

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139050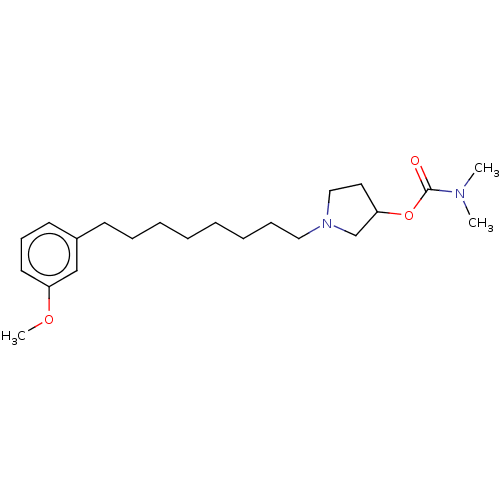 (CHEMBL3753906) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139006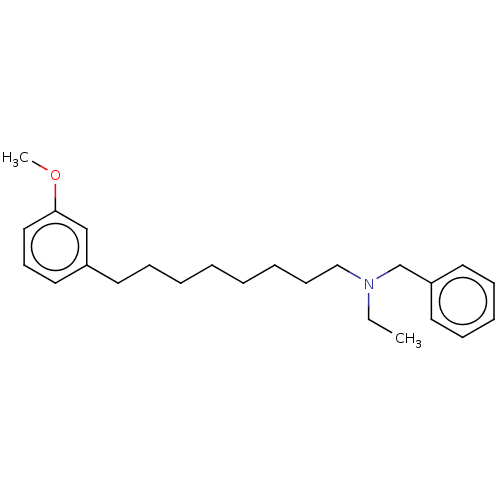 (CHEMBL3753607) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139052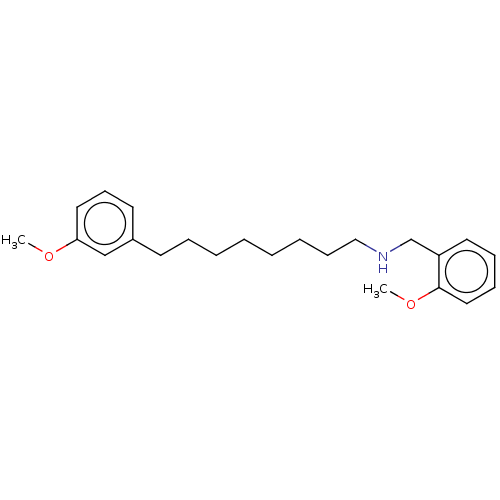 (CHEMBL3754700) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139049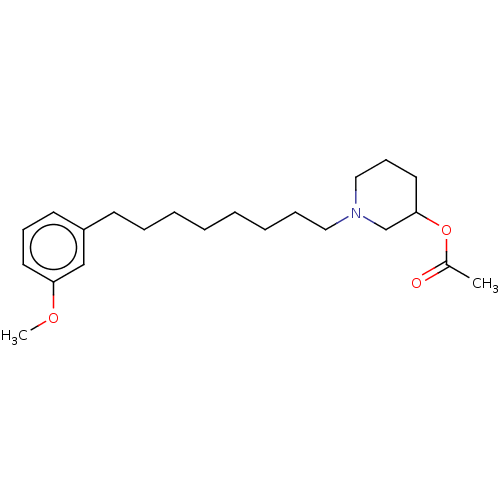 (CHEMBL3753732) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139051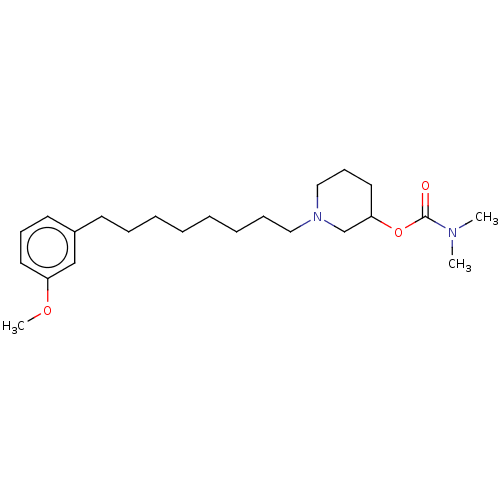 (CHEMBL3753070) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139007 (CHEMBL3754287) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Mixed inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139053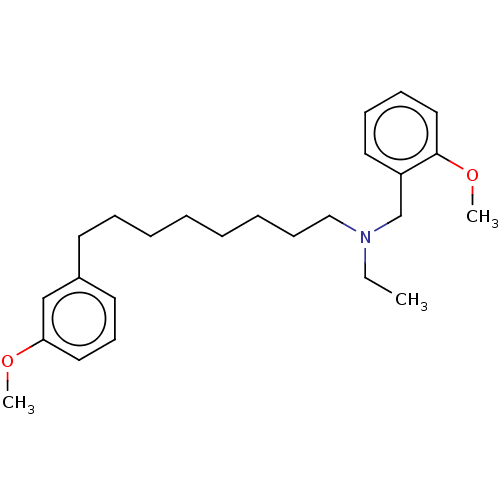 (CHEMBL3752227) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase preincubated for 10 mins followed by the addition of 550 uM of acetylcholine iodide as substrate measured ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139052 (CHEMBL3754700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50597595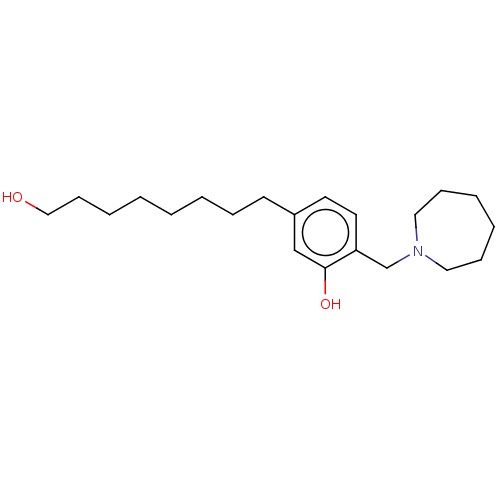 (CHEMBL5190179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139053 (CHEMBL3752227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50139053 (CHEMBL3752227) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50139053 (CHEMBL3752227) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase preincubated for 10 mins followed by the addition of 550 uM of acetylcholine iodide as substrate measured ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50597594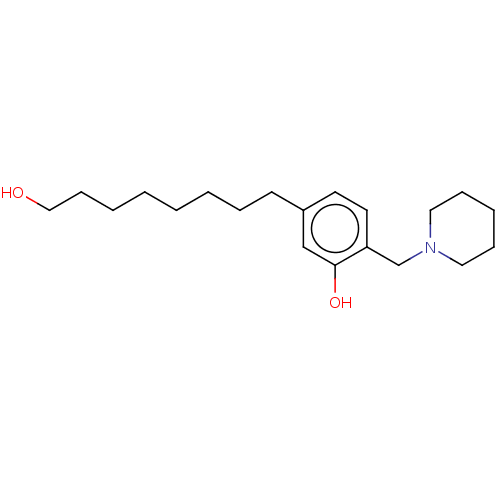 (CHEMBL5183866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139053 (CHEMBL3752227) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50597596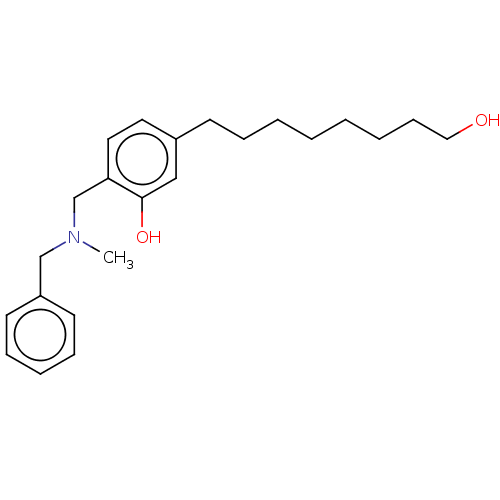 (CHEMBL5195029) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139006 (CHEMBL3753607) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50597593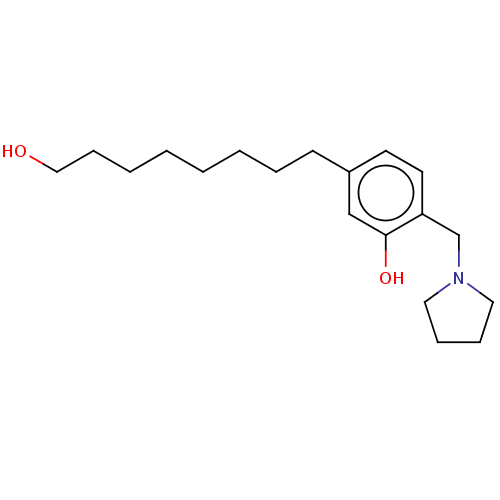 (CHEMBL5192958) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139050 (CHEMBL3753906) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139051 (CHEMBL3753070) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139049 (CHEMBL3753732) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139006 (CHEMBL3753607) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139007 (CHEMBL3754287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139052 (CHEMBL3754700) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50597597 (CHEMBL5176423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139049 (CHEMBL3753732) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139007 (CHEMBL3754287) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139050 (CHEMBL3753906) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139051 (CHEMBL3753070) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50597594 (CHEMBL5183866) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50597593 (CHEMBL5192958) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50597595 (CHEMBL5190179) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00046b BindingDB Entry DOI: 10.7270/Q2WM1JG5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||