 Found 476 hits Enz. Inhib. hit(s) with Target = 'P2Y purinoceptor 14'
Found 476 hits Enz. Inhib. hit(s) with Target = 'P2Y purinoceptor 14' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14R |
J Med Chem 63: 9563-9589 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00745
BindingDB Entry DOI: 10.7270/Q20R9SZP |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14 receptor expressed in CHO cells by fluorescence assay |
Eur J Med Chem 175: 34-39 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.068
BindingDB Entry DOI: 10.7270/Q29C71R4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Rattus norvegicus) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y14R in rat C6 cells assessed as suppression of UDP-glucose-mediated inhibition of forskolin-stimulated [3H]cyclic-AMP accum... |
J Med Chem 61: 4860-4882 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00168
BindingDB Entry DOI: 10.7270/Q2X069NH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human P2Y14 expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP accumulation incubated for 15 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01964
BindingDB Entry DOI: 10.7270/Q2611470 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Mus musculus) | BDBM50343888

((R)-4-(4-(2,2-difluoro-1-hydroxyethyl)phenyl)-7-(4...)Show SMILES O[C@@H](C(F)F)c1ccc(cc1)-c1cc(cc2cc(ccc12)-c1ccc(cc1)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C26H17F5O3/c27-24(28)23(32)16-3-1-15(2-4-16)22-13-19(25(33)34)12-18-11-17(7-10-21(18)22)14-5-8-20(9-6-14)26(29,30)31/h1-13,23-24,32H,(H,33,34)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mouse P2Y14 receptor |
Eur J Med Chem 175: 34-39 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.068
BindingDB Entry DOI: 10.7270/Q29C71R4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Mus musculus) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mouse P2Y14 receptor in presence of 2% HSA |
Eur J Med Chem 175: 34-39 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.068
BindingDB Entry DOI: 10.7270/Q29C71R4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50614414
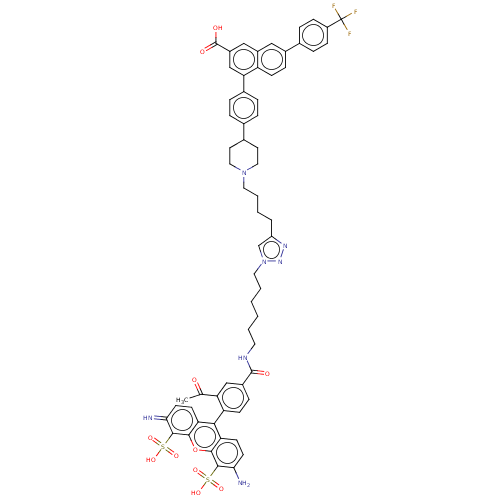
(CHEMBL5271821) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50343128
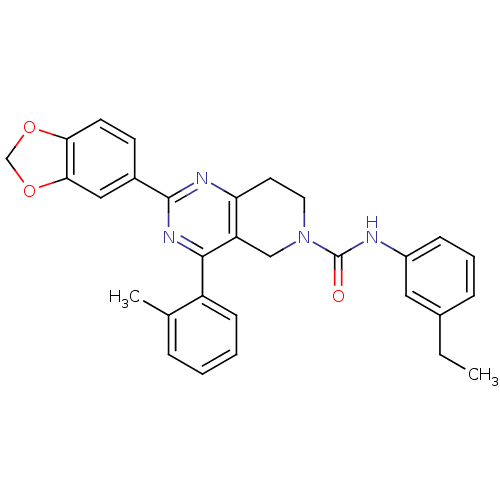
(2-(benzo[d][1,3]dioxol-5-yl)-N-(3-ethylphenyl)-4-o...)Show SMILES CCc1cccc(NC(=O)N2CCc3nc(nc(c3C2)-c2ccccc2C)-c2ccc3OCOc3c2)c1 Show InChI InChI=1S/C30H28N4O3/c1-3-20-8-6-9-22(15-20)31-30(35)34-14-13-25-24(17-34)28(23-10-5-4-7-19(23)2)33-29(32-25)21-11-12-26-27(16-21)37-18-36-26/h4-12,15-16H,3,13-14,17-18H2,1-2H3,(H,31,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Mus musculus) | BDBM50343888

