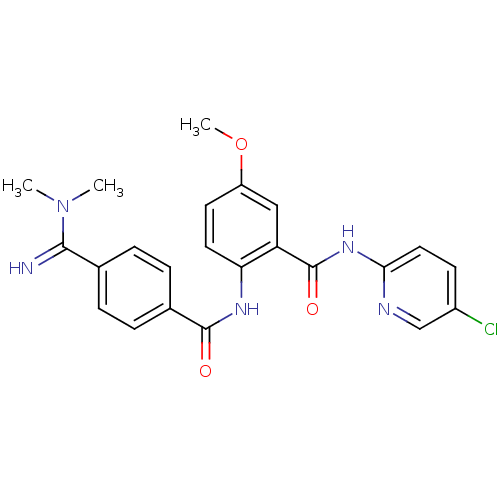
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124975
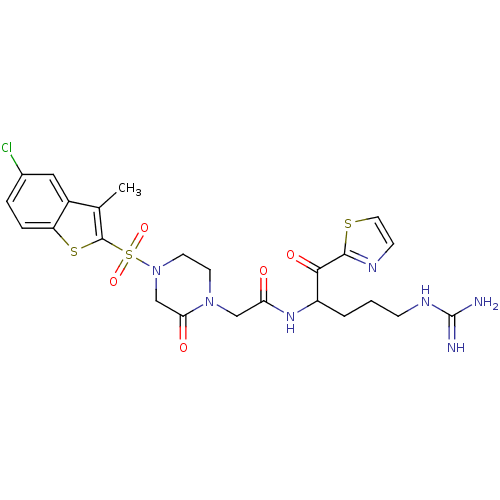
(2-[4-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfon...)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)N1CCN(CC(=O)NC(CCCNC(N)=N)C(=O)c2nccs2)C(=O)C1 Show InChI InChI=1S/C24H28ClN7O5S3/c1-14-16-11-15(25)4-5-18(16)39-23(14)40(36,37)32-9-8-31(20(34)13-32)12-19(33)30-17(3-2-6-29-24(26)27)21(35)22-28-7-10-38-22/h4-5,7,10-11,17H,2-3,6,8-9,12-13H2,1H3,(H,30,33)(H4,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113598
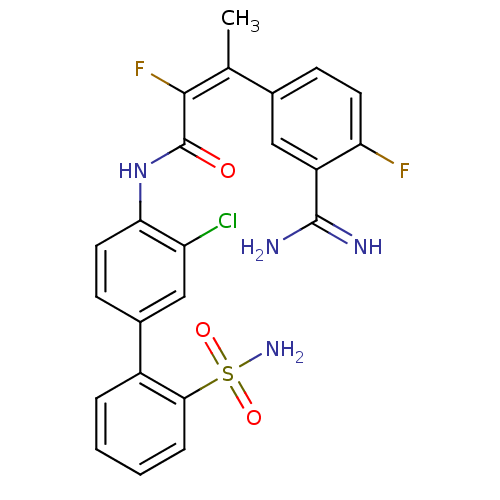
((Z)-3-(3-Carbamimidoyl-4-fluoro-phenyl)-2-fluoro-b...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Cl)-c1ccccc1S(N)(=O)=O)c1ccc(F)c(c1)C(N)=N Show InChI InChI=1S/C23H19ClF2N4O3S/c1-12(13-6-8-18(25)16(10-13)22(27)28)21(26)23(31)30-19-9-7-14(11-17(19)24)15-4-2-3-5-20(15)34(29,32)33/h2-11H,1H3,(H3,27,28)(H,30,31)(H2,29,32,33)/b21-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against factor Xa,activity expressed as Ki nM |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142129
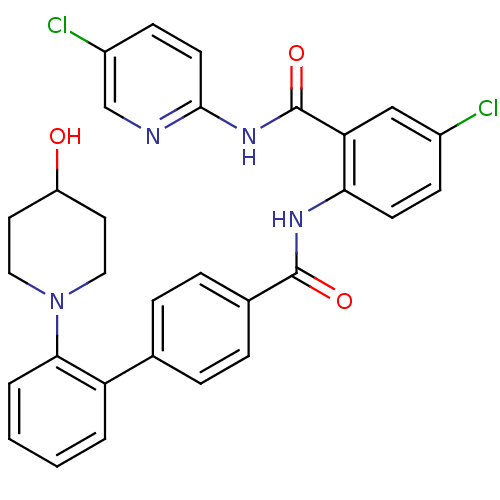
(2'-(4-Hydroxy-piperidin-1-yl)-biphenyl-4-carboxyli...)Show SMILES OC1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C30H26Cl2N4O3/c31-21-9-11-26(25(17-21)30(39)35-28-12-10-22(32)18-33-28)34-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)36-15-13-23(37)14-16-36/h1-12,17-18,23,37H,13-16H2,(H,34,38)(H,33,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 (837-1142) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142118
