 Found 392 hits with Last Name = 'zimmerlin' and Initial = 'a'
Found 392 hits with Last Name = 'zimmerlin' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 4F2
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CYP4F2 in human liver microsomes assessed as fingolimod metabolism |
Drug Metab Dispos 39: 191-8 (2011)
Article DOI: 10.1124/dmd.110.035378
BindingDB Entry DOI: 10.7270/Q28917MJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546172

(CHEMBL4762397)Show SMILES CN(Cc1c[nH]c2ncnc(-c3cccc(NC(=O)c4ccc(cc4)C(C)(C)C)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546180
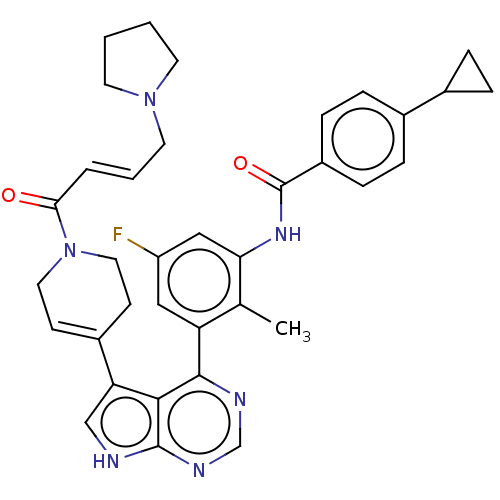
(CHEMBL4749522)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)\C=C\CN3CCCC3)c12 |t:31| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355393
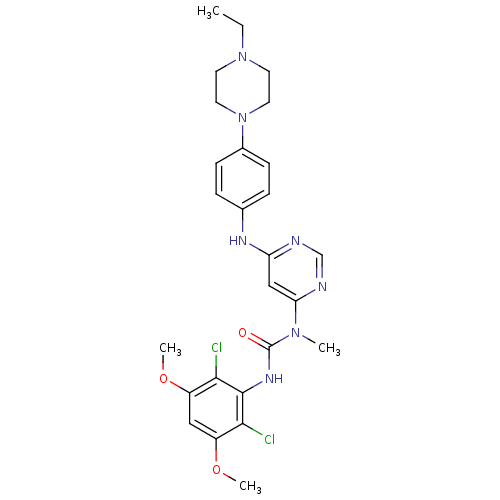
(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259415
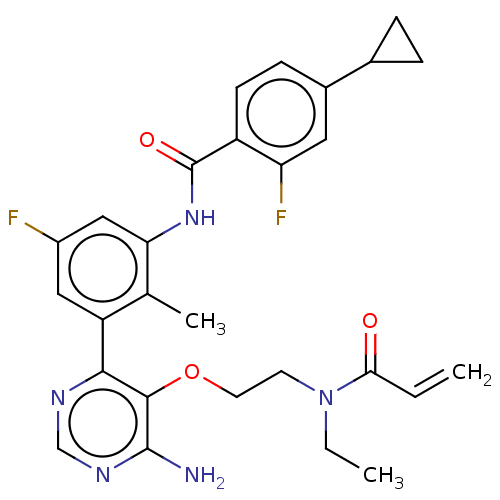
(US10457647, Example 14 | US11180460, Example 14 | ...)Show SMILES CCN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H29F2N5O3/c1-4-24(36)35(5-2)10-11-38-26-25(32-15-33-27(26)31)21-13-19(29)14-23(16(21)3)34-28(37)20-9-8-18(12-22(20)30)17-6-7-17/h4,8-9,12-15,17H,1,5-7,10-11H2,2-3H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR1 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50030448
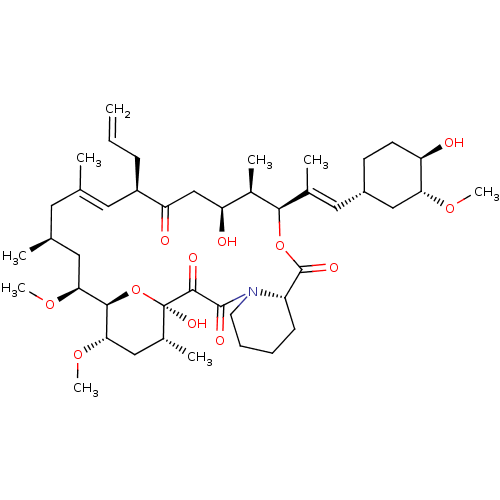
(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR3 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546179

