 Found 502 hits for monomerid = 136570,136574,112777,50041457,50056190,50062614,50075373,50090677,50105417,50110590,50139181,50151865,50218116,50225074,50265079
Found 502 hits for monomerid = 136570,136574,112777,50041457,50056190,50062614,50075373,50090677,50105417,50110590,50139181,50151865,50218116,50225074,50265079 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677

(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Binding affinity measured on human cytochrome P450 2C9 (CYP2C9) enzyme |
J Med Chem 43: 2789-96 (2000)
BindingDB Entry DOI: 10.7270/Q2K64H9Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50062614
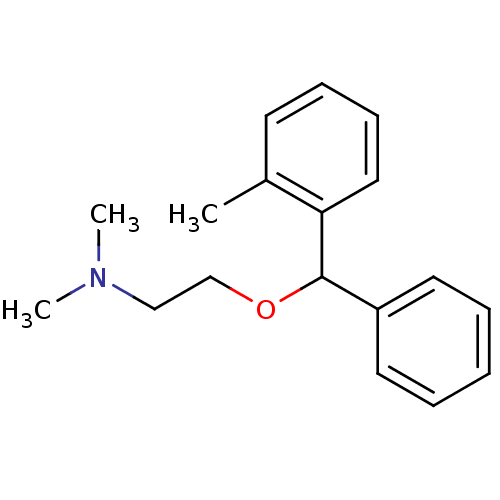
(CHEMBL900 | Dimethyl-[2-(phenyl-o-tolyl-methoxy)-e...)Show InChI InChI=1S/C18H23NO/c1-15-9-7-8-12-17(15)18(20-14-13-19(2)3)16-10-5-4-6-11-16/h4-12,18H,13-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 2352-4 (1993)
Article DOI: 10.1016/0006-2952(93)90211-e
BindingDB Entry DOI: 10.7270/Q2F76B2C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Chick) | BDBM50062614

(CHEMBL900 | Dimethyl-[2-(phenyl-o-tolyl-methoxy)-e...)Show InChI InChI=1S/C18H23NO/c1-15-9-7-8-12-17(15)18(20-14-13-19(2)3)16-10-5-4-6-11-16/h4-12,18H,13-14H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 38: 805-15 (1990)
BindingDB Entry DOI: 10.7270/Q2H993P0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells after 1.5 hrs by liquid scintillation counting |
J Med Chem 55: 5704-19 (2012)
Article DOI: 10.1021/jm2011657
BindingDB Entry DOI: 10.7270/Q2NV9KB2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 114: 559-65 (1994)
Article DOI: 10.1007/BF02244985
BindingDB Entry DOI: 10.7270/Q25X27FZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 317 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Biol Psychiatry 55: 320-2 (2004)
Article DOI: 10.1016/j.biopsych.2003.07.006
BindingDB Entry DOI: 10.7270/Q2K64GMB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677