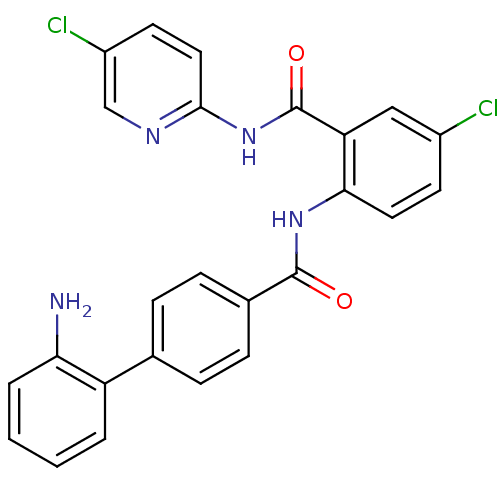
(2'-Amino-biphenyl-4-carboxylic acid [4-chloro-2-(5...)Show SMILES Nc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H18Cl2N4O2/c26-17-9-11-22(20(13-17)25(33)31-23-12-10-18(27)14-29-23)30-24(32)16-7-5-15(6-8-16)19-3-1-2-4-21(19)28/h1-14H,28H2,(H,30,32)(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142169
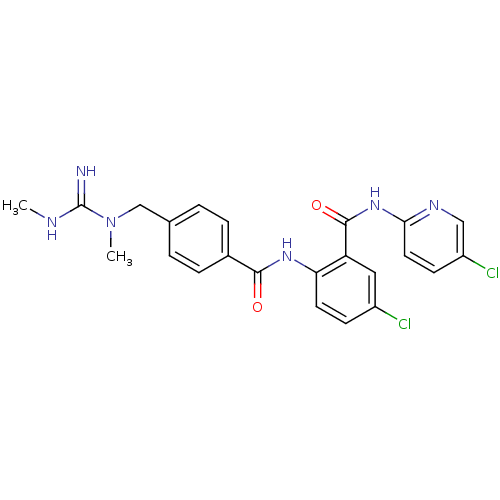
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N'-dime...)Show SMILES CNC(=N)N(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22Cl2N6O2/c1-27-23(26)31(2)13-14-3-5-15(6-4-14)21(32)29-19-9-7-16(24)11-18(19)22(33)30-20-10-8-17(25)12-28-20/h3-12H,13H2,1-2H3,(H2,26,27)(H,29,32)(H,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142161
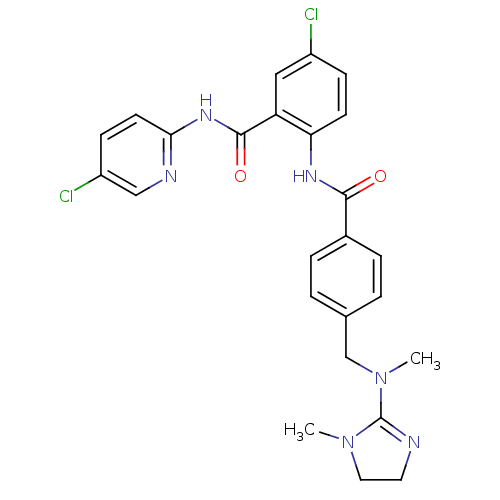
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-(4-{[methyl-(...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C1=NCCN1C |t:32| Show InChI InChI=1S/C25H24Cl2N6O2/c1-32-12-11-28-25(32)33(2)15-16-3-5-17(6-4-16)23(34)30-21-9-7-18(26)13-20(21)24(35)31-22-10-8-19(27)14-29-22/h3-10,13-14H,11-12,15H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142142

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N',N'-dim...)Show SMILES CN(C)NCc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H21Cl2N5O2/c1-29(2)26-12-14-3-5-15(6-4-14)21(30)27-19-9-7-16(23)11-18(19)22(31)28-20-10-8-17(24)13-25-20/h3-11,13,26H,12H2,1-2H3,(H,27,30)(H,25,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50399016

