 Found 15 hits of ec50 for UniProtKB: P39086
Found 15 hits of ec50 for UniProtKB: P39086 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50126761
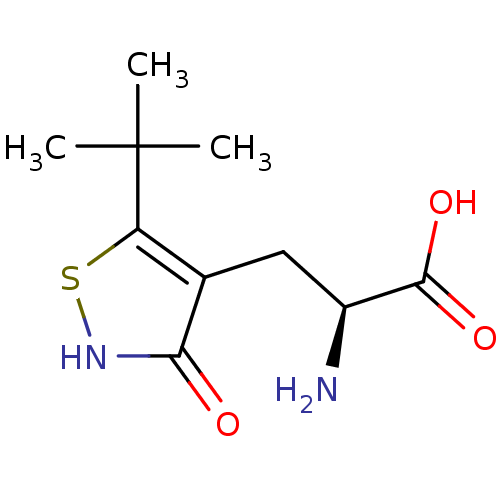
((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...)Show InChI InChI=1S/C10H16N2O3S/c1-10(2,3)7-5(8(13)12-16-7)4-6(11)9(14)15/h6H,4,11H2,1-3H3,(H,12,13)(H,14,15)/t6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at GluK1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50088222

((2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pe...)Show SMILES N[C@@H](C[C@@H](C\C=C\c1ccc2ccccc2c1)C(O)=O)C(O)=O Show InChI InChI=1S/C18H19NO4/c19-16(18(22)23)11-15(17(20)21)7-3-4-12-8-9-13-5-1-2-6-14(13)10-12/h1-6,8-10,15-16H,7,11,19H2,(H,20,21)(H,22,23)/b4-3+/t15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd
Curated by ChEMBL
| Assay Description
Compound was tested for agonistic activity at Glutamate receptor 6 using HEK293 cells |
Bioorg Med Chem Lett 10: 1807-10 (2000)
BindingDB Entry DOI: 10.7270/Q2765FVB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50088222

((2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pe...)Show SMILES N[C@@H](C[C@@H](C\C=C\c1ccc2ccccc2c1)C(O)=O)C(O)=O Show InChI InChI=1S/C18H19NO4/c19-16(18(22)23)11-15(17(20)21)7-3-4-12-8-9-13-5-1-2-6-14(13)10-12/h1-6,8-10,15-16H,7,11,19H2,(H,20,21)(H,22,23)/b4-3+/t15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at GluK1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50088222