(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by ChEMBL
| Assay Description
Binding affinity towards cytochrome P450 2C9 |
J Med Chem 47: 907-14 (2004)
Article DOI: 10.1021/jm030972s
BindingDB Entry DOI: 10.7270/Q2ZK5HF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Equus caballus (horse) serum butyrylcholinesterase (BChE) assessed as inhibition of BTCh hydrolysis by Ellman method |
Citation and Details
Article DOI: 10.1007/s00044-005-0140-0
BindingDB Entry DOI: 10.7270/Q2CJ8HD5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Equus caballus (horse) serum butyrylcholinesterase (BChE) assessed as inhibition of BTCh hydrolysis by Ellman method |
Citation and Details
Article DOI: 10.1007/s00044-005-0140-0
BindingDB Entry DOI: 10.7270/Q2CJ8HD5 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States
| Assay Description
Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO... |
ACS Chem Biol 9: 1284-93 (2014)
Article DOI: 10.1021/cb500018s
BindingDB Entry DOI: 10.7270/Q2FN14VF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Eur J Pharmacol 340: 249-58 (1997)
Article DOI: 10.1016/s0014-2999(97)01393-9
BindingDB Entry DOI: 10.7270/Q2V69H3D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Biol Psychiatry 55: 320-2 (2004)
Article DOI: 10.1016/j.biopsych.2003.07.006
BindingDB Entry DOI: 10.7270/Q2K64GMB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.,
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 226: 686-700 (1983)
BindingDB Entry DOI: 10.7270/Q2XP73D5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Electrophorus electricus (electric eel) acetylcholinesterase (AChE) assessed as inhibition of ATCh hydrolysis by Ellman method |
Citation and Details
Article DOI: 10.1007/s00044-005-0140-0
BindingDB Entry DOI: 10.7270/Q2CJ8HD5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Electrophorus electricus (electric eel) acetylcholinesterase (AChE) assessed as inhibition of ATCh hydrolysis by Ellman method |
Citation and Details
Article DOI: 10.1007/s00044-005-0140-0
BindingDB Entry DOI: 10.7270/Q2CJ8HD5 |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, liver
(Rattus norvegicus (Rat)) | BDBM50110590
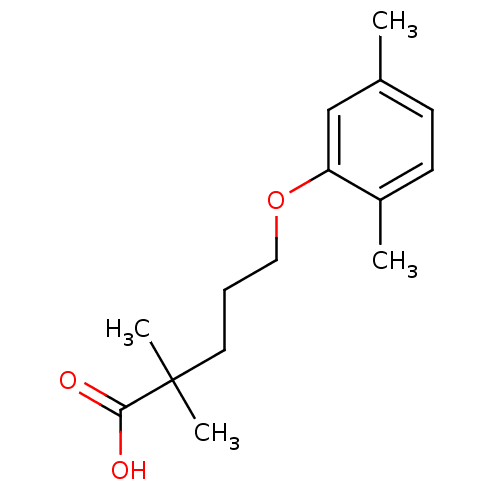
(2,2-Dimethyl-5-(2,5-dimethylphenoxy)valeriansaeure...)Show InChI InChI=1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of 1-anilinonaphthalene-8-sulphonic acid from rat recombinant L-FABP high binding affinity site expressed in Escherichia coli BL21 by co... |
J Med Chem 51: 3755-64 (2008)
Article DOI: 10.1021/jm701192w
BindingDB Entry DOI: 10.7270/Q2KH0P7G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells |
ACS Med Chem Lett 4: 1037-41 (2013)
Article DOI: 10.1021/ml400015f
BindingDB Entry DOI: 10.7270/Q2M61P6M |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
J Pharm Pharmacol 38: 374-9 (1986)
Article DOI: 10.1111/j.2042-7158.1986.tb04590.x
BindingDB Entry DOI: 10.7270/Q2JS9NXW |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 114: 559-65 (1994)
Article DOI: 10.1007/BF02244985
BindingDB Entry DOI: 10.7270/Q25X27FZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 114: 559-65 (1994)
Article DOI: 10.1007/BF02244985
BindingDB Entry DOI: 10.7270/Q25X27FZ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States
| Assay Description
Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO... |
ACS Chem Biol 9: 1284-93 (2014)
Article DOI: 10.1021/cb500018s
BindingDB Entry DOI: 10.7270/Q2FN14VF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Biol Psychiatry 55: 320-2 (2004)
Article DOI: 10.1016/j.biopsych.2003.07.006
BindingDB Entry DOI: 10.7270/Q2K64GMB |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 940-951 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.021
BindingDB Entry DOI: 10.7270/Q2ZC8597 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States
| Assay Description
Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... |
ACS Chem Biol 9: 1284-93 (2014)
Article DOI: 10.1021/cb500018s
BindingDB Entry DOI: 10.7270/Q2FN14VF |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.075
BindingDB Entry DOI: 10.7270/Q2BR8T25 |
More data for this
Ligand-Target Pair | |
CDGSH iron-sulfur domain-containing protein 1
(Homo sapiens (Human)) | BDBM50110590