(CHEMBL2178803 | US8637526, 225)Show SMILES Cc1ccc(C)c(c1)-c1nn(C)cc1NC(=O)c1cnn2ccc(N)nc12 Show InChI InChI=1S/C19H19N7O/c1-11-4-5-12(2)13(8-11)17-15(10-25(3)24-17)22-19(27)14-9-21-26-7-6-16(20)23-18(14)26/h4-10H,1-3H3,(H2,20,23)(H,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc.
US Patent
| Assay Description
To determine the inhibition constants (Ki), compounds were diluted serially in DMSO and added to 50 kinase reactions containing 0.2 nM purified JAK2 ... |
US Patent US8637526 (2014)
BindingDB Entry DOI: 10.7270/Q25719Q3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140394
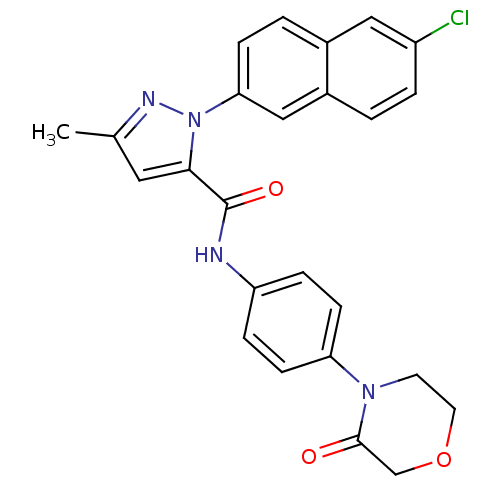
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)N2CCOCC2=O)n(n1)-c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H21ClN4O3/c1-16-12-23(30(28-16)22-7-3-17-13-19(26)4-2-18(17)14-22)25(32)27-20-5-8-21(9-6-20)29-10-11-33-15-24(29)31/h2-9,12-14H,10-11,15H2,1H3,(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142126
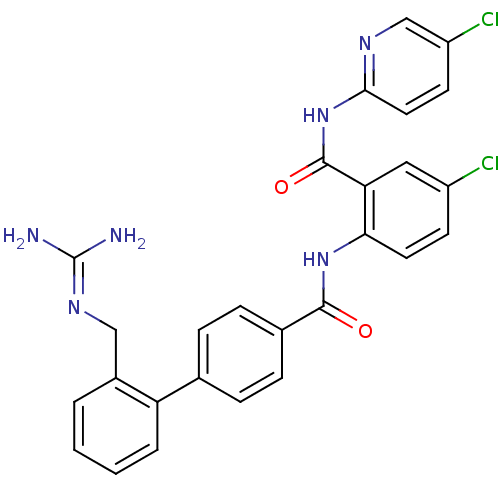
(2'-Guanidinomethyl-biphenyl-4-carboxylic acid [4-c...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1ccccc1-c1ccc(cc1)-[#6](=O)-[#7]-c1ccc(Cl)cc1-[#6](=O)-[#7]-c1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N6O2/c28-19-9-11-23(22(13-19)26(37)35-24-12-10-20(29)15-32-24)34-25(36)17-7-5-16(6-8-17)21-4-2-1-3-18(21)14-33-27(30)31/h1-13,15H,14H2,(H,34,36)(H4,30,31,33)(H,32,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM99582
