 Found 27 hits of ki for UniProtKB: P05093
Found 27 hits of ki for UniProtKB: P05093 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063477

((3S,10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-2,3,4...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h3,6,11-12,14,16-19,25H,4-5,7-10,13H2,1-2H3/t16-,17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50435992
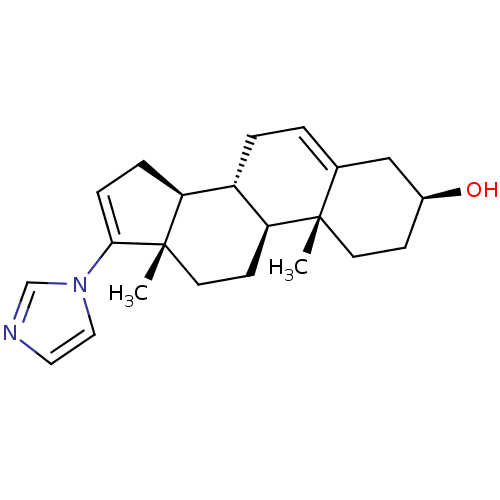
(CHEMBL2392006)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC=C2n1ccnc1 |r,c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h3,6,11-12,14,16-19,25H,4-5,7-10,13H2,1-2H3/t16-,17-,18-,19-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 using 17alpha-hydroxypregnenolone substrate |
J Med Chem 58: 2077-87 (2015)
Article DOI: 10.1021/jm501239f
BindingDB Entry DOI: 10.7270/Q2MS3VFF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50075389
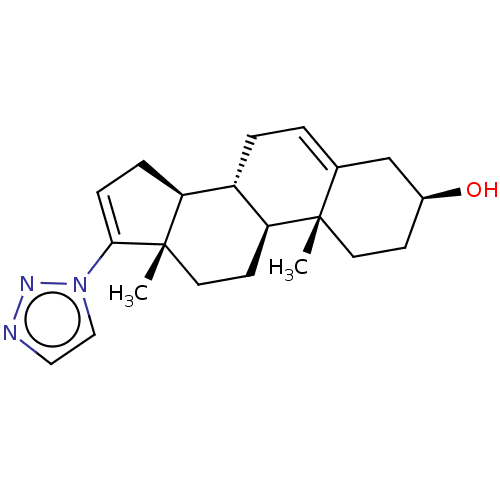
(CHEMBL3415119)Show SMILES [H][C@@]12CC=C(n3ccnn3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |r,t:3,22| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3/t15-,16-,17-,18-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 using 17alpha-hydroxypregnenolone substrate |
J Med Chem 58: 2077-87 (2015)
Article DOI: 10.1021/jm501239f
BindingDB Entry DOI: 10.7270/Q2MS3VFF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063476

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063475
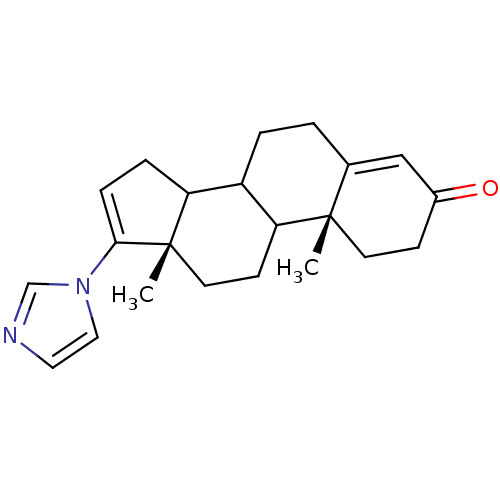
((10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h6,11-14,17-19H,3-5,7-10H2,1-2H3/t17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50075391
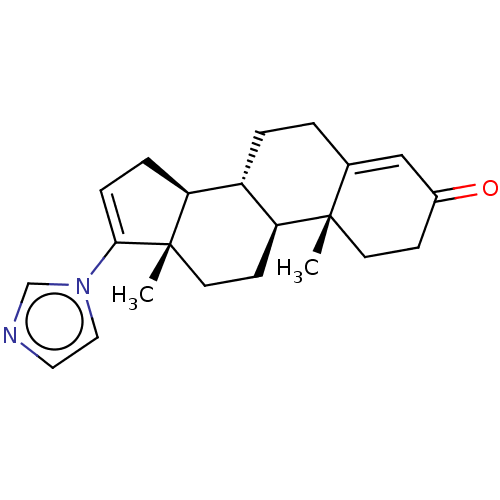
(CHEMBL3415124)Show SMILES [H][C@@]12CC=C(n3ccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:3,23| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h6,11-14,17-19H,3-5,7-10H2,1-2H3/t17-,18-,19-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 using 17alpha-hydroxypregnenolone substrate |