(CHEMBL4739958)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(CC3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50357312
(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BMX (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546175

(CHEMBL4795673)Show SMILES CN(C(=O)Cc1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259407
(US10457647, Example 6 | US11180460, Example 6 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C27H27F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h4,7-8,11-14,16H,1,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546176

(CHEMBL4746262)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(c12)C1(O)CN(C1)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259423
(US10457647, Example 22 | US11180460, Example 22 | ...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C29H29F2N5O3/c1-3-25(37)36-10-4-5-20(36)14-39-27-26(33-15-34-28(27)32)22-12-19(30)13-24(16(22)2)35-29(38)21-9-8-18(11-23(21)31)17-6-7-17/h3,8-9,11-13,15,17,20H,1,4-7,10,14H2,2H3,(H,35,38)(H2,32,33,34)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FGFR2 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50153090

(CHEMBL411735 | Macrolide derivative)Show SMILES COC1C[C@H](CC(C)[C@H]2OC(=O)C3CCCCN3C(=O)C(=O)[C@]3(O)CC([C@H](C[C@H]3C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)CC[C@H]1CC(=O)Nc1ccc(CCCC(=O)OC)cc1 |t:40| Show InChI InChI=1S/C58H88N2O13/c1-11-15-42-27-35(2)26-36(3)28-50(70-8)45-34-58(68,38(5)30-51(45)71-9)55(65)56(66)60-25-13-12-17-46(60)57(67)73-54(39(6)47(61)33-48(42)62)37(4)29-41-19-22-43(49(31-41)69-7)32-52(63)59-44-23-20-40(21-24-44)16-14-18-53(64)72-10/h11,20-21,23-24,27,36-39,41-43,45-47,49-51,54,61,68H,1,12-19,22,25-26,28-34H2,2-10H3,(H,59,63)/b35-27+/t36-,37?,38+,39+,41-,42+,43-,45?,46?,47-,49?,50-,51-,54+,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259409
(US10457647, Example 8 | US11180460, Example 8 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C#C Show InChI InChI=1S/C27H25F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h1,7-8,11-14,16H,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514634

(CHEMBL4450082)Show SMILES CN(CC\C=C\c1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C29H30FN5O2/c1-4-26(36)35(3)14-6-5-7-23-27(32-17-33-28(23)31)24-15-22(30)16-25(18(24)2)34-29(37)21-12-10-20(11-13-21)19-8-9-19/h4-5,7,10-13,15-17,19H,1,6,8-9,14H2,2-3H3,(H,34,37)(H2,31,32,33)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50153091
(CHEMBL265123 | Macrolide derivative)Show SMILES COC1C[C@H](CC(C)[C@H]2OC(=O)C3CCCCN3C(=O)C(=O)[C@]3(O)CC([C@H](C[C@H]3C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)CC[C@H]1CC(=O)Nc1ccc(CCCC(O)=O)cc1 |t:40| Show InChI InChI=1S/C57H86N2O13/c1-10-14-41-26-34(2)25-35(3)27-49(70-8)44-33-57(68,37(5)29-50(44)71-9)54(65)55(66)59-24-12-11-16-45(59)56(67)72-53(38(6)46(60)32-47(41)61)36(4)28-40-18-21-42(48(30-40)69-7)31-51(62)58-43-22-19-39(20-23-43)15-13-17-52(63)64/h10,19-20,22-23,26,35-38,40-42,44-46,48-50,53,60,68H,1,11-18,21,24-25,27-33H2,2-9H3,(H,58,62)(H,63,64)/b34-26+/t35-,36?,37+,38+,40-,41+,42-,44?,45?,46-,48?,49-,50-,53+,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514645
(CHEMBL4546324)Show SMILES CN(C\C=C\c1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H27F2N5O2/c1-4-25(36)35(3)11-5-6-21-26(32-15-33-27(21)31)22-13-19(29)14-24(16(22)2)34-28(37)20-10-9-18(12-23(20)30)17-7-8-17/h4-6,9-10,12-15,17H,1,7-8,11H2,2-3H3,(H,34,37)(H2,31,32,33)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR2 juxtamembrane domain-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514637