((R)-4-(4-(2,2-difluoro-1-hydroxyethyl)phenyl)-7-(4...)Show SMILES O[C@@H](C(F)F)c1ccc(cc1)-c1cc(cc2cc(ccc12)-c1ccc(cc1)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C26H17F5O3/c27-24(28)23(32)16-3-1-15(2-4-16)22-13-19(25(33)34)12-18-11-17(7-10-21(18)22)14-5-8-20(9-6-14)26(29,30)31/h1-13,23-24,32H,(H,33,34)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mouse P2Y14 receptor in presence of 2% HSA |
Eur J Med Chem 175: 34-39 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.068
BindingDB Entry DOI: 10.7270/Q29C71R4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50429538
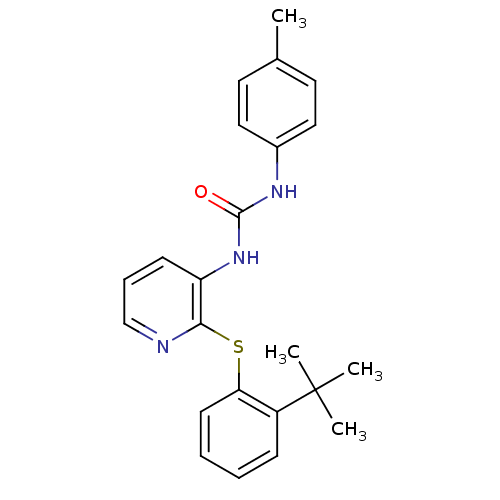
(CHEMBL2333767)Show SMILES Cc1ccc(NC(=O)Nc2cccnc2Sc2ccccc2C(C)(C)C)cc1 Show InChI InChI=1S/C23H25N3OS/c1-16-11-13-17(14-12-16)25-22(27)26-19-9-7-15-24-21(19)28-20-10-6-5-8-18(20)23(2,3)4/h5-15H,1-4H3,(H2,25,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14 receptor |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50429534
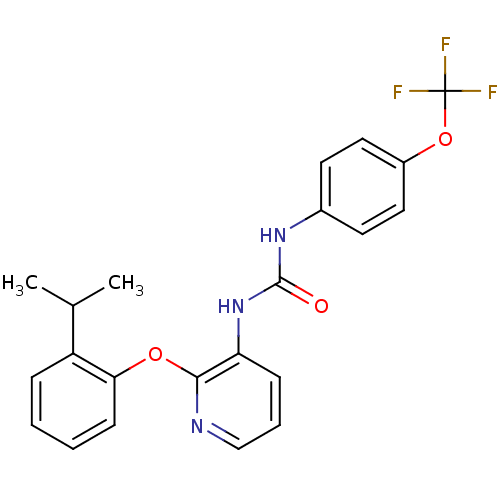
(CHEMBL2333773)Show SMILES CC(C)c1ccccc1Oc1ncccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C22H20F3N3O3/c1-14(2)17-6-3-4-8-19(17)30-20-18(7-5-13-26-20)28-21(29)27-15-9-11-16(12-10-15)31-22(23,24)25/h3-14H,1-2H3,(H2,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14 receptor |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50429537
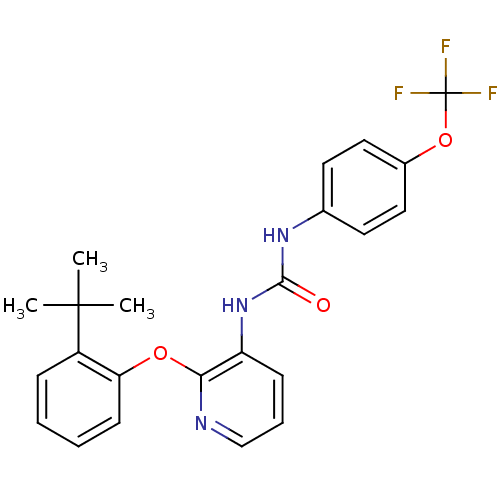
(CHEMBL2333770)Show SMILES CC(C)(C)c1ccccc1Oc1ncccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H22F3N3O3/c1-22(2,3)17-7-4-5-9-19(17)31-20-18(8-6-14-27-20)29-21(30)28-15-10-12-16(13-11-15)32-23(24,25)26/h4-14H,1-3H3,(H2,28,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14 receptor |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50429536
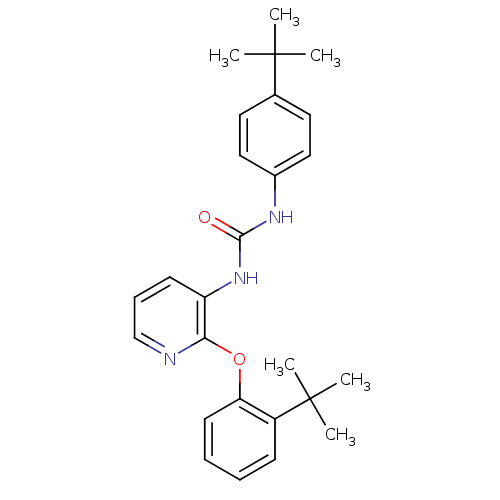
(CHEMBL2333771)Show SMILES CC(C)(C)c1ccc(NC(=O)Nc2cccnc2Oc2ccccc2C(C)(C)C)cc1 Show InChI InChI=1S/C26H31N3O2/c1-25(2,3)18-13-15-19(16-14-18)28-24(30)29-21-11-9-17-27-23(21)31-22-12-8-7-10-20(22)26(4,5)6/h7-17H,1-6H3,(H2,28,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14 receptor |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50429535
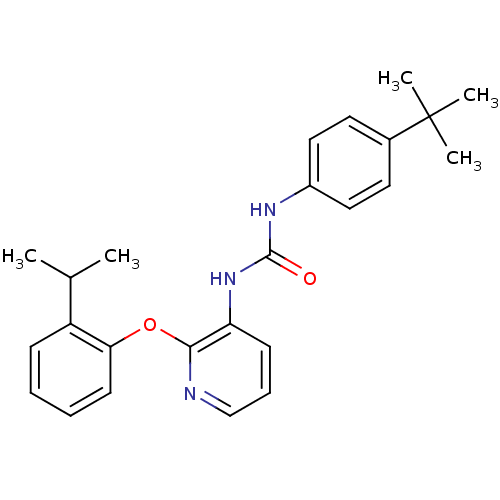
(CHEMBL2333772)Show SMILES CC(C)c1ccccc1Oc1ncccc1NC(=O)Nc1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C25H29N3O2/c1-17(2)20-9-6-7-11-22(20)30-23-21(10-8-16-26-23)28-24(29)27-19-14-12-18(13-15-19)25(3,4)5/h6-17H,1-5H3,(H2,27,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14 receptor |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity human P2Y14R expressed in African green monkey COS7 cells assessed as inhibition of UDPG-induced [3H]inositol phosphate accumulat... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity human P2Y14R expressed in African green monkey COS7 cells assessed as inhibition of UDPG-induced [3H]inositol phosphate accumulat... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Mus musculus) | BDBM50343131