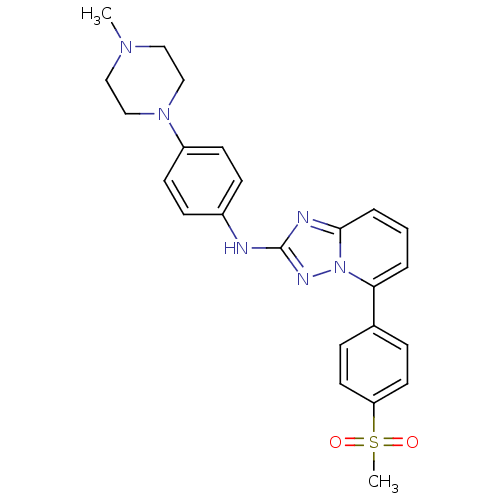
(US8501936, 81)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc3cccc(-c4ccc(cc4)S(C)(=O)=O)n3n2)cc1 Show InChI InChI=1S/C24H26N6O2S/c1-28-14-16-29(17-15-28)20-10-8-19(9-11-20)25-24-26-23-5-3-4-22(30(23)27-24)18-6-12-21(13-7-18)33(2,31)32/h3-13H,14-17H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 5014-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.008
BindingDB Entry DOI: 10.7270/Q29S1SF2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
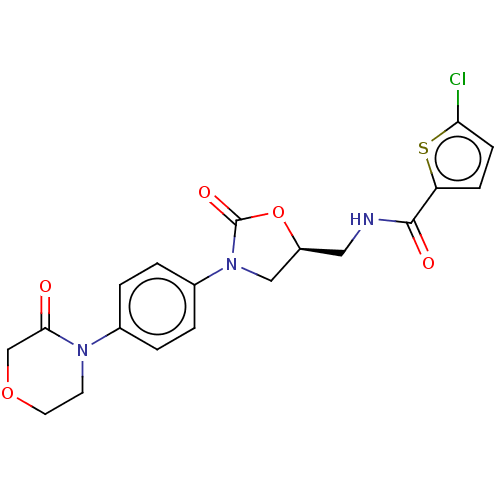
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused JAK3 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 3... |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363990
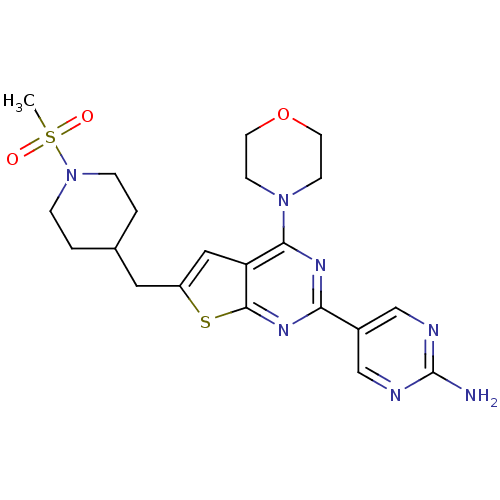
(CHEMBL1949915)Show SMILES CS(=O)(=O)N1CCC(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-4-2-14(3-5-28)10-16-11-17-19(27-6-8-31-9-7-27)25-18(26-20(17)32-16)15-12-23-21(22)24-13-15/h11-14H,2-10H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM153667
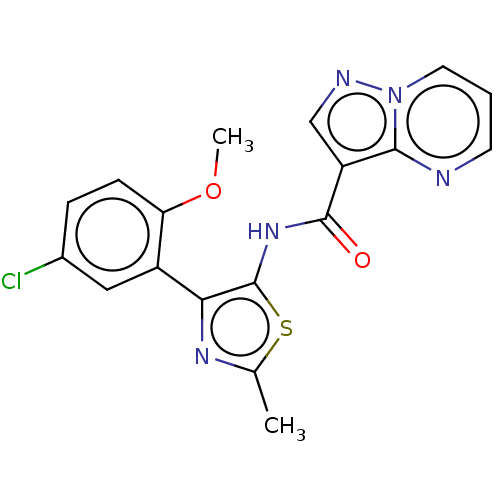
(US8999998, 170)Show SMILES COc1ccc(Cl)cc1-c1nc(C)sc1NC(=O)c1cnn2cccnc12 Show InChI InChI=1S/C18H14ClN5O2S/c1-10-22-15(12-8-11(19)4-5-14(12)26-2)18(27-10)23-17(25)13-9-21-24-7-3-6-20-16(13)24/h3-9H,1-2H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Genentech, Inc.
