

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human CYP4F2 in human liver microsomes assessed as fingolimod metabolism | Drug Metab Dispos 39: 191-8 (2011) Article DOI: 10.1124/dmd.110.035378 BindingDB Entry DOI: 10.7270/Q28917MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50030448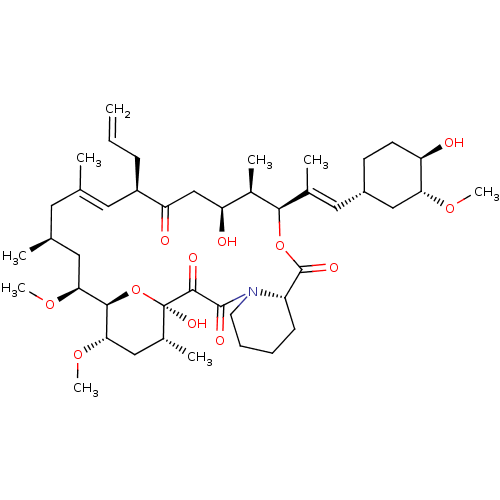 (8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development Curated by ChEMBL | Assay Description Inhibitory activity against macrophilin (FKBP-12) | J Med Chem 47: 4950-7 (2004) Article DOI: 10.1021/jm031101l BindingDB Entry DOI: 10.7270/Q21N81WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50153090 (CHEMBL411735 | Macrolide derivative) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development Curated by ChEMBL | Assay Description Inhibitory activity against macrophilin (FKBP-12) | J Med Chem 47: 4950-7 (2004) Article DOI: 10.1021/jm031101l BindingDB Entry DOI: 10.7270/Q21N81WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50153091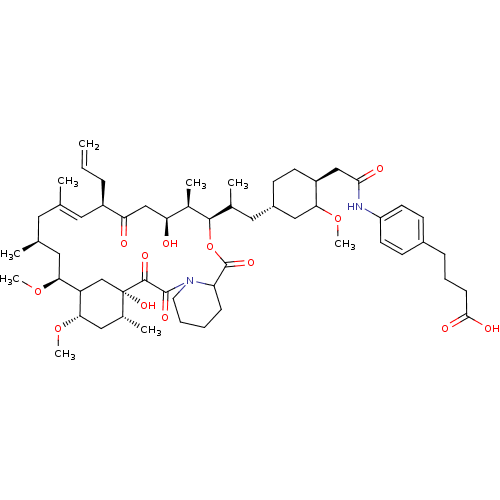 (CHEMBL265123 | Macrolide derivative) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development Curated by ChEMBL | Assay Description Inhibitory activity against macrophilin (FKBP-12) | J Med Chem 47: 4950-7 (2004) Article DOI: 10.1021/jm031101l BindingDB Entry DOI: 10.7270/Q21N81WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP4F2 assessed as fingolimod metabolism | Drug Metab Dispos 39: 191-8 (2011) Article DOI: 10.1124/dmd.110.035378 BindingDB Entry DOI: 10.7270/Q28917MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50158336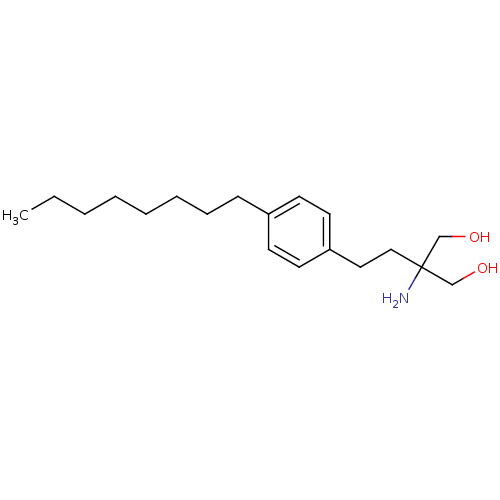 (2-(4-octylphenethyl)-2-aminopropane-1,3-diol | 2-A...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-2 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50158336 (2-(4-octylphenethyl)-2-aminopropane-1,3-diol | 2-A...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-4 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-2 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50158336 (2-(4-octylphenethyl)-2-aminopropane-1,3-diol | 2-A...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-3 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM23164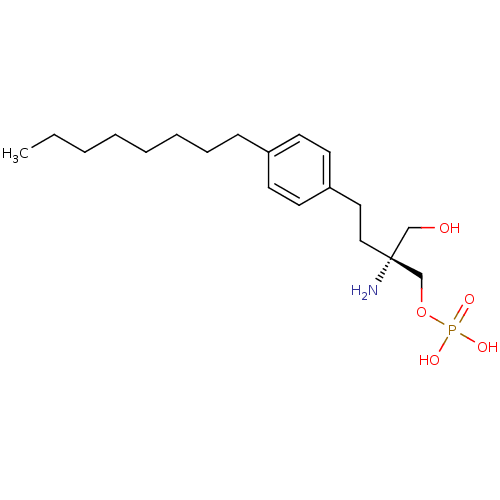 (CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 29 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-3 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-3 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM23164 (CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-5 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-2 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-3 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM23164 (CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-2 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-4 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-4 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-5 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM23164 (CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-4 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23164 (CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-1 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50158336 (2-(4-octylphenethyl)-2-aminopropane-1,3-diol | 2-A...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-1 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-5 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50158336 (2-(4-octylphenethyl)-2-aminopropane-1,3-diol | 2-A...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-5 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-1 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonism of human S1P-1 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand | J Med Chem 48: 5373-7 (2005) Article DOI: 10.1021/jm050242f BindingDB Entry DOI: 10.7270/Q218361R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||