(CHEMBL4463185)Show SMILES Cc1c(NC(=O)c2ccc(c(F)c2F)C(C)(C)O)cc(F)cc1-c1ncnc(N)c1OC1CN(C1)C(=O)C=C Show InChI InChI=1S/C27H26F3N5O4/c1-5-20(36)35-10-15(11-35)39-24-23(32-12-33-25(24)31)17-8-14(28)9-19(13(17)2)34-26(37)16-6-7-18(27(3,4)38)22(30)21(16)29/h5-9,12,15,38H,1,10-11H2,2-4H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259407

(US10457647, Example 6 | US11180460, Example 6 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C27H27F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h4,7-8,11-14,16H,1,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT... |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259434
(US10457647, Example 33 | US11180460, Example 33 | ...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC[C@@H]1CCN1C(=O)C=C |r| Show InChI InChI=1S/C28H27F2N5O3/c1-3-24(36)35-9-8-19(35)13-38-26-25(32-14-33-27(26)31)21-11-18(29)12-23(15(21)2)34-28(37)20-7-6-17(10-22(20)30)16-4-5-16/h3,6-7,10-12,14,16,19H,1,4-5,8-9,13H2,2H3,(H,34,37)(H2,31,32,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514644

(CHEMBL4463650)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc(N)c1OC1CN(C1)C(=O)C=C Show InChI InChI=1S/C27H26FN5O3/c1-3-23(34)33-12-20(13-33)36-25-24(30-14-31-26(25)29)21-10-19(28)11-22(15(21)2)32-27(35)18-8-6-17(7-9-18)16-4-5-16/h3,6-11,14,16,20H,1,4-5,12-13H2,2H3,(H,32,35)(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259422
(US10457647, Example 21 | US11180460, Example 21 | ...)Show SMILES CN(CCCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H29F2N5O3/c1-4-24(36)35(3)10-5-11-38-26-25(32-15-33-27(26)31)21-13-19(29)14-23(16(21)2)34-28(37)20-9-8-18(12-22(20)30)17-6-7-17/h4,8-9,12-15,17H,1,5-7,10-11H2,2-3H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259406
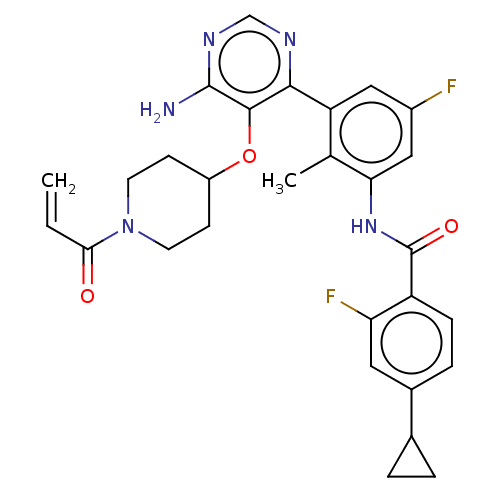
(US10457647, Example 5 | US11180460, Example 5 | US...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C29H29F2N5O3/c1-3-25(37)36-10-8-20(9-11-36)39-27-26(33-15-34-28(27)32)22-13-19(30)14-24(16(22)2)35-29(38)21-7-6-18(12-23(21)31)17-4-5-17/h3,6-7,12-15,17,20H,1,4-5,8-11H2,2H3,(H,35,38)(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514635
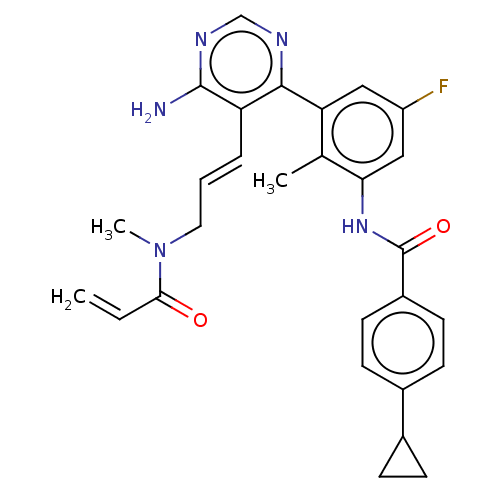
(CHEMBL4559123)Show SMILES CN(C\C=C\c1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H28FN5O2/c1-4-25(35)34(3)13-5-6-22-26(31-16-32-27(22)30)23-14-21(29)15-24(17(23)2)33-28(36)20-11-9-19(10-12-20)18-7-8-18/h4-6,9-12,14-16,18H,1,7-8,13H2,2-3H3,(H,33,36)(H2,30,31,32)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50116037