US Patent
| Assay Description
The activity of the isolated JAK1, JAK2 or TYK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-Ala-Leu-... |
US Patent US8999998 (2015)
BindingDB Entry DOI: 10.7270/Q2J67FN6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50438673
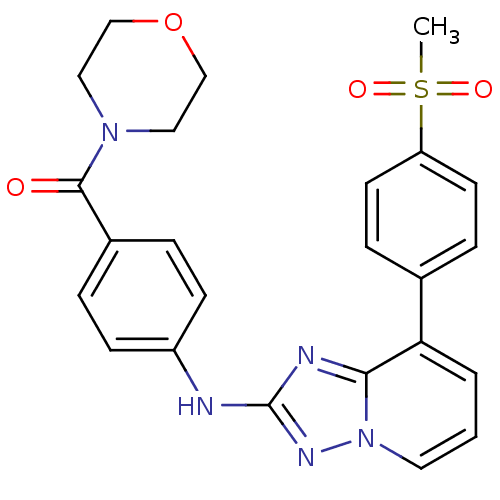
(CHEMBL2414535)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cccn2nc(Nc3ccc(cc3)C(=O)N3CCOCC3)nc12 Show InChI InChI=1S/C24H23N5O4S/c1-34(31,32)20-10-6-17(7-11-20)21-3-2-12-29-22(21)26-24(27-29)25-19-8-4-18(5-9-19)23(30)28-13-15-33-16-14-28/h2-12H,13-16H2,1H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 5014-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.008
BindingDB Entry DOI: 10.7270/Q29S1SF2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580024

(CHEMBL5092858)Show SMILES FC(F)(F)c1ccc(cc1)-n1ncc2CCC\C(=C/CNC(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50438670
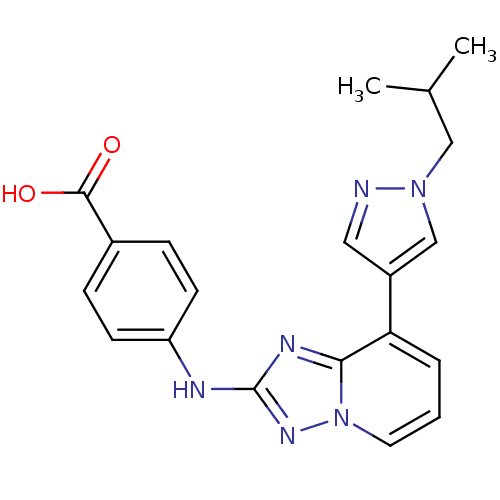
(CHEMBL2414538)Show SMILES CC(C)Cn1cc(cn1)-c1cccn2nc(Nc3ccc(cc3)C(O)=O)nc12 Show InChI InChI=1S/C20H20N6O2/c1-13(2)11-25-12-15(10-21-25)17-4-3-9-26-18(17)23-20(24-26)22-16-7-5-14(6-8-16)19(27)28/h3-10,12-13H,11H2,1-2H3,(H,22,24)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 5014-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.008
BindingDB Entry DOI: 10.7270/Q29S1SF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50438667

(CHEMBL2414541)Show SMILES OC(=O)c1ccc(Nc2nc3c(cccn3n2)-c2cnn(c2)C2CCCCC2)cc1 Show InChI InChI=1S/C22H22N6O2/c29-21(30)15-8-10-17(11-9-15)24-22-25-20-19(7-4-12-27(20)26-22)16-13-23-28(14-16)18-5-2-1-3-6-18/h4,7-14,18H,1-3,5-6H2,(H,24,26)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 5014-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.008
BindingDB Entry DOI: 10.7270/Q29S1SF2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50438666