(2-(2-cyanopyrimidin-5-yl)-N-(3-ethylphenyl)-4-o-to...)Show SMILES CCc1cccc(NC(=O)N2CCc3nc(nc(c3C2)-c2ccccc2C)-c2cnc(nc2)C#N)c1 Show InChI InChI=1S/C28H25N7O/c1-3-19-8-6-9-21(13-19)32-28(36)35-12-11-24-23(17-35)26(22-10-5-4-7-18(22)2)34-27(33-24)20-15-30-25(14-29)31-16-20/h4-10,13,15-16H,3,11-12,17H2,1-2H3,(H,32,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... |
Bioorg Med Chem Lett 21: 2832-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.084
BindingDB Entry DOI: 10.7270/Q20865N7 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50515480
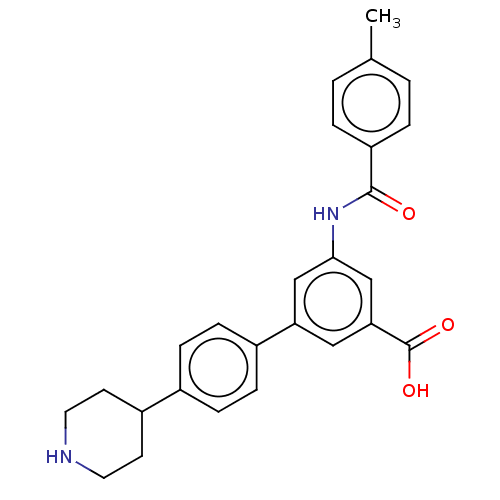
(CHEMBL4436879)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(cc(c1)-c1ccc(cc1)C1CCNCC1)C(O)=O Show InChI InChI=1S/C26H26N2O3/c1-17-2-4-21(5-3-17)25(29)28-24-15-22(14-23(16-24)26(30)31)19-8-6-18(7-9-19)20-10-12-27-13-11-20/h2-9,14-16,20,27H,10-13H2,1H3,(H,28,29)(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R stably expressed in human forskolin treated THP1 cells assessed as reduction in P2Y14R agonist UDPG-induced inhib... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111564
BindingDB Entry DOI: 10.7270/Q20005D2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50515480