(3,5-Difluoro-4-[5-(4-fluoro-phenyl)-4-pyridin-4-yl...)Show SMILES Nc1nc(N)c(F)c(-c2cc(c([nH]2)-c2ccc(F)cc2)-c2ccncc2)c1F Show InChI InChI=1S/C20H14F3N5/c21-12-3-1-11(2-4-12)18-13(10-5-7-26-8-6-10)9-14(27-18)15-16(22)19(24)28-20(25)17(15)23/h1-9,27H,(H4,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vivo inhibition of murine Mitogen-activated protein kinase p38 alpha activity, GST-ATF-2 as substrate in the presence of 120 microM ATP |
Bioorg Med Chem Lett 12: 2109-12 (2002)
BindingDB Entry DOI: 10.7270/Q2610ZNX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50116031
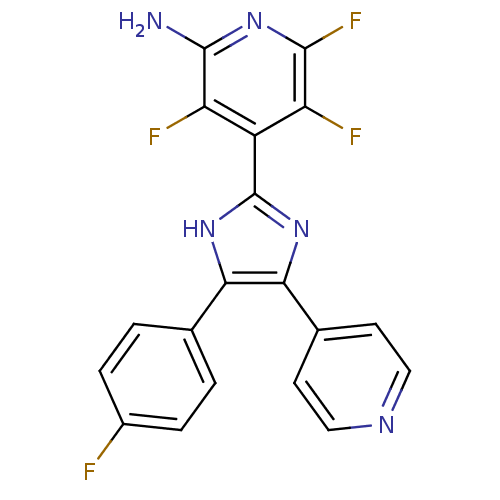
(3,5,6-Trifluoro-4-[4-(4-fluoro-phenyl)-5-pyridin-4...)Show SMILES Nc1nc(F)c(F)c(-c2nc(c([nH]2)-c2ccc(F)cc2)-c2ccncc2)c1F Show InChI InChI=1S/C19H11F4N5/c20-11-3-1-9(2-4-11)15-16(10-5-7-25-8-6-10)27-19(26-15)12-13(21)17(23)28-18(24)14(12)22/h1-8H,(H2,24,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vivo inhibition of murine Mitogen-activated protein kinase p38 alpha activity, GST-ATF-2 as substrate in the presence of 120 microM ATP |
Bioorg Med Chem Lett 12: 2109-12 (2002)
BindingDB Entry DOI: 10.7270/Q2610ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259402
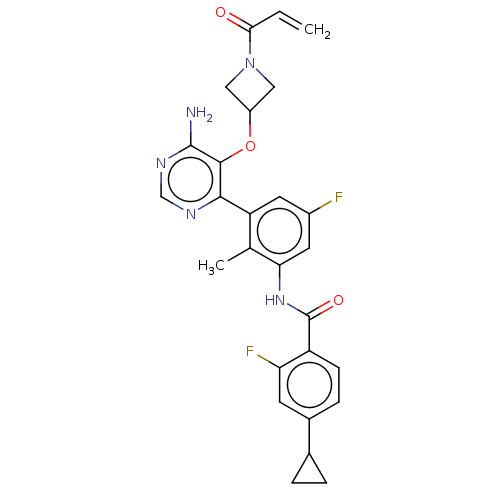
(US10457647, Example 1 | US11180460, Example 1 | US...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC1CN(C1)C(=O)C=C Show InChI InChI=1S/C27H25F2N5O3/c1-3-23(35)34-11-18(12-34)37-25-24(31-13-32-26(25)30)20-9-17(28)10-22(14(20)2)33-27(36)19-7-6-16(8-21(19)29)15-4-5-15/h3,6-10,13,15,18H,1,4-5,11-12H2,2H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type FGFR1 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa... |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of wild type FGFR2 expressed in HEK293 cells assessed as inhibition of autophosphorylation of tyrosine residue after 40 mins by ELISA assa... |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50116038
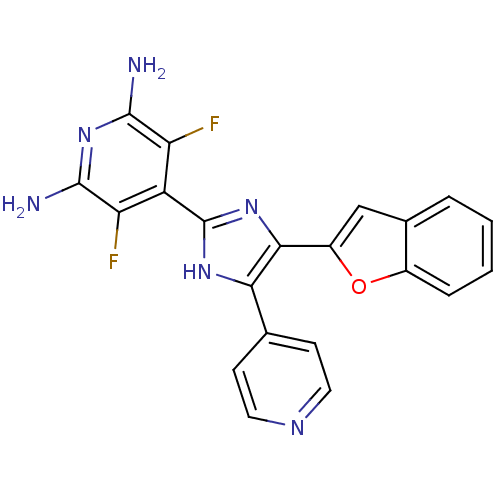
(4-(4-Benzofuran-2-yl-5-pyridin-4-yl-1H-imidazol-2-...)Show SMILES Nc1nc(N)c(F)c(-c2nc(-c3cc4ccccc4o3)c([nH]2)-c2ccncc2)c1F Show InChI InChI=1S/C21H14F2N6O/c22-15-14(16(23)20(25)29-19(15)24)21-27-17(10-5-7-26-8-6-10)18(28-21)13-9-11-3-1-2-4-12(11)30-13/h1-9H,(H,27,28)(H4,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vivo inhibition of murine Mitogen-activated protein kinase p38 alpha activity, GST-ATF-2 as substrate in the presence of 120 microM ATP |
Bioorg Med Chem Lett 12: 2109-12 (2002)
BindingDB Entry DOI: 10.7270/Q2610ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259434