(CHEMBL2414542)Show SMILES CC(C)n1cc(cn1)-c1cccn2nc(Nc3ccc(cc3)C(O)=O)nc12 Show InChI InChI=1S/C19H18N6O2/c1-12(2)25-11-14(10-20-25)16-4-3-9-24-17(16)22-19(23-24)21-15-7-5-13(6-8-15)18(26)27/h3-12H,1-2H3,(H,21,23)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 5014-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.008
BindingDB Entry DOI: 10.7270/Q29S1SF2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140388
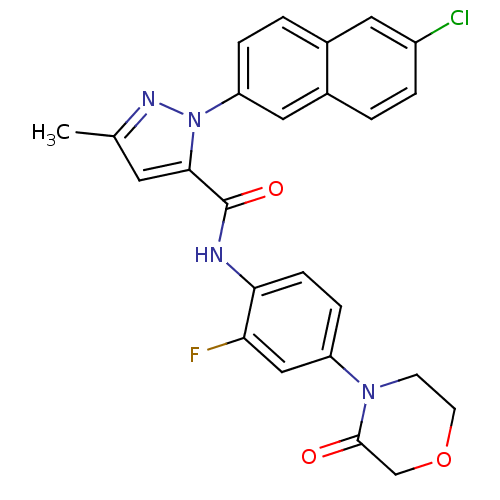
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)N2CCOCC2=O)n(n1)-c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C25H20ClFN4O3/c1-15-10-23(31(29-15)20-5-3-16-11-18(26)4-2-17(16)12-20)25(33)28-22-7-6-19(13-21(22)27)30-8-9-34-14-24(30)32/h2-7,10-13H,8-9,14H2,1H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140371
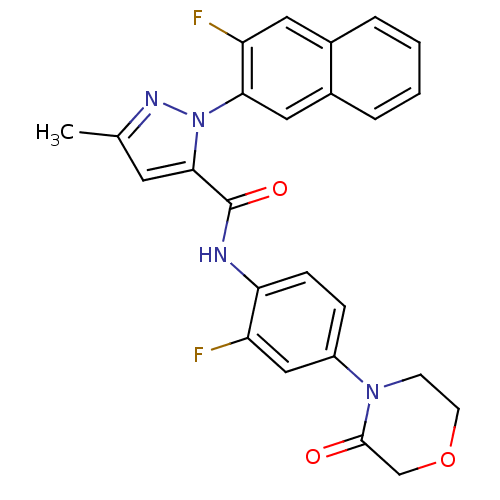
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)N2CCOCC2=O)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C25H20F2N4O3/c1-15-10-23(31(29-15)22-12-17-5-3-2-4-16(17)11-20(22)27)25(33)28-21-7-6-18(13-19(21)26)30-8-9-34-14-24(30)32/h2-7,10-13H,8-9,14H2,1H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Coagulation factor X |
Bioorg Med Chem Lett 14: 1221-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.053
BindingDB Entry DOI: 10.7270/Q2VD6XV5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142158

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(2-imino-i...)Show SMILES NC1=NCCN1Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |t:1| Show InChI InChI=1S/C23H20Cl2N6O2/c24-16-5-7-19(18(11-16)22(33)30-20-8-6-17(25)12-28-20)29-21(32)15-3-1-14(2-4-15)13-31-10-9-27-23(31)26/h1-8,11-12H,9-10,13H2,(H2,26,27)(H,29,32)(H,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50580013

(CHEMBL5086191)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)NC\C=C1/CCCc2cnn(Cc3ccc(Cl)cc3Cl)c12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor assessed as inhibition constant incubated for 2 hrs by TopCount scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00594
BindingDB Entry DOI: 10.7270/Q2HT2T6X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data