((2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pe...)Show SMILES N[C@@H](C[C@@H](C\C=C\c1ccc2ccccc2c1)C(O)=O)C(O)=O Show InChI InChI=1S/C18H19NO4/c19-16(18(22)23)11-15(17(20)21)7-3-4-12-8-9-13-5-1-2-6-14(13)10-12/h1-6,8-10,15-16H,7,11,19H2,(H,20,21)(H,22,23)/b4-3+/t15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Effective concentration against GluR5 expressed in HEK293 cells |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50088197
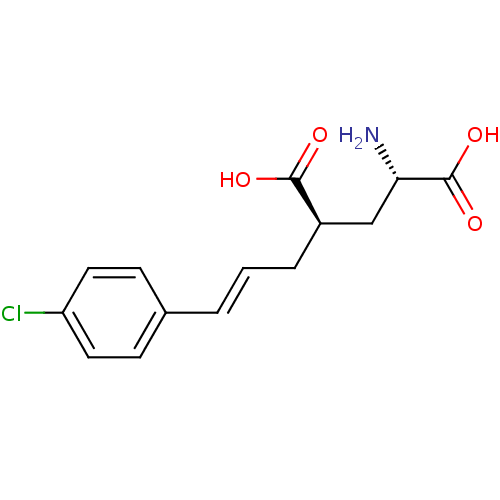
(CHEMBL58590 | E-2-Amino-4-[3-(4-chloro-phenyl)-all...)Show SMILES N[C@@H](C[C@@H](C\C=C\c1ccc(Cl)cc1)C(O)=O)C(O)=O Show InChI InChI=1S/C14H16ClNO4/c15-11-6-4-9(5-7-11)2-1-3-10(13(17)18)8-12(16)14(19)20/h1-2,4-7,10,12H,3,8,16H2,(H,17,18)(H,19,20)/b2-1+/t10-,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Effective concentration of the compound required for evoking response in HEK293 cell |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50088216
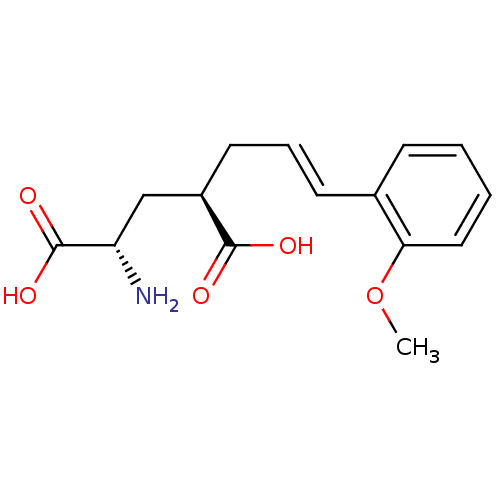
(CHEMBL59284 | E-2-Amino-4-[3-(2-methoxy-phenyl)-al...)Show SMILES COc1ccccc1\C=C\C[C@H](C[C@H](N)C(O)=O)C(O)=O Show InChI InChI=1S/C15H19NO5/c1-21-13-8-3-2-5-10(13)6-4-7-11(14(17)18)9-12(16)15(19)20/h2-6,8,11-12H,7,9,16H2,1H3,(H,17,18)(H,19,20)/b6-4+/t11-,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Effective concentration against GluR5 expressed in HEK293 cells |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50088213

((2S,4R)-4-allyl glutamate | 2-Allyl-4-amino-pentan...)Show InChI InChI=1S/C8H13NO4/c1-2-3-5(7(10)11)4-6(9)8(12)13/h2,5-6H,1,3-4,9H2,(H,10,11)(H,12,13)/t5-,6+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Effective concentration of the compound required for evoking response in HEK293 cell |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50088212

(2-Amino-4-but-2-enyl-pentanedioic acid(LY339180) |...)Show InChI InChI=1S/C9H15NO4/c1-2-3-4-6(8(11)12)5-7(10)9(13)14/h2-3,6-7H,4-5,10H2,1H3,(H,11,12)(H,13,14)/b3-2+/t6-,7+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Effective concentration of the compound required for evoking response in HEK293 cell |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50091477
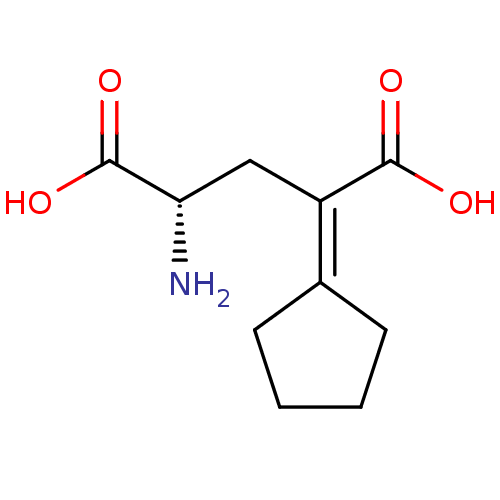
((S)-2-Amino-4-cyclopentylidene-pentanedioic acid |...)Show SMILES [#7]-[#6@@H](-[#6]\[#6](-[#6](-[#8])=O)=[#6]-1\[#6]-[#6]-[#6]-[#6]-1)-[#6](-[#8])=O Show InChI InChI=1S/C10H15NO4/c11-8(10(14)15)5-7(9(12)13)6-3-1-2-4-6/h8H,1-5,11H2,(H,12,13)(H,14,15)/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd
Curated by ChEMBL
| Assay Description
Compound was tested for agonistic activity at Glutamate receptor 5 using HEK293 cells |
Bioorg Med Chem Lett 10: 1807-10 (2000)
BindingDB Entry DOI: 10.7270/Q2765FVB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50091481
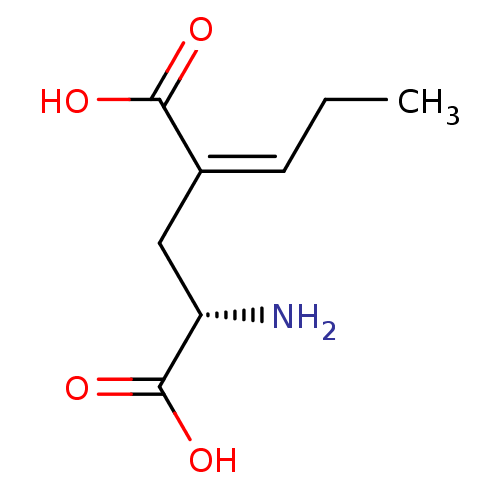
((S)-2-Amino-4-propylidene-pentanedioicacid | CHEMB...)Show InChI InChI=1S/C8H13NO4/c1-2-3-5(7(10)11)4-6(9)8(12)13/h3,6H,2,4,9H2,1H3,(H,10,11)(H,12,13)/b5-3-/t6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd
Curated by ChEMBL
| Assay Description
Compound was tested for agonistic activity at Glutamate receptor 5 using HEK293 cells |
Bioorg Med Chem Lett 10: 1807-10 (2000)
BindingDB Entry DOI: 10.7270/Q2765FVB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50002369
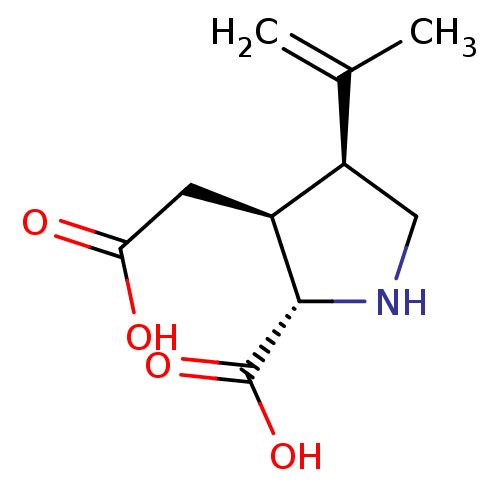
((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd
Curated by ChEMBL
| Assay Description
Compound was tested for agonistic activity at Glutamate receptor 5 using HEK293 cells |
Bioorg Med Chem Lett 10: 1807-10 (2000)
BindingDB Entry DOI: 10.7270/Q2765FVB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Effective concentration against GluR5 expressed in HEK293 cells |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM26431
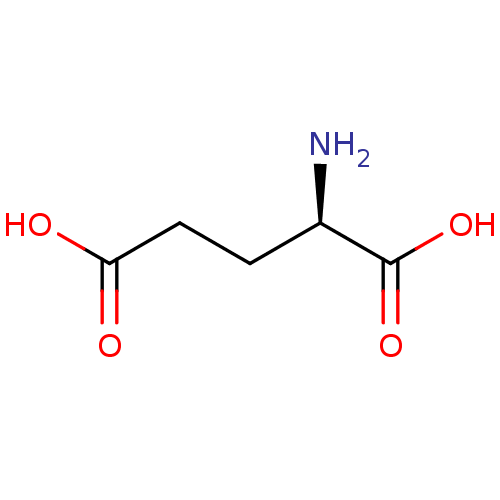
((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a |
University Walk
Curated by ChEMBL
| Assay Description
Induction of calcium influx in HEK293 cells expressing human GLUK5 by FLIPR assay |
J Med Chem 49: 2579-92 (2006)
Article DOI: 10.1021/jm051086f
BindingDB Entry DOI: 10.7270/Q2FQ9W76 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd
Curated by ChEMBL
| Assay Description
Compound was tested for agonistic activity at Glutamate receptor 6 using HEK293 cells |
Bioorg Med Chem Lett 10: 1807-10 (2000)
BindingDB Entry DOI: 10.7270/Q2765FVB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM26431

((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Effective concentration against GluR5 expressed in HEK293 cells |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data