(US10457647, Example 33 | US11180460, Example 33 | ...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC[C@@H]1CCN1C(=O)C=C |r| Show InChI InChI=1S/C28H27F2N5O3/c1-3-24(36)35-9-8-19(35)13-38-26-25(32-14-33-27(26)31)21-11-18(29)12-23(15(21)2)34-28(37)20-7-6-17(10-22(20)30)16-4-5-16/h3,6-7,10-12,14,16,19H,1,4-5,8-9,13H2,2H3,(H,34,37)(H2,31,32,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT... |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546177
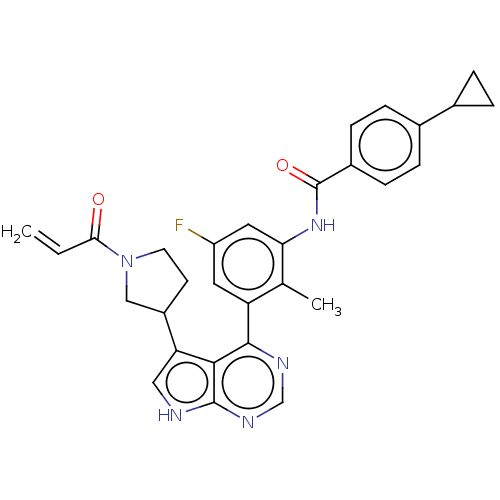
(CHEMBL4740933)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(C3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by im... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259415