(CHEMBL4436879)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(cc(c1)-c1ccc(cc1)C1CCNCC1)C(O)=O Show InChI InChI=1S/C26H26N2O3/c1-17-2-4-21(5-3-17)25(29)28-24-15-22(14-23(16-24)26(30)31)19-8-6-18(7-9-19)20-10-12-27-13-11-20/h2-9,14-16,20,27H,10-13H2,1H3,(H,28,29)(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2Y14 (unknown origin) expressed in HEK293 cells assessed as reduction in cAMP production incubated for 30 mins by ADP-glo ass... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113313
BindingDB Entry DOI: 10.7270/Q2D50RQK |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602122
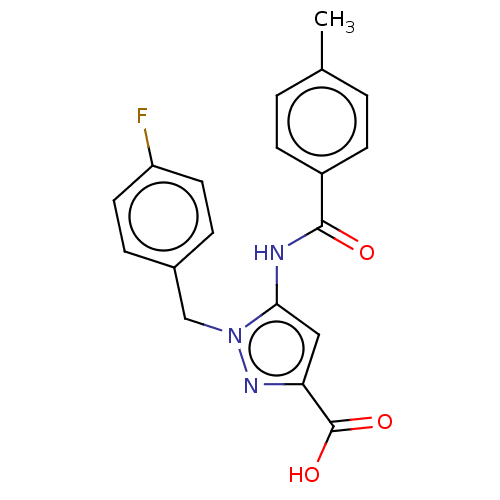
(CHEMBL5208130)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(nn1Cc1ccc(F)cc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50596039

(CHEMBL5186555)Show SMILES COc1ccc(CC(=O)Nc2cccc(c2)-c2nc3ccccc3o2)cc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113933
BindingDB Entry DOI: 10.7270/Q200063F |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R stably expressed in human forskolin treated THP1 cells assessed as reduction in P2Y14R agonist UDPG-induced inhib... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111564
BindingDB Entry DOI: 10.7270/Q20005D2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2Y14 (unknown origin) expressed in HEK293 cells assessed as reduction in cAMP production incubated for 30 mins by ADP-glo ass... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113313
BindingDB Entry DOI: 10.7270/Q2D50RQK |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50569005
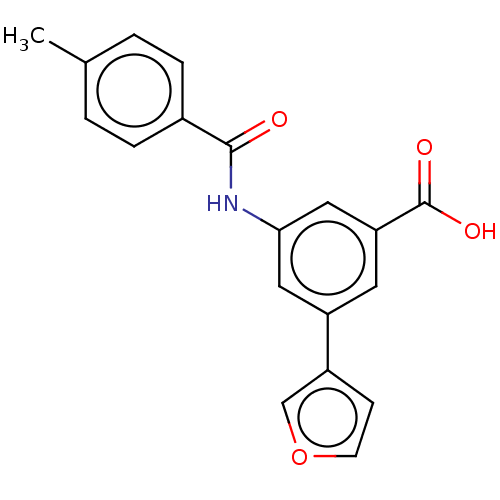
(CHEMBL4854775)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(cc(c1)-c1ccoc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2Y14 (unknown origin) expressed in HEK293 cells assessed as reduction in cAMP production incubated for 30 mins by ADP-glo ass... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113313
BindingDB Entry DOI: 10.7270/Q2D50RQK |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50569007