J Med Chem 58: 2077-87 (2015)
Article DOI: 10.1021/jm501239f
BindingDB Entry DOI: 10.7270/Q2MS3VFF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409039

(CHEMBL2111951)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)C1=CC[C@@H]2[C@H]1CN1 |r,c:19,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,5,14-15,17-19,22-23H,4,6-12H2,1-2H3/t14-,15-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50094191
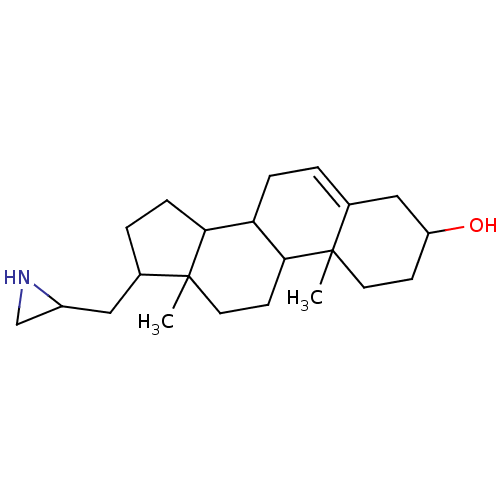
(17-Aziridin-2-ylmethyl-10,13-dimethyl-2,3,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H35NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16-20,23-24H,4-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063479
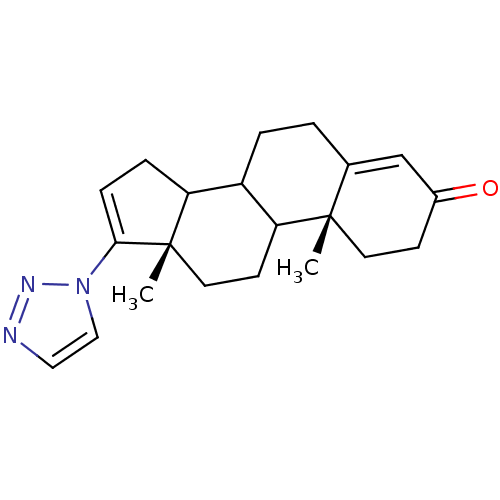
((10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl-1,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:8| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50075392
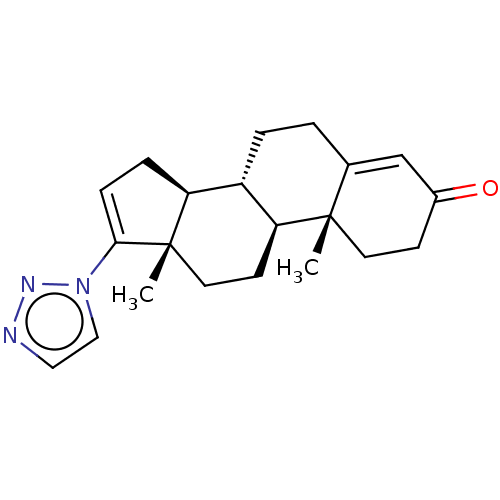
(CHEMBL3415125)Show SMILES [H][C@@]12CC=C(n3ccnn3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:3,23| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16-,17-,18-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 using 17alpha-hydroxypregnenolone substrate |
J Med Chem 58: 2077-87 (2015)
Article DOI: 10.1021/jm501239f
BindingDB Entry DOI: 10.7270/Q2MS3VFF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063480

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1cncn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50075388
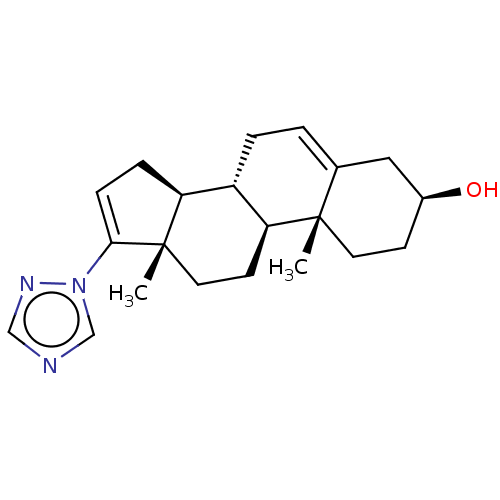
(CHEMBL3415117)Show SMILES [H][C@@]12CC=C(n3cncn3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |r,t:3,22| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3/t15-,16-,17-,18-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 using 17alpha-hydroxypregnenolone substrate |
J Med Chem 58: 2077-87 (2015)
Article DOI: 10.1021/jm501239f
BindingDB Entry DOI: 10.7270/Q2MS3VFF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768
(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 using 17alpha-hydroxypregnenolone substrate |
J Med Chem 58: 2077-87 (2015)
Article DOI: 10.1021/jm501239f
BindingDB Entry DOI: 10.7270/Q2MS3VFF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063478
((10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl-1,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1cncn1 |c:21,t:8| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50075390
(CHEMBL3415123)Show SMILES [H][C@@]12CC=C(n3cncn3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:3,23| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16-,17-,18-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human CYP17 using 17alpha-hydroxypregnenolone substrate |
J Med Chem 58: 2077-87 (2015)
Article DOI: 10.1021/jm501239f
BindingDB Entry DOI: 10.7270/Q2MS3VFF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044425
(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 17alpha,20-lyase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50128548
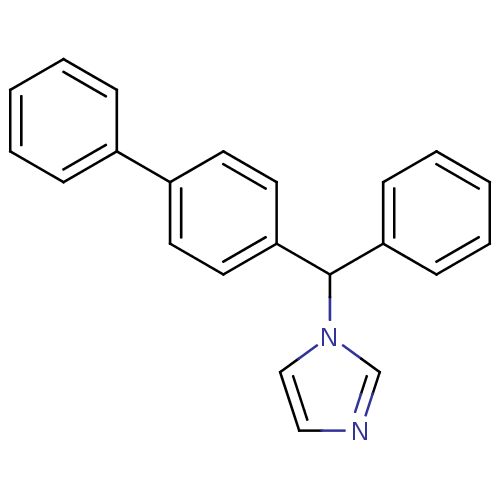
(1-(1-Biphenyl-4-yl-2-phenyl-methyl)-1H-imidazole |...)Show InChI InChI=1S/C22H18N2/c1-3-7-18(8-4-1)19-11-13-21(14-12-19)22(24-16-15-23-17-24)20-9-5-2-6-10-20/h1-17,22H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 56.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Cytochrome P450 17A1 activity |
J Med Chem 46: 2345-51 (2003)
Article DOI: 10.1021/jm020576u
BindingDB Entry DOI: 10.7270/Q2WD41B7 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31774
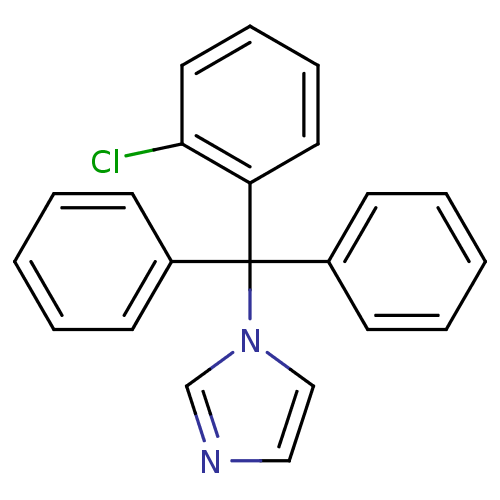
(CHEMBL104 | Canesten | Clotrimazole | Lotrimin | M...)Show InChI InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 81.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Cytochrome P450 17A1 activity |
J Med Chem 46: 2345-51 (2003)
Article DOI: 10.1021/jm020576u
BindingDB Entry DOI: 10.7270/Q2WD41B7 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for cholesterol 17-alpha-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31772