(US10457647, Example 14 | US11180460, Example 14 | ...)Show SMILES CCN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H29F2N5O3/c1-4-24(36)35(5-2)10-11-38-26-25(32-15-33-27(26)31)21-13-19(29)14-23(16(21)3)34-28(37)20-9-8-18(12-22(20)30)17-6-7-17/h4,8-9,12-15,17H,1,5-7,10-11H2,2-3H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT... |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM194087
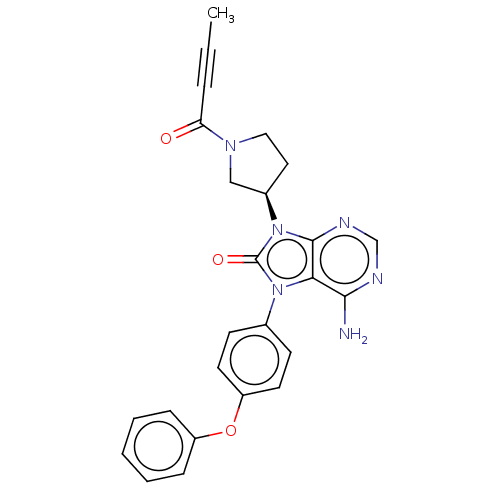
(US20230364079, Example Tirabrutinib | US9199997, 9...)Show SMILES CC#CC(=O)N1CC[C@H](C1)n1c2ncnc(N)c2n(-c2ccc(Oc3ccccc3)cc2)c1=O |r| Show InChI InChI=1S/C25H22N6O3/c1-2-6-21(32)29-14-13-18(15-29)31-24-22(23(26)27-16-28-24)30(25(31)33)17-9-11-20(12-10-17)34-19-7-4-3-5-8-19/h3-5,7-12,16,18H,13-15H2,1H3,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259411
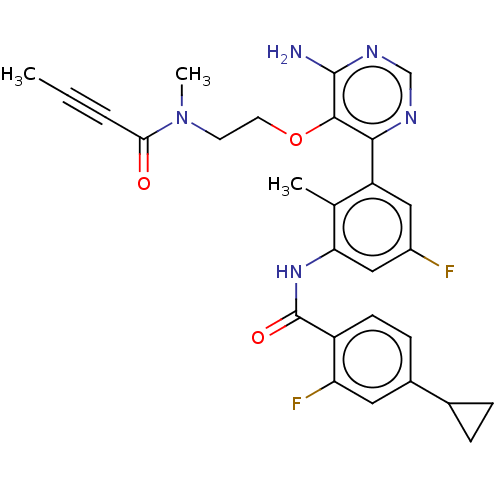
(US10457647, Example 10 | US11180460, Example 10 | ...)Show SMILES CC#CC(=O)N(C)CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C Show InChI InChI=1S/C28H27F2N5O3/c1-4-5-24(36)35(3)10-11-38-26-25(32-15-33-27(26)31)21-13-19(29)14-23(16(21)2)34-28(37)20-9-8-18(12-22(20)30)17-6-7-17/h8-9,12-15,17H,6-7,10-11H2,1-3H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546181
(CHEMBL4789435)Show SMILES C\C=C\C(=O)N1CCC(=CC1)c1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12 |c:8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638
(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT... |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259409

(US10457647, Example 8 | US11180460, Example 8 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C#C Show InChI InChI=1S/C27H25F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h1,7-8,11-14,16H,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT... |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ERBB4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data