(CHEMBL4858280)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(cc(c1)-c1cc[nH]n1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2Y14 (unknown origin) expressed in HEK293 cells assessed as reduction in cAMP production incubated for 30 mins by ADP-glo ass... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113313
BindingDB Entry DOI: 10.7270/Q2D50RQK |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50569006

(CHEMBL4875670)Show SMILES Cc1noc(C)c1-c1cc(NC(=O)c2ccc(C)cc2)cc(c1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2Y14 (unknown origin) expressed in HEK293 cells assessed as reduction in cAMP production incubated for 30 mins by ADP-glo ass... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113313
BindingDB Entry DOI: 10.7270/Q2D50RQK |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Mus musculus) | BDBM50343129

(2-(4-cyanopyridin-3-yl)-N-(3-ethylphenyl)-4-o-toly...)Show SMILES CCc1cccc(NC(=O)N2CCc3nc(nc(c3C2)-c2ccccc2C)-c2cnccc2C#N)c1 Show InChI InChI=1S/C29H26N6O/c1-3-20-8-6-9-22(15-20)32-29(36)35-14-12-26-25(18-35)27(23-10-5-4-7-19(23)2)34-28(33-26)24-17-31-13-11-21(24)16-30/h4-11,13,15,17H,3,12,14,18H2,1-2H3,(H,32,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... |
Bioorg Med Chem Lett 21: 2832-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.084
BindingDB Entry DOI: 10.7270/Q20865N7 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50584891
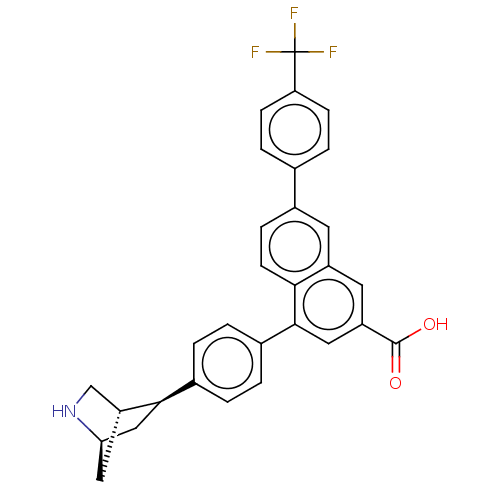
(CHEMBL5085823)Show SMILES [H][C@@]12C[C@H](c3ccc(cc3)-c3cc(cc4cc(ccc34)-c3ccc(cc3)C(F)(F)F)C(O)=O)[C@@]([H])(CN1)C2 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of fluorescent antagonist binding to human P2Y14R expressed in CHO cells preincubated for 30 mins followed by 6-amino-9-(2-carboxy-4-((6-(... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01964
BindingDB Entry DOI: 10.7270/Q2611470 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50584891

(CHEMBL5085823)Show SMILES [H][C@@]12C[C@H](c3ccc(cc3)-c3cc(cc4cc(ccc34)-c3ccc(cc3)C(F)(F)F)C(O)=O)[C@@]([H])(CN1)C2 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of fluorescent antagonist binding to human P2Y14R expressed in CHO cells preincubated for 30 mins followed by 6-amino-9-(2-carboxy-4-((6-(... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01964
BindingDB Entry DOI: 10.7270/Q2611470 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50515476