(2,2-Dimethyl-5-(2,5-dimethylphenoxy)valeriansaeure...)Show InChI InChI=1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
West Virginia University
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from recombinant human C-terminal His-tagged MitoNEET cytosolic domain (32 to 108 residues) expressed in Escherichi... |
Bioorg Med Chem Lett 26: 5350-5353 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.009
BindingDB Entry DOI: 10.7270/Q2ZP483C |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member
(Danio rerio (Zebrafish)) | BDBM50110590

(2,2-Dimethyl-5-(2,5-dimethylphenoxy)valeriansaeure...)Show InChI InChI=1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rudjer Boskovic Institute
| Assay Description
In the inhibition experiments, the cells were preincubated for 20 s with test compounds, followed by a 5-min incubation with [3H]E3S (5 nM) or 30-min... |
J Biol Chem 288: 33894-911 (2013)
Article DOI: 10.1074/jbc.M113.518506
BindingDB Entry DOI: 10.7270/Q29Z93RK |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Eur J Pharmacol 340: 249-58 (1997)
Article DOI: 10.1016/s0014-2999(97)01393-9
BindingDB Entry DOI: 10.7270/Q2V69H3D |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Mol Pharmacol 12: 568-80 (1976)
BindingDB Entry DOI: 10.7270/Q2ZK5F5P |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Eur J Pharmacol 340: 249-58 (1997)
Article DOI: 10.1016/s0014-2999(97)01393-9
BindingDB Entry DOI: 10.7270/Q2V69H3D |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.,
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 226: 686-700 (1983)
BindingDB Entry DOI: 10.7270/Q2XP73D5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Eur J Pharmacol 340: 249-58 (1997)
Article DOI: 10.1016/s0014-2999(97)01393-9
BindingDB Entry DOI: 10.7270/Q2V69H3D |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Rattus norvegicus) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem Lett 18: 2916-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.075
BindingDB Entry DOI: 10.7270/Q2BR8T25 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 |
J Med Chem 54: 8236-50 (2011)
Article DOI: 10.1021/jm201048w
BindingDB Entry DOI: 10.7270/Q2D50NFR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM112777

(NORTRIPTYLINE | US8629135, SW-02)Show SMILES [#6]-[#7]-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-11,20H,6,12-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 4.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using human liver microsomes |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México
Curated by ChEMBL
| Assay Description
Inhibitory constant against HMG-CoA reductase with alpha asarone |
Bioorg Med Chem Lett 15: 989-94 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.046
BindingDB Entry DOI: 10.7270/Q2BC3Z1P |
More data for this
Ligand-Target Pair | |
Fatty acid-binding protein, liver
(Rattus norvegicus (Rat)) | BDBM50110590

(2,2-Dimethyl-5-(2,5-dimethylphenoxy)valeriansaeure...)Show InChI InChI=1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Displacement of 1-anilinonaphthalene-8-sulphonic acid from rat recombinant L-FABP low binding affinity site expressed in Escherichia coli BL21 by com... |
J Med Chem 51: 3755-64 (2008)
Article DOI: 10.1021/jm701192w
BindingDB Entry DOI: 10.7270/Q2KH0P7G |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 2
(Homo sapiens (Human)) | BDBM50062614

(CHEMBL900 | Dimethyl-[2-(phenyl-o-tolyl-methoxy)-e...)Show InChI InChI=1S/C18H23NO/c1-15-9-7-8-12-17(15)18(20-14-13-19(2)3)16-10-5-4-6-11-16/h4-12,18H,13-14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human OCT2 expressed in HEK-293-Flp-In cells incubated for 3 mins by ASP+ substrate uptake assay |
J Med Chem 54: 4548-58 (2011)
Article DOI: 10.1021/jm2001629
BindingDB Entry DOI: 10.7270/Q26W9C5N |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 1
(Homo sapiens (Human)) | BDBM50062614