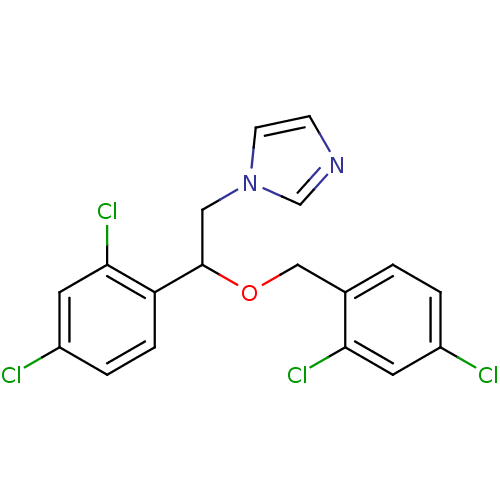
(1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...)Show SMILES Clc1ccc(COC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Cytochrome P450 17A1 activity |
J Med Chem 46: 2345-51 (2003)
Article DOI: 10.1021/jm020576u
BindingDB Entry DOI: 10.7270/Q2WD41B7 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31773
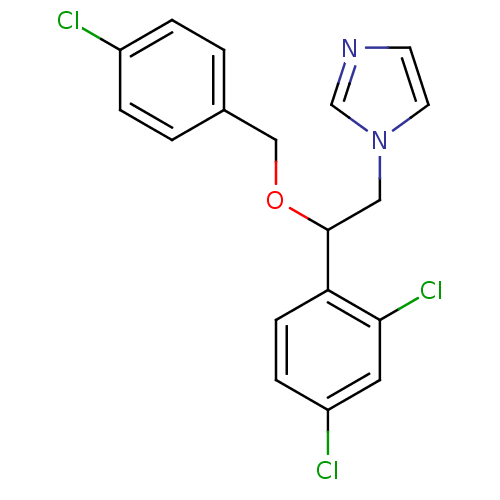
(ECONAZOLE | Econazole nitrate | Gyno-pevaryl | Pev...)Show InChI InChI=1S/C18H15Cl3N2O/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Cytochrome P450 17A1 activity |
J Med Chem 46: 2345-51 (2003)
Article DOI: 10.1021/jm020576u
BindingDB Entry DOI: 10.7270/Q2WD41B7 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044424
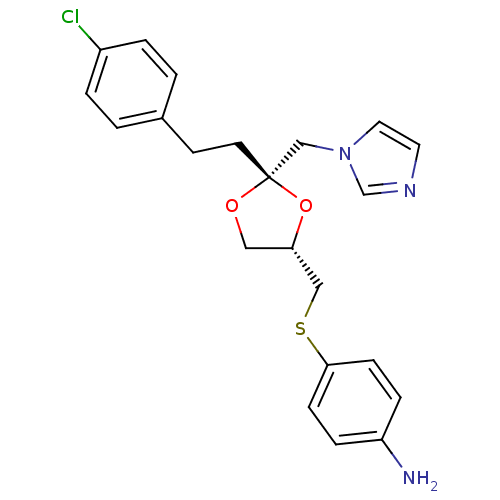
(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 17-alpha,20-lyase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50370218
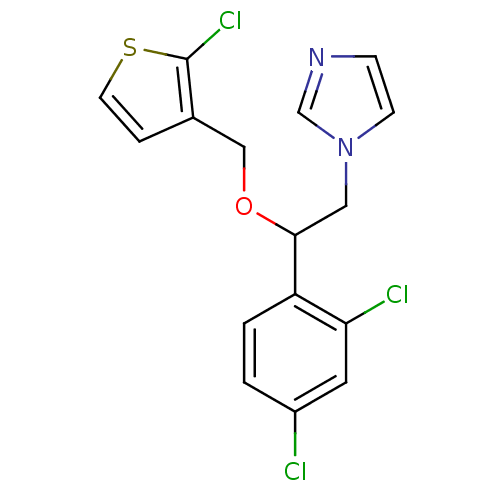
(TIOCONAZOLE)Show InChI InChI=1S/C16H13Cl3N2OS/c17-12-1-2-13(14(18)7-12)15(8-21-5-4-20-10-21)22-9-11-3-6-23-16(11)19/h1-7,10,15H,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Cytochrome P450 17A1 activity |
J Med Chem 46: 2345-51 (2003)
Article DOI: 10.1021/jm020576u
BindingDB Entry DOI: 10.7270/Q2WD41B7 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50128554
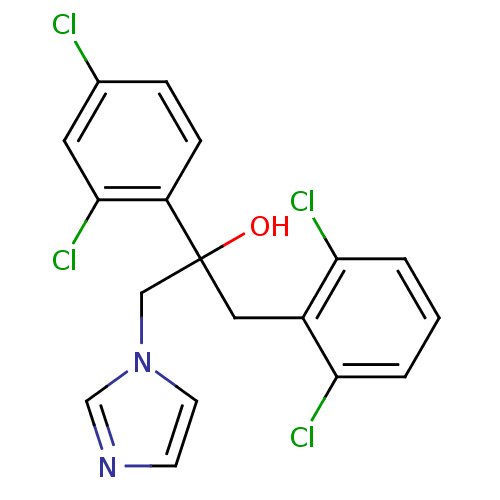
(1-(2,6-Dichloro-phenyl)-2-(2,4-dichloro-phenyl)-3-...)Show SMILES OC(Cc1c(Cl)cccc1Cl)(Cn1ccnc1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C18H14Cl4N2O/c19-12-4-5-14(17(22)8-12)18(25,10-24-7-6-23-11-24)9-13-15(20)2-1-3-16(13)21/h1-8,11,25H,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Cytochrome P450 17A1 activity |
J Med Chem 46: 2345-51 (2003)
Article DOI: 10.1021/jm020576u
BindingDB Entry DOI: 10.7270/Q2WD41B7 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for cholesterol 17-alpha-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data