(CHEMBL4577585)Show SMILES OC(=O)c1cc(NC(=O)c2ccncc2)cc(c1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C24H23N3O3/c28-23(19-7-11-26-12-8-19)27-22-14-20(13-21(15-22)24(29)30)17-3-1-16(2-4-17)18-5-9-25-10-6-18/h1-4,7-8,11-15,18,25H,5-6,9-10H2,(H,27,28)(H,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R stably expressed in human forskolin treated THP1 cells assessed as reduction in P2Y14R agonist UDPG-induced inhib... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111564
BindingDB Entry DOI: 10.7270/Q20005D2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602119
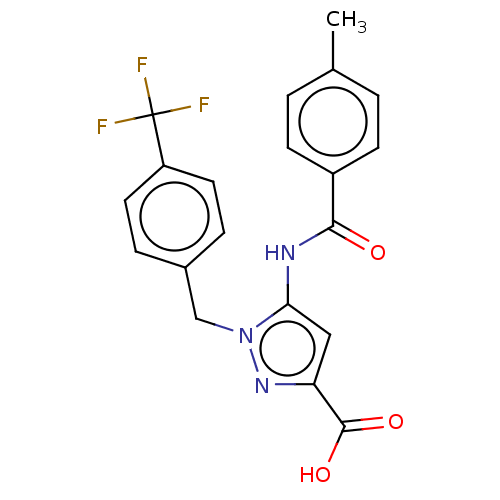
(CHEMBL5194881)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(nn1Cc1ccc(cc1)C(F)(F)F)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602131
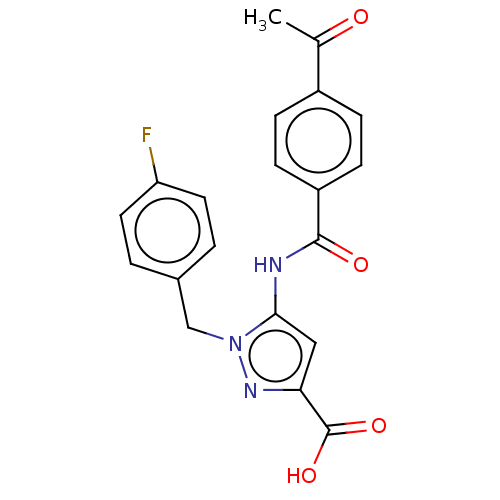
(CHEMBL5203559)Show SMILES CC(=O)c1ccc(cc1)C(=O)Nc1cc(nn1Cc1ccc(F)cc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
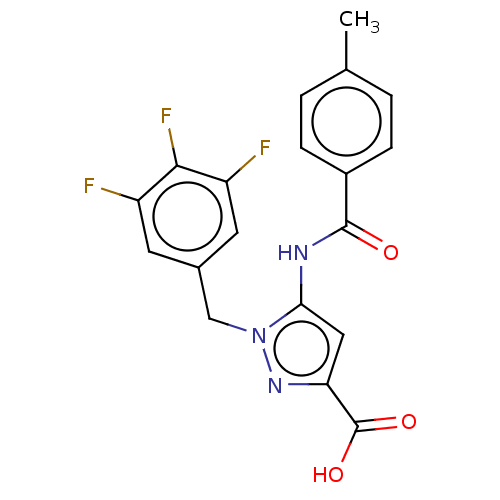
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602108
(CHEMBL5182459)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(nn1Cc1cc(F)c(F)c(F)c1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Mus musculus) | BDBM50343132

(2-(3,5-dimethylisoxazol-4-yl)-N-(3-ethylphenyl)-4-...)Show SMILES CCc1cccc(NC(=O)N2CCc3nc(nc(c3C2)-c2ccccc2C)-c2c(C)noc2C)c1 Show InChI InChI=1S/C28H29N5O2/c1-5-20-10-8-11-21(15-20)29-28(34)33-14-13-24-23(16-33)26(22-12-7-6-9-17(22)2)31-27(30-24)25-18(3)32-35-19(25)4/h6-12,15H,5,13-14,16H2,1-4H3,(H,29,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... |
Bioorg Med Chem Lett 21: 2832-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.084
BindingDB Entry DOI: 10.7270/Q20865N7 |
More data for this
Ligand-Target Pair | |
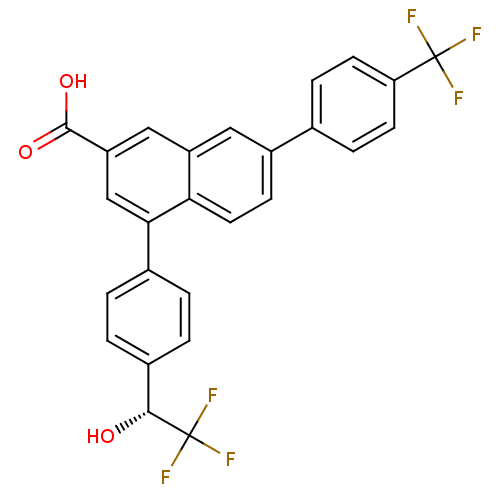
P2Y purinoceptor 14
(Mus musculus) | BDBM50343886
((R)-4-(4-(2,2,2-trifluoro-1-hydroxyethyl)phenyl)-7...)Show SMILES O[C@H](c1ccc(cc1)-c1cc(cc2cc(ccc12)-c1ccc(cc1)C(F)(F)F)C(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C26H16F6O3/c27-25(28,29)20-8-5-14(6-9-20)17-7-10-21-18(11-17)12-19(24(34)35)13-22(21)15-1-3-16(4-2-15)23(33)26(30,31)32/h1-13,23,33H,(H,34,35)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay |
Bioorg Med Chem Lett 21: 2836-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.081
BindingDB Entry DOI: 10.7270/Q2NP24S4 |
More data for this
Ligand-Target Pair | |
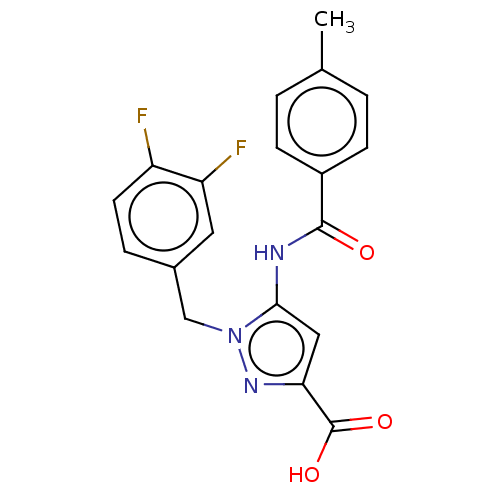
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602109
(CHEMBL5175291)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(nn1Cc1ccc(F)c(F)c1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50515477
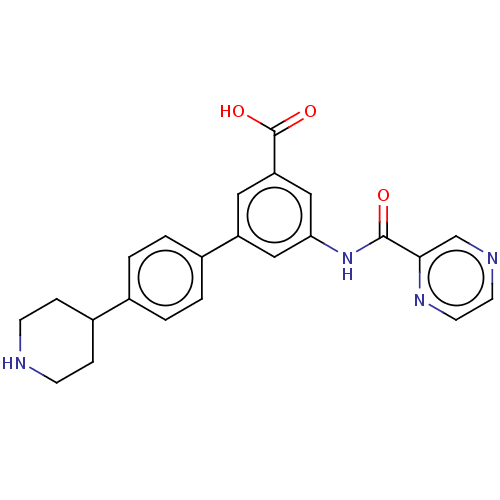
(CHEMBL4538561)Show SMILES OC(=O)c1cc(NC(=O)c2cnccn2)cc(c1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C23H22N4O3/c28-22(21-14-25-9-10-26-21)27-20-12-18(11-19(13-20)23(29)30)16-3-1-15(2-4-16)17-5-7-24-8-6-17/h1-4,9-14,17,24H,5-8H2,(H,27,28)(H,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R stably expressed in human forskolin treated THP1 cells assessed as reduction in P2Y14R agonist UDPG-induced inhib... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111564
BindingDB Entry DOI: 10.7270/Q20005D2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50515478
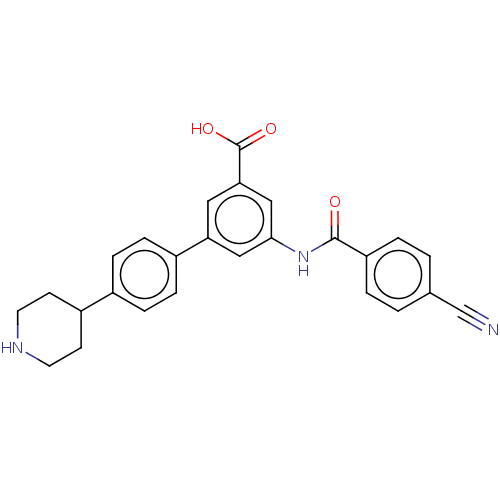
(CHEMBL4455311)Show SMILES OC(=O)c1cc(NC(=O)c2ccc(cc2)C#N)cc(c1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C26H23N3O3/c27-16-17-1-3-21(4-2-17)25(30)29-24-14-22(13-23(15-24)26(31)32)19-7-5-18(6-8-19)20-9-11-28-12-10-20/h1-8,13-15,20,28H,9-12H2,(H,29,30)(H,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R stably expressed in human forskolin treated THP1 cells assessed as reduction in P2Y14R agonist UDPG-induced inhib... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111564
BindingDB Entry DOI: 10.7270/Q20005D2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602115
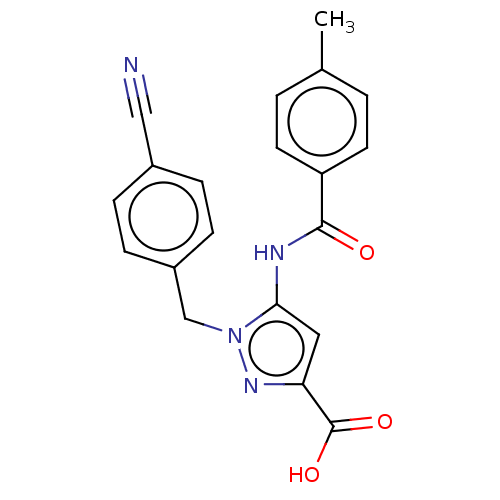
(CHEMBL5192153)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(nn1Cc1ccc(cc1)C#N)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602130

(CHEMBL5189241)Show SMILES CCc1ccc(cc1)C(=O)Nc1cc(nn1Cc1ccc(F)cc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602117
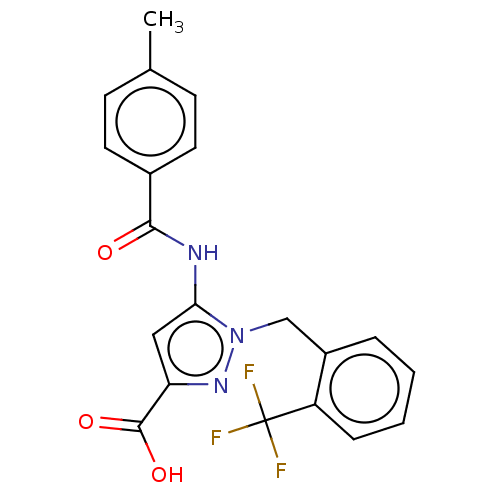
(CHEMBL5185002)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(nn1Cc1ccccc1C(F)(F)F)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50596040
(CHEMBL5193065)Show SMILES FC(F)(F)Oc1ccc(CC(=O)Nc2cccc(c2)-c2nc3ccccc3o2)cc1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113933
BindingDB Entry DOI: 10.7270/Q200063F |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50569003
(CHEMBL4857372)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(cc(c1)-c1ccccc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2Y14 (unknown origin) expressed in HEK293 cells assessed as reduction in cAMP production incubated for 30 mins by ADP-glo ass... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113313
BindingDB Entry DOI: 10.7270/Q2D50RQK |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50515475

(CHEMBL4458922)Show SMILES OC(=O)c1cc(NC(=O)c2ccccc2)cc(c1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C25H24N2O3/c28-24(20-4-2-1-3-5-20)27-23-15-21(14-22(16-23)25(29)30)18-8-6-17(7-9-18)19-10-12-26-13-11-19/h1-9,14-16,19,26H,10-13H2,(H,27,28)(H,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R stably expressed in human forskolin treated THP1 cells assessed as reduction in P2Y14R agonist UDPG-induced inhib... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111564
BindingDB Entry DOI: 10.7270/Q20005D2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50602116

(CHEMBL5195243)Show SMILES Cc1ccc(cc1)C(=O)Nc1cc(nn1Cc1ccc(cc1)[N+]([O-])=O)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01632
BindingDB Entry DOI: 10.7270/Q26D5Z20 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50515479

(CHEMBL4526840)Show SMILES OC(=O)c1cc(NC(=O)c2ccc(cc2)[N+]([O-])=O)cc(c1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C25H23N3O5/c29-24(19-5-7-23(8-6-19)28(32)33)27-22-14-20(13-21(15-22)25(30)31)17-3-1-16(2-4-17)18-9-11-26-12-10-18/h1-8,13-15,18,26H,9-12H2,(H,27,29)(H,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R stably expressed in human forskolin treated THP1 cells assessed as reduction in P2Y14R agonist UDPG-induced inhib... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111564
BindingDB Entry DOI: 10.7270/Q20005D2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data