(CHEMBL900 | Dimethyl-[2-(phenyl-o-tolyl-methoxy)-e...)Show InChI InChI=1S/C18H23NO/c1-15-9-7-8-12-17(15)18(20-14-13-19(2)3)16-10-5-4-6-11-16/h4-12,18H,13-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human OCT1 expressed in HEK-293-Flp-In cells incubated for 3 mins by ASP+ substrate uptake assay |
J Med Chem 54: 4548-58 (2011)
Article DOI: 10.1021/jm2001629
BindingDB Entry DOI: 10.7270/Q26W9C5N |
More data for this
Ligand-Target Pair | |
Multidrug and toxin extrusion protein 1
(Homo sapiens (Human)) | BDBM50062614

(CHEMBL900 | Dimethyl-[2-(phenyl-o-tolyl-methoxy)-e...)Show InChI InChI=1S/C18H23NO/c1-15-9-7-8-12-17(15)18(20-14-13-19(2)3)16-10-5-4-6-11-16/h4-12,18H,13-14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human MATE1 expressed in HEK-293-Flp-In cells incubated for 3 mins by ASP+ substrate uptake assay |
J Med Chem 54: 4548-58 (2011)
Article DOI: 10.1021/jm2001629
BindingDB Entry DOI: 10.7270/Q26W9C5N |
More data for this
Ligand-Target Pair | |
Multidrug and toxin extrusion protein 2
(Homo sapiens (Human)) | BDBM50062614

(CHEMBL900 | Dimethyl-[2-(phenyl-o-tolyl-methoxy)-e...)Show InChI InChI=1S/C18H23NO/c1-15-9-7-8-12-17(15)18(20-14-13-19(2)3)16-10-5-4-6-11-16/h4-12,18H,13-14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Inhibition of human MATE2-K expressed in HEK-293-Flp-In cells incubated for 3 mins by ASP+ substrate uptake assay |
J Med Chem 54: 4548-58 (2011)
Article DOI: 10.1021/jm2001629
BindingDB Entry DOI: 10.7270/Q26W9C5N |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50062614

(CHEMBL900 | Dimethyl-[2-(phenyl-o-tolyl-methoxy)-e...)Show InChI InChI=1S/C18H23NO/c1-15-9-7-8-12-17(15)18(20-14-13-19(2)3)16-10-5-4-6-11-16/h4-12,18H,13-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a | n/a |
TCG Lifesciences Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 46: 618-30 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.042
BindingDB Entry DOI: 10.7270/Q2WQ052W |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50265079
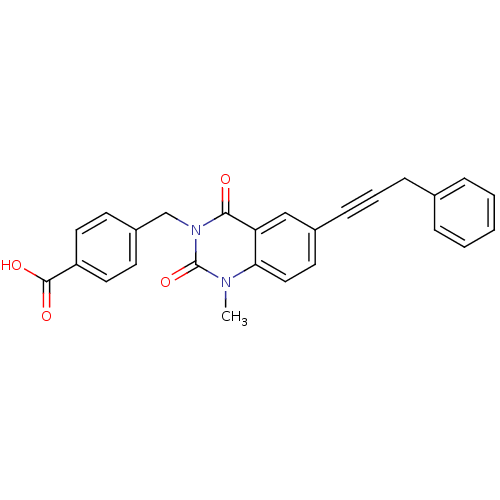
((4-[1-methyl-2,4-dioxo-6-(3-phenyl-prop-1-ynyl)-1,...)Show SMILES Cn1c2ccc(cc2c(=O)n(Cc2ccc(cc2)C(O)=O)c1=O)C#CCc1ccccc1 Show InChI InChI=1S/C26H20N2O4/c1-27-23-15-12-19(9-5-8-18-6-3-2-4-7-18)16-22(23)24(29)28(26(27)32)17-20-10-13-21(14-11-20)25(30)31/h2-4,6-7,10-16H,8,17H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique
| Assay Description
Enzyme assay using human matrix metalloproteases or ADAMTS. |
J Biol Chem 287: 26647-56 (2012)
Article DOI: 10.1074/jbc.M112.380782
BindingDB Entry DOI: 10.7270/Q